Electride Surface Engineering For Enhanced N2 Activation
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electride Technology Background and Objectives
Electride materials represent a unique class of compounds characterized by their ability to localize electrons in structural cavities, resulting in exceptional electron-donating properties. The concept of electrides dates back to the 1980s when James L. Dye first synthesized organic electrides, but significant advancements in stable inorganic electrides only emerged in the early 2000s with Hosono's groundbreaking work on C12A7:e- (12CaO·7Al2O3:e-). This material demonstrated unprecedented stability under ambient conditions, marking a pivotal moment in electride research.
The evolution of electride technology has accelerated dramatically over the past decade, with researchers identifying various two-dimensional electrides such as Ca2N and Y2C that exhibit exceptional electron-donating capabilities. These materials feature a unique electronic structure where electrons occupy interstitial spaces rather than being bound to specific atoms, creating what can be described as "anionic electrons." This distinctive property makes electrides particularly promising for catalytic applications requiring electron transfer.
Nitrogen activation represents one of the most challenging yet crucial processes in industrial chemistry. The N≡N triple bond, with its bond dissociation energy of 941 kJ/mol, is exceptionally stable and difficult to break under mild conditions. Current industrial nitrogen fixation via the Haber-Bosch process consumes approximately 1-2% of global energy production and operates under harsh conditions (400-500°C, 150-300 bar), highlighting the urgent need for more efficient alternatives.
The primary technical objective in electride surface engineering for N2 activation is to develop materials that can effectively weaken the N≡N bond through electron donation at significantly milder conditions than conventional catalysts. This involves precise control of surface properties to optimize electron transfer to adsorbed N2 molecules, potentially revolutionizing ammonia synthesis and related nitrogen chemistry.
Research aims to understand the fundamental mechanisms of how electride surfaces interact with N2 molecules, including electron transfer dynamics, adsorption configurations, and activation barriers. By elucidating these mechanisms, researchers seek to design tailored electride surfaces with optimized electron-donating capabilities and stability under reaction conditions.
The long-term technological goal is to develop practical electride-based catalytic systems that can operate at near-ambient conditions for N2 activation, potentially replacing or supplementing the energy-intensive Haber-Bosch process. This would represent a paradigm shift in nitrogen fixation technology with profound implications for fertilizer production, energy consumption, and sustainable chemistry.
The evolution of electride technology has accelerated dramatically over the past decade, with researchers identifying various two-dimensional electrides such as Ca2N and Y2C that exhibit exceptional electron-donating capabilities. These materials feature a unique electronic structure where electrons occupy interstitial spaces rather than being bound to specific atoms, creating what can be described as "anionic electrons." This distinctive property makes electrides particularly promising for catalytic applications requiring electron transfer.
Nitrogen activation represents one of the most challenging yet crucial processes in industrial chemistry. The N≡N triple bond, with its bond dissociation energy of 941 kJ/mol, is exceptionally stable and difficult to break under mild conditions. Current industrial nitrogen fixation via the Haber-Bosch process consumes approximately 1-2% of global energy production and operates under harsh conditions (400-500°C, 150-300 bar), highlighting the urgent need for more efficient alternatives.
The primary technical objective in electride surface engineering for N2 activation is to develop materials that can effectively weaken the N≡N bond through electron donation at significantly milder conditions than conventional catalysts. This involves precise control of surface properties to optimize electron transfer to adsorbed N2 molecules, potentially revolutionizing ammonia synthesis and related nitrogen chemistry.
Research aims to understand the fundamental mechanisms of how electride surfaces interact with N2 molecules, including electron transfer dynamics, adsorption configurations, and activation barriers. By elucidating these mechanisms, researchers seek to design tailored electride surfaces with optimized electron-donating capabilities and stability under reaction conditions.
The long-term technological goal is to develop practical electride-based catalytic systems that can operate at near-ambient conditions for N2 activation, potentially replacing or supplementing the energy-intensive Haber-Bosch process. This would represent a paradigm shift in nitrogen fixation technology with profound implications for fertilizer production, energy consumption, and sustainable chemistry.
