Roadmap For Translating Electride Research Into Industrial Catalysts
AUG 28, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electride Catalysis Background and Objectives
Electride materials represent a revolutionary class of compounds characterized by their unique electron configuration, where electrons serve as anions without being bound to specific atomic nuclei. This distinctive property has positioned electrides as promising candidates for catalytic applications, particularly in reactions requiring electron donation or transfer. The evolution of electride research traces back to the 1980s with the pioneering work of James L. Dye on alkali metal electrides, but significant breakthroughs in stable, air-resistant electrides only emerged in the early 2000s with the development of mayenite-based electrides (C12A7:e-) by Hosono and colleagues.
The technological trajectory of electride catalysis has accelerated dramatically over the past decade, with remarkable demonstrations in ammonia synthesis, nitrogen reduction, and hydrogenation reactions. These materials have shown potential to operate under milder conditions than conventional catalysts, potentially revolutionizing energy-intensive chemical processes. The fundamental advantage lies in their ability to donate electrons with minimal energy barriers, facilitating bond activation in ways traditional catalysts cannot achieve.
Current research objectives in electride catalysis focus on bridging the considerable gap between laboratory discoveries and industrial implementation. Primary goals include enhancing the stability of electride catalysts under industrial conditions, scaling up synthesis methods while maintaining consistent properties, and developing cost-effective production techniques that can compete with established catalytic systems. Additionally, researchers aim to expand the reaction scope beyond the currently demonstrated processes to include other industrially significant transformations.
Another critical objective involves understanding the precise mechanistic details of electride-mediated catalysis. While the electron-donating properties are well-established, the exact nature of active sites, reaction intermediates, and deactivation pathways remains incompletely understood. Developing this fundamental knowledge is essential for rational catalyst design and optimization.
The translation of electride research into viable industrial catalysts also necessitates addressing engineering challenges related to reactor design, catalyst formulation, and process integration. Current objectives include developing supported electride catalysts with optimized morphologies, investigating regeneration protocols for deactivated materials, and establishing performance metrics that accurately predict industrial viability.
Looking forward, the field aims to establish clear design principles for next-generation electride catalysts with tailored electronic properties, enhanced stability, and optimized activity. This includes exploring new classes of electride materials beyond the current mayenite and 2D electride systems, potentially incorporating computational screening and machine learning approaches to accelerate discovery and development timelines.
The technological trajectory of electride catalysis has accelerated dramatically over the past decade, with remarkable demonstrations in ammonia synthesis, nitrogen reduction, and hydrogenation reactions. These materials have shown potential to operate under milder conditions than conventional catalysts, potentially revolutionizing energy-intensive chemical processes. The fundamental advantage lies in their ability to donate electrons with minimal energy barriers, facilitating bond activation in ways traditional catalysts cannot achieve.
Current research objectives in electride catalysis focus on bridging the considerable gap between laboratory discoveries and industrial implementation. Primary goals include enhancing the stability of electride catalysts under industrial conditions, scaling up synthesis methods while maintaining consistent properties, and developing cost-effective production techniques that can compete with established catalytic systems. Additionally, researchers aim to expand the reaction scope beyond the currently demonstrated processes to include other industrially significant transformations.
Another critical objective involves understanding the precise mechanistic details of electride-mediated catalysis. While the electron-donating properties are well-established, the exact nature of active sites, reaction intermediates, and deactivation pathways remains incompletely understood. Developing this fundamental knowledge is essential for rational catalyst design and optimization.
The translation of electride research into viable industrial catalysts also necessitates addressing engineering challenges related to reactor design, catalyst formulation, and process integration. Current objectives include developing supported electride catalysts with optimized morphologies, investigating regeneration protocols for deactivated materials, and establishing performance metrics that accurately predict industrial viability.
Looking forward, the field aims to establish clear design principles for next-generation electride catalysts with tailored electronic properties, enhanced stability, and optimized activity. This includes exploring new classes of electride materials beyond the current mayenite and 2D electride systems, potentially incorporating computational screening and machine learning approaches to accelerate discovery and development timelines.
Market Analysis for Industrial Electride Catalysts
The global market for industrial catalysts is projected to reach $40 billion by 2025, with a compound annual growth rate of 4.5%. Within this expanding sector, electride-based catalysts represent an emerging segment with significant growth potential due to their superior performance characteristics. Current market penetration remains limited, primarily confined to research applications and pilot-scale implementations, but industry analysts forecast rapid adoption as manufacturing processes mature.
Ammonia synthesis represents the most immediate and substantial market opportunity for electride catalysts. The global ammonia market, valued at approximately $70 billion, relies heavily on the Haber-Bosch process, which consumes nearly 2% of global energy production. Electride catalysts demonstrating the ability to operate at lower temperatures and pressures could disrupt this market by offering substantial energy savings and operational cost reductions of up to 30%.