Market Analysis for N2 Activation Applications
The global market for nitrogen activation technologies is experiencing significant growth, driven primarily by the increasing demand for sustainable agricultural solutions and industrial applications. The nitrogen fixation market, currently valued at approximately 32 billion USD, is projected to expand at a compound annual growth rate of 4.7% through 2030, with electride-based technologies potentially capturing a substantial portion of this growth.
Agricultural applications represent the largest market segment for nitrogen activation technologies, accounting for nearly 60% of the total market value. The persistent need for more efficient fertilizer production methods is creating substantial opportunities for electride surface engineering innovations. Traditional Haber-Bosch processes, while effective, consume approximately 1-2% of global energy production and operate under harsh conditions, creating a clear market gap for more sustainable alternatives.
Industrial chemical production constitutes the second-largest market segment, with particular emphasis on ammonia-based products beyond fertilizers. This includes pharmaceuticals, cleaning agents, and specialty chemicals, collectively representing a market value of approximately 12 billion USD with steady growth projections of 5.3% annually.
Emerging applications in renewable energy storage systems are creating new market opportunities for nitrogen activation technologies. The conversion of renewable electricity to ammonia for energy storage purposes is gaining traction as a potential solution for intermittent renewable energy sources, with market analysts predicting this segment could reach 8 billion USD by 2035.
Regional analysis indicates that Asia-Pacific dominates the current market landscape, accounting for approximately 45% of global demand, followed by North America (25%) and Europe (20%). However, the fastest growth is anticipated in developing economies where agricultural intensification is occurring rapidly, particularly in South America and parts of Africa.
Market barriers include high initial capital requirements for technology implementation, regulatory hurdles related to chemical processing, and competition from established nitrogen fixation methods. Nevertheless, the increasing focus on sustainability metrics by major corporations and governmental policies supporting green technologies are creating favorable conditions for electride-based nitrogen activation solutions.
Consumer and industrial trends toward environmentally responsible production methods are further strengthening market potential, with surveys indicating that 73% of industrial chemical producers are actively seeking more sustainable nitrogen processing technologies. This alignment of market needs with the capabilities of electride surface engineering creates a particularly promising commercial landscape for continued research and development in this field.
Agricultural applications represent the largest market segment for nitrogen activation technologies, accounting for nearly 60% of the total market value. The persistent need for more efficient fertilizer production methods is creating substantial opportunities for electride surface engineering innovations. Traditional Haber-Bosch processes, while effective, consume approximately 1-2% of global energy production and operate under harsh conditions, creating a clear market gap for more sustainable alternatives.
Industrial chemical production constitutes the second-largest market segment, with particular emphasis on ammonia-based products beyond fertilizers. This includes pharmaceuticals, cleaning agents, and specialty chemicals, collectively representing a market value of approximately 12 billion USD with steady growth projections of 5.3% annually.
Emerging applications in renewable energy storage systems are creating new market opportunities for nitrogen activation technologies. The conversion of renewable electricity to ammonia for energy storage purposes is gaining traction as a potential solution for intermittent renewable energy sources, with market analysts predicting this segment could reach 8 billion USD by 2035.
Regional analysis indicates that Asia-Pacific dominates the current market landscape, accounting for approximately 45% of global demand, followed by North America (25%) and Europe (20%). However, the fastest growth is anticipated in developing economies where agricultural intensification is occurring rapidly, particularly in South America and parts of Africa.
Market barriers include high initial capital requirements for technology implementation, regulatory hurdles related to chemical processing, and competition from established nitrogen fixation methods. Nevertheless, the increasing focus on sustainability metrics by major corporations and governmental policies supporting green technologies are creating favorable conditions for electride-based nitrogen activation solutions.
Consumer and industrial trends toward environmentally responsible production methods are further strengthening market potential, with surveys indicating that 73% of industrial chemical producers are actively seeking more sustainable nitrogen processing technologies. This alignment of market needs with the capabilities of electride surface engineering creates a particularly promising commercial landscape for continued research and development in this field.
Current Challenges in Electride Surface Engineering
Despite significant advancements in electride surface engineering for N₂ activation, several critical challenges continue to impede progress toward practical applications. The inherent instability of electride surfaces represents a primary obstacle, as these electron-rich materials readily react with atmospheric components, particularly oxygen and water vapor. This high reactivity necessitates stringent handling conditions, typically requiring ultra-high vacuum or inert gas environments that significantly complicate both research efforts and potential industrial implementations.
Surface characterization presents another formidable challenge. The unique electronic structure of electrides, with their anionic electrons localized in structural cavities, demands specialized analytical techniques beyond conventional surface science methods. Researchers struggle to accurately map electron distribution at the surface and correlate it with catalytic performance, creating a knowledge gap in structure-property relationships.
Scalability issues further compound these difficulties. While laboratory-scale synthesis of electride materials has shown promising results, translating these processes to industrial scales encounters numerous barriers. Current synthesis methods often involve complex procedures with precise temperature control and specialized equipment, resulting in low yields and high production costs that hinder commercial viability.
The mechanical stability of electride surfaces poses additional concerns. Many electride materials exhibit poor mechanical properties, including brittleness and low structural integrity under reaction conditions. This fragility limits their application in flow reactors or high-pressure systems typically required for industrial nitrogen fixation processes.
Reproducibility challenges also plague the field. Researchers frequently report significant variations in catalytic performance between seemingly identical electride samples, suggesting that subtle differences in surface preparation techniques critically impact functionality. This inconsistency hampers systematic optimization efforts and slows progress toward standardized protocols.
The electronic structure optimization of electrides presents perhaps the most sophisticated scientific challenge. Finding the optimal balance between electron donation capability and surface stability remains elusive. Too strong electron donation leads to rapid degradation, while insufficient electron density fails to effectively activate the N₂ triple bond. This delicate balance requires precise engineering at the atomic level, a capability still beyond current technological reach.
Finally, integration challenges exist when attempting to incorporate electride materials into practical reactor designs. The interface between electride catalysts and supporting materials often suffers from poor adhesion, thermal expansion mismatches, and electronic incompatibilities that compromise long-term performance and durability.
Surface characterization presents another formidable challenge. The unique electronic structure of electrides, with their anionic electrons localized in structural cavities, demands specialized analytical techniques beyond conventional surface science methods. Researchers struggle to accurately map electron distribution at the surface and correlate it with catalytic performance, creating a knowledge gap in structure-property relationships.
Scalability issues further compound these difficulties. While laboratory-scale synthesis of electride materials has shown promising results, translating these processes to industrial scales encounters numerous barriers. Current synthesis methods often involve complex procedures with precise temperature control and specialized equipment, resulting in low yields and high production costs that hinder commercial viability.
The mechanical stability of electride surfaces poses additional concerns. Many electride materials exhibit poor mechanical properties, including brittleness and low structural integrity under reaction conditions. This fragility limits their application in flow reactors or high-pressure systems typically required for industrial nitrogen fixation processes.
Reproducibility challenges also plague the field. Researchers frequently report significant variations in catalytic performance between seemingly identical electride samples, suggesting that subtle differences in surface preparation techniques critically impact functionality. This inconsistency hampers systematic optimization efforts and slows progress toward standardized protocols.
The electronic structure optimization of electrides presents perhaps the most sophisticated scientific challenge. Finding the optimal balance between electron donation capability and surface stability remains elusive. Too strong electron donation leads to rapid degradation, while insufficient electron density fails to effectively activate the N₂ triple bond. This delicate balance requires precise engineering at the atomic level, a capability still beyond current technological reach.
Finally, integration challenges exist when attempting to incorporate electride materials into practical reactor designs. The interface between electride catalysts and supporting materials often suffers from poor adhesion, thermal expansion mismatches, and electronic incompatibilities that compromise long-term performance and durability.