Hydrogenation processes across pharmaceutical, food, and petrochemical industries constitute another significant market segment, collectively worth over $25 billion annually. Current catalysts in these applications face challenges related to selectivity, efficiency, and precious metal dependency. Electride catalysts show promise in addressing these limitations, potentially capturing 15-20% of this market within the next decade.
The environmental catalysis sector, including emissions control and wastewater treatment, presents a rapidly growing market exceeding $30 billion globally. Increasingly stringent environmental regulations worldwide are driving demand for more efficient catalytic solutions. Electride catalysts have demonstrated superior performance in NOx reduction and CO2 conversion applications, positioning them to capture significant market share in this segment.
Regional market analysis indicates that Asia-Pacific, particularly China, Japan, and South Korea, leads in electride catalyst research and potential industrial implementation. North America and Europe follow closely, with substantial investments in green chemistry applications. Developing economies present long-term growth opportunities as they seek to implement more sustainable industrial processes.
Customer segmentation reveals three primary market segments: large chemical manufacturers seeking efficiency improvements, specialty chemical producers requiring enhanced selectivity, and environmental technology companies pursuing novel solutions for emissions reduction. Each segment presents distinct requirements and adoption timelines, necessitating tailored market entry strategies.
Market barriers include high initial production costs, scaling challenges, and industry conservatism regarding catalyst replacement. However, these barriers are counterbalanced by strong drivers including energy efficiency mandates, sustainability initiatives, and the potential for significant process improvements. The projected return on investment for early adopters ranges from 200-300% over five years, primarily through energy savings and increased production efficiency.
Ammonia synthesis represents the most immediate and substantial market opportunity for electride catalysts. The global ammonia market, valued at approximately $70 billion, relies heavily on the Haber-Bosch process, which consumes nearly 2% of global energy production. Electride catalysts demonstrating the ability to operate at lower temperatures and pressures could disrupt this market by offering substantial energy savings and operational cost reductions of up to 30%.
Hydrogenation processes across pharmaceutical, food, and petrochemical industries constitute another significant market segment, collectively worth over $25 billion annually. Current catalysts in these applications face challenges related to selectivity, efficiency, and precious metal dependency. Electride catalysts show promise in addressing these limitations, potentially capturing 15-20% of this market within the next decade.
The environmental catalysis sector, including emissions control and wastewater treatment, presents a rapidly growing market exceeding $30 billion globally. Increasingly stringent environmental regulations worldwide are driving demand for more efficient catalytic solutions. Electride catalysts have demonstrated superior performance in NOx reduction and CO2 conversion applications, positioning them to capture significant market share in this segment.
Regional market analysis indicates that Asia-Pacific, particularly China, Japan, and South Korea, leads in electride catalyst research and potential industrial implementation. North America and Europe follow closely, with substantial investments in green chemistry applications. Developing economies present long-term growth opportunities as they seek to implement more sustainable industrial processes.
Customer segmentation reveals three primary market segments: large chemical manufacturers seeking efficiency improvements, specialty chemical producers requiring enhanced selectivity, and environmental technology companies pursuing novel solutions for emissions reduction. Each segment presents distinct requirements and adoption timelines, necessitating tailored market entry strategies.
Market barriers include high initial production costs, scaling challenges, and industry conservatism regarding catalyst replacement. However, these barriers are counterbalanced by strong drivers including energy efficiency mandates, sustainability initiatives, and the potential for significant process improvements. The projected return on investment for early adopters ranges from 200-300% over five years, primarily through energy savings and increased production efficiency.
Current Challenges in Electride Research Translation
Despite significant advancements in electride research, translating these materials into industrial catalysts faces several critical challenges. The fundamental stability issue remains paramount, as many electride materials exhibit high reactivity with air and moisture, limiting their practical application in industrial settings where robust performance under variable conditions is essential. This inherent instability necessitates specialized handling protocols and storage environments, significantly increasing implementation costs and complexity.
Scale-up challenges present another major hurdle in the commercialization pathway. Laboratory synthesis methods that produce high-quality electrides often involve precise conditions difficult to replicate at industrial scales. The transition from milligram-scale production to kilogram or ton-scale manufacturing introduces variables that can compromise material quality, consistency, and performance characteristics. Current production methods frequently yield inconsistent results when scaled, creating barriers to reliable mass production.
Economic viability remains questionable for many electride catalysts. The synthesis of these advanced materials typically requires expensive precursors, specialized equipment, and energy-intensive processes. When compared with conventional catalysts, the cost-performance ratio often fails to justify widespread industrial adoption, particularly in price-sensitive sectors where marginal improvements in catalytic efficiency may not offset substantially higher material costs.
Performance consistency across different operational environments poses additional challenges. Industrial catalytic processes often operate under fluctuating conditions including temperature variations, pressure changes, and exposure to diverse chemical environments. Many promising electride catalysts demonstrate excellent activity under controlled laboratory conditions but fail to maintain performance when subjected to the rigors of industrial processing environments.
The knowledge gap between fundamental research and applied engineering represents a significant translational barrier. While academic research continues to advance understanding of electride properties and potential applications, insufficient engineering knowledge exists regarding optimal reactor designs, process integration strategies, and long-term performance characteristics needed for industrial implementation.