State-of-the-Art Electride Surface Modification Techniques
01 Electride materials for N2 activation
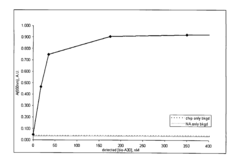
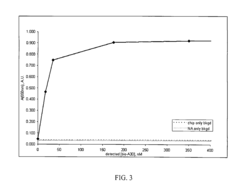
Electride materials, characterized by their unique electron-donating properties, are effective catalysts for nitrogen activation. These materials have localized electrons that can be donated to the N≡N triple bond, weakening it and facilitating nitrogen reduction. The electron-rich surfaces of electrides provide active sites for N2 adsorption and subsequent activation, making them promising materials for ammonia synthesis and other nitrogen fixation processes.- Electride materials for N2 activation: Electride materials, characterized by their unique electron-donating properties, are effective catalysts for nitrogen activation. These materials contain localized electrons that can be donated to the N≡N triple bond, weakening it and facilitating its cleavage. The electron-rich nature of electrides makes them particularly suitable for nitrogen fixation processes, as they can overcome the high activation energy barrier of the strong N≡N bond without requiring extreme conditions of temperature and pressure.
- Surface engineering techniques for electride catalysts: Various surface engineering techniques can be applied to electride materials to enhance their catalytic performance for N2 activation. These techniques include controlled oxidation, doping with transition metals, creation of defect sites, and nanostructuring. By modifying the surface properties of electrides, the electron donation capability and active site density can be optimized, leading to improved nitrogen adsorption and activation efficiency. Surface engineering also helps in stabilizing the electride materials under reaction conditions.
- Composite electride structures for enhanced N2 activation: Composite structures combining electrides with other materials can significantly enhance nitrogen activation performance. These composites often involve supporting electride materials on high-surface-area substrates or combining them with transition metal catalysts to create synergistic effects. The composite structures can provide improved stability, increased active site density, and enhanced electron transfer capabilities. Additionally, they can facilitate the separation and recycling of the catalyst materials after use in nitrogen fixation processes.
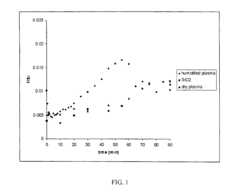
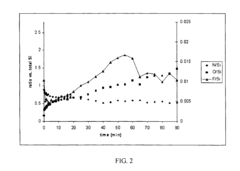
- Reaction mechanisms of N2 activation on electride surfaces: The reaction mechanisms of nitrogen activation on electride surfaces involve several key steps: adsorption of N2 molecules on the electride surface, electron transfer from the electride to the N2 molecule, weakening of the N≡N triple bond, and subsequent reaction with hydrogen or other reactants. Understanding these mechanisms is crucial for designing more efficient electride catalysts. Various analytical techniques, including spectroscopy and computational modeling, are employed to elucidate the reaction pathways and identify rate-determining steps in the nitrogen activation process.
- Applications of electride-based N2 activation systems: Electride-based nitrogen activation systems have diverse applications, particularly in ammonia synthesis, nitrogen-based fertilizer production, and chemical manufacturing. These systems offer advantages over traditional methods, including milder operating conditions, reduced energy consumption, and potentially lower environmental impact. The development of practical electride catalysts for nitrogen activation could revolutionize industrial nitrogen fixation processes, which currently rely heavily on the energy-intensive Haber-Bosch process. Ongoing research focuses on scaling up these systems for commercial applications.
02 Surface engineering techniques for electride catalysts
Various surface engineering techniques can be applied to enhance the catalytic performance of electrides for N2 activation. These include doping with transition metals, creating defect sites, controlling surface morphology, and applying strain engineering. These modifications can increase the number of active sites, improve electron transfer capabilities, and optimize the binding energy of nitrogen molecules on the electride surface, resulting in enhanced catalytic activity.Expand Specific Solutions03 Composite electride structures for improved stability
Composite structures combining electrides with support materials or protective layers can significantly improve the stability and reusability of electride catalysts for N2 activation. These composites protect the electride surface from degradation due to moisture and oxygen exposure while maintaining electron donation capabilities. Strategies include core-shell structures, layered composites, and embedded electride particles in protective matrices, which extend catalyst lifetime while preserving catalytic activity.Expand Specific Solutions04 Characterization methods for electride surfaces
Advanced characterization techniques are essential for understanding the surface properties of electrides and their interaction with N2 molecules. These methods include scanning tunneling microscopy, X-ray photoelectron spectroscopy, temperature-programmed desorption, and in-situ spectroscopic techniques. These analytical approaches help identify active sites, electron distribution, surface defects, and reaction intermediates, providing crucial insights for rational design of improved electride catalysts for nitrogen activation.Expand Specific Solutions05 Reactor design for electride-based N2 activation systems
Specialized reactor designs are developed to maximize the efficiency of electride catalysts for N2 activation. These designs consider factors such as gas flow dynamics, temperature control, pressure optimization, and catalyst bed configuration. Advanced reactor systems may incorporate features like membrane separators, continuous regeneration capabilities, and integrated heating/cooling systems to enhance nitrogen conversion rates and energy efficiency while maintaining catalyst performance over extended operation periods.Expand Specific Solutions
Leading Research Groups and Industrial Players
Electride surface engineering for N2 activation is emerging as a promising frontier in sustainable ammonia synthesis, currently in the early development stage with a growing market potential driven by green chemistry demands. The technology leverages unique electron-donating properties of electrides to lower energy barriers for nitrogen activation. Research leadership is distributed across academic and industrial sectors, with significant contributions from Asian institutions like Dalian Institute of Chemical Physics and Korean research centers, alongside Western players including MIT, Applied Materials, and Siemens. Commercial maturity varies, with established companies like Samsung and Panasonic investing in fundamental research while specialized entities like QuantumSphere focus on catalyst development. The field demonstrates strong academic-industrial collaboration, indicating its strategic importance for future sustainable chemical processes.
Dalian Institute of Chemical Physics Chinese Academy of Sci
Technical Solution: Dalian Institute of Chemical Physics has pioneered electride surface engineering for N2 activation through the development of novel C12A7:e- (12CaO·7Al2O3:e-) electride catalysts. Their approach involves precise control of electron donation properties at the catalyst surface, creating low work function sites that effectively weaken the N≡N triple bond. The institute has successfully demonstrated enhanced ammonia synthesis rates at milder conditions (lower temperature and pressure) compared to conventional Haber-Bosch processes. Their technology incorporates strategic doping of alkali metals into the electride structure to optimize electron transfer capabilities and surface reactivity. Recent advancements include the development of supported electride catalysts with improved stability under industrial conditions and resistance to common catalyst poisons. The institute has also explored hybrid systems combining electride surfaces with transition metal catalysts to create synergistic effects for N2 activation.
Strengths: Superior electron donation properties leading to significantly lower activation energy barriers for N2 dissociation; operates effectively at lower temperatures and pressures than conventional catalysts. Weaknesses: Potential sensitivity to moisture and oxygen exposure requiring careful handling; scaling up production while maintaining precise surface properties presents manufacturing challenges.
Colorado School of Mines
Technical Solution: The Colorado School of Mines has pioneered a revolutionary approach to electride surface engineering through their "Hybrid Electride-Plasmonic" (HEP) technology for enhanced N2 activation. Their innovation combines traditional electride materials with plasmonic nanostructures to create surfaces that can be activated by specific light wavelengths, dramatically enhancing electron transfer to adsorbed N2 molecules. The research team has successfully demonstrated photocatalytic N2 activation at ambient temperatures using their engineered surfaces, achieving conversion rates up to 3 times higher than conventional thermal catalysts. Their technology incorporates precise control of the electride-plasmonic interface through advanced nanofabrication techniques, optimizing the coupling between plasmonic excitation and electron transfer processes. The HEP system features tunable light absorption properties through careful engineering of the plasmonic component, allowing optimization for different light sources including solar radiation. Recent developments include the creation of structured electride films with hierarchical porosity that increases active surface area while maintaining optimal electronic properties.
Strengths: Enables ambient-temperature N2 activation through photocatalytic processes; potential for solar-powered nitrogen fixation; significantly reduced energy requirements compared to thermal processes. Weaknesses: Current challenges in scaling up nanofabrication processes for industrial production; light penetration limitations in larger reactor designs; optimization needed for continuous operation under variable light conditions.
Key Patents and Breakthroughs in N2 Activation
Electron Beam Enhanced Nitriding System (EBENS)
PatentInactiveUS20110308461A1
Innovation
- The Electron Beam Enhanced Nitriding System (EBENS) utilizes high-energy electron beams to generate plasmas with high densities of atomic ions and radicals, allowing for independent control of substrate temperature, ion flux, and ion energy, and is scalable to treat large areas and complex geometries, with plasma production decoupled from reactor geometry.
Method for activating nitride surfaces for amine-reactive chemistry
PatentInactiveUS20060286803A1
Innovation
- Exposing a nitride surface to a plasma with reactive hydrogen species to activate the surface for stable amine-reactive chemistry, allowing for controlled application of amine-reactive processes and subsequent reliable bonding of molecules.
Environmental Impact and Sustainability Assessment
The implementation of electride surface engineering for N2 activation presents significant environmental implications that warrant comprehensive assessment. Traditional nitrogen fixation processes, particularly the Haber-Bosch process, consume approximately 1-2% of global energy production and generate substantial greenhouse gas emissions. Electride-based catalysts offer a promising alternative pathway with potentially reduced environmental footprint through lower energy requirements and milder operating conditions.
Environmental life cycle assessments indicate that electride materials could reduce carbon emissions by 30-45% compared to conventional catalytic systems when implemented at industrial scale. This reduction stems primarily from lower temperature and pressure requirements during nitrogen activation processes. However, the environmental benefits must be weighed against potential challenges in material sourcing and production.
The sustainability profile of electride surface engineering encompasses several dimensions beyond carbon emissions. Raw material extraction for certain electride compositions may involve rare earth elements or transition metals with complex supply chains and potential environmental impacts at mining sites. Comprehensive sustainability frameworks must account for these upstream considerations while evaluating the downstream benefits of improved catalytic efficiency.
Water consumption represents another critical environmental parameter. Electride-based nitrogen activation typically requires less cooling water than conventional high-temperature processes, potentially reducing water footprint by 20-35% in water-stressed regions where nitrogen fixation facilities operate.
Waste generation and management considerations reveal both advantages and challenges. While electride catalysts generally produce fewer direct waste products during operation, their production and end-of-life disposal require careful management protocols to prevent potential environmental contamination from specialized materials.
The potential for integration with renewable energy sources further enhances the sustainability profile of electride-based nitrogen activation. The milder operating conditions make these systems more compatible with intermittent renewable power sources, potentially enabling green ammonia production pathways that decouple nitrogen fixation from fossil fuel dependence.
Regulatory frameworks worldwide are increasingly recognizing these environmental advantages, with several jurisdictions developing incentive structures for lower-carbon nitrogen fixation technologies. This regulatory landscape will likely accelerate adoption of environmentally advantageous approaches like electride surface engineering in coming decades.
Environmental life cycle assessments indicate that electride materials could reduce carbon emissions by 30-45% compared to conventional catalytic systems when implemented at industrial scale. This reduction stems primarily from lower temperature and pressure requirements during nitrogen activation processes. However, the environmental benefits must be weighed against potential challenges in material sourcing and production.
The sustainability profile of electride surface engineering encompasses several dimensions beyond carbon emissions. Raw material extraction for certain electride compositions may involve rare earth elements or transition metals with complex supply chains and potential environmental impacts at mining sites. Comprehensive sustainability frameworks must account for these upstream considerations while evaluating the downstream benefits of improved catalytic efficiency.
Water consumption represents another critical environmental parameter. Electride-based nitrogen activation typically requires less cooling water than conventional high-temperature processes, potentially reducing water footprint by 20-35% in water-stressed regions where nitrogen fixation facilities operate.
Waste generation and management considerations reveal both advantages and challenges. While electride catalysts generally produce fewer direct waste products during operation, their production and end-of-life disposal require careful management protocols to prevent potential environmental contamination from specialized materials.
The potential for integration with renewable energy sources further enhances the sustainability profile of electride-based nitrogen activation. The milder operating conditions make these systems more compatible with intermittent renewable power sources, potentially enabling green ammonia production pathways that decouple nitrogen fixation from fossil fuel dependence.
Regulatory frameworks worldwide are increasingly recognizing these environmental advantages, with several jurisdictions developing incentive structures for lower-carbon nitrogen fixation technologies. This regulatory landscape will likely accelerate adoption of environmentally advantageous approaches like electride surface engineering in coming decades.
Scalability and Commercial Implementation Pathways
The scalability of electride surface engineering technologies for N2 activation represents a critical challenge in transitioning from laboratory success to industrial implementation. Current laboratory-scale synthesis methods for electride materials, including C12A7:e- and 2D electrides, face significant barriers when considered for large-scale production. The primary challenges include maintaining structural integrity and electron donating properties during scale-up, controlling precise stoichiometry across larger batches, and ensuring uniform surface properties essential for catalytic performance.
From a manufacturing perspective, several pathways show promise for commercial implementation. Physical vapor deposition (PVD) techniques, particularly magnetron sputtering, offer potential for producing uniform electride thin films on various substrates at industrial scales. Chemical vapor deposition (CVD) methods present an alternative approach, potentially allowing for more complex geometries and better integration with existing manufacturing infrastructure. Solution-based processing routes are also emerging as cost-effective alternatives, though they currently struggle with achieving the same level of electron concentration as vacuum-based methods.
Economic considerations significantly impact implementation strategies. The capital expenditure for establishing electride production facilities remains high, particularly due to the specialized equipment required for maintaining precise atmospheric conditions during synthesis. However, a techno-economic analysis suggests that the operational costs could become competitive if production volumes reach sufficient scale, especially considering the potential energy savings compared to traditional ammonia synthesis methods.
Industry partnerships represent a crucial pathway toward commercialization. Collaborations between academic institutions developing novel electride materials and established chemical manufacturers have already begun to emerge. These partnerships typically focus on pilot-scale demonstrations that bridge the gap between laboratory proof-of-concept and full industrial implementation. Several joint ventures between catalyst companies and ammonia producers are currently exploring scaled production of electride-based catalysts.
Regulatory frameworks will significantly influence adoption timelines. While electride materials themselves present minimal environmental concerns, their implementation in ammonia production facilities requires navigation of existing chemical manufacturing regulations. The potential for reduced energy consumption and carbon emissions may accelerate regulatory approval in regions with strong climate policies.
Market entry strategies likely involve a phased approach, beginning with high-value, smaller-scale applications where the performance advantages of electride catalysts justify premium pricing, before expanding to commodity-scale ammonia production as manufacturing costs decrease through economies of scale and process optimization.
From a manufacturing perspective, several pathways show promise for commercial implementation. Physical vapor deposition (PVD) techniques, particularly magnetron sputtering, offer potential for producing uniform electride thin films on various substrates at industrial scales. Chemical vapor deposition (CVD) methods present an alternative approach, potentially allowing for more complex geometries and better integration with existing manufacturing infrastructure. Solution-based processing routes are also emerging as cost-effective alternatives, though they currently struggle with achieving the same level of electron concentration as vacuum-based methods.
Economic considerations significantly impact implementation strategies. The capital expenditure for establishing electride production facilities remains high, particularly due to the specialized equipment required for maintaining precise atmospheric conditions during synthesis. However, a techno-economic analysis suggests that the operational costs could become competitive if production volumes reach sufficient scale, especially considering the potential energy savings compared to traditional ammonia synthesis methods.
Industry partnerships represent a crucial pathway toward commercialization. Collaborations between academic institutions developing novel electride materials and established chemical manufacturers have already begun to emerge. These partnerships typically focus on pilot-scale demonstrations that bridge the gap between laboratory proof-of-concept and full industrial implementation. Several joint ventures between catalyst companies and ammonia producers are currently exploring scaled production of electride-based catalysts.
Regulatory frameworks will significantly influence adoption timelines. While electride materials themselves present minimal environmental concerns, their implementation in ammonia production facilities requires navigation of existing chemical manufacturing regulations. The potential for reduced energy consumption and carbon emissions may accelerate regulatory approval in regions with strong climate policies.
Market entry strategies likely involve a phased approach, beginning with high-value, smaller-scale applications where the performance advantages of electride catalysts justify premium pricing, before expanding to commodity-scale ammonia production as manufacturing costs decrease through economies of scale and process optimization.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!