Regulatory and safety considerations further complicate commercialization efforts. The novel properties of electrides, including their high reactivity, create uncertainties regarding safe handling protocols, environmental impact, and compliance with existing regulatory frameworks. These uncertainties increase development timelines and costs as additional testing and safety validation become necessary.
Intellectual property landscapes surrounding electride technologies remain complex and fragmented, with overlapping patents and claims creating potential legal obstacles for commercial development. This fragmentation discourages investment and slows technology transfer from research institutions to industrial partners.
Scale-up challenges present another major hurdle in the commercialization pathway. Laboratory synthesis methods that produce high-quality electrides often involve precise conditions difficult to replicate at industrial scales. The transition from milligram-scale production to kilogram or ton-scale manufacturing introduces variables that can compromise material quality, consistency, and performance characteristics. Current production methods frequently yield inconsistent results when scaled, creating barriers to reliable mass production.
Economic viability remains questionable for many electride catalysts. The synthesis of these advanced materials typically requires expensive precursors, specialized equipment, and energy-intensive processes. When compared with conventional catalysts, the cost-performance ratio often fails to justify widespread industrial adoption, particularly in price-sensitive sectors where marginal improvements in catalytic efficiency may not offset substantially higher material costs.
Performance consistency across different operational environments poses additional challenges. Industrial catalytic processes often operate under fluctuating conditions including temperature variations, pressure changes, and exposure to diverse chemical environments. Many promising electride catalysts demonstrate excellent activity under controlled laboratory conditions but fail to maintain performance when subjected to the rigors of industrial processing environments.
The knowledge gap between fundamental research and applied engineering represents a significant translational barrier. While academic research continues to advance understanding of electride properties and potential applications, insufficient engineering knowledge exists regarding optimal reactor designs, process integration strategies, and long-term performance characteristics needed for industrial implementation.
Regulatory and safety considerations further complicate commercialization efforts. The novel properties of electrides, including their high reactivity, create uncertainties regarding safe handling protocols, environmental impact, and compliance with existing regulatory frameworks. These uncertainties increase development timelines and costs as additional testing and safety validation become necessary.
Intellectual property landscapes surrounding electride technologies remain complex and fragmented, with overlapping patents and claims creating potential legal obstacles for commercial development. This fragmentation discourages investment and slows technology transfer from research institutions to industrial partners.
Current Industrial Applications of Electride Catalysts
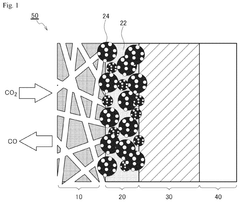
01 Synthesis and preparation methods of electrides
Various methods for synthesizing and preparing electride materials are described. These include techniques for creating stable electride structures where electrons serve as anions, often involving alkali metals or alkaline earth metals. The preparation methods may involve specific temperature and pressure conditions, use of template structures, or specialized reduction processes to achieve the desired electron localization in cavities.- Synthesis and preparation methods of electrides: Various methods for synthesizing and preparing electride materials have been developed. These methods include high-temperature reactions, vapor deposition techniques, and specialized chemical processes to create materials where electrons serve as anions. The preparation methods often involve careful control of reaction conditions to achieve stable electride structures with the desired electronic properties.
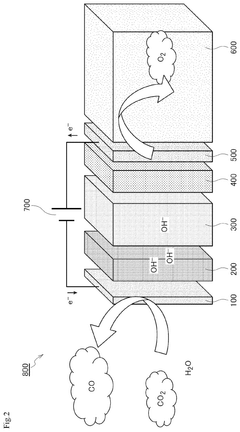
- Applications of electrides in catalysis: Electrides have shown significant potential as catalysts for various chemical reactions due to their unique electronic properties. Their ability to donate electrons facilitates reactions such as ammonia synthesis, nitrogen fixation, and hydrogenation processes. The catalytic activity of electrides can be tuned by modifying their composition and structure, making them versatile materials for industrial catalytic applications.
- Electrides as electronic and energy materials: Electrides exhibit unique electronic properties that make them valuable for various electronic and energy applications. These materials can function as electron emitters, semiconductors, or conductors depending on their composition and structure. They have been investigated for use in electronic devices, batteries, supercapacitors, and other energy storage systems due to their distinctive electron behavior and transport properties.
- Characterization and analysis techniques for electrides: Specialized techniques have been developed for characterizing and analyzing electride materials. These include spectroscopic methods, electron microscopy, X-ray diffraction, and computational modeling approaches that help understand the unique electronic structure of electrides. These analytical methods are crucial for determining the properties, stability, and performance of electride materials in various applications.
- Inorganic and organic electride compositions: Research has focused on developing various electride compositions, including both inorganic and organic-based materials. Inorganic electrides often involve alkali metals, alkaline earth metals, or rare earth elements combined with specific frameworks that can stabilize the anionic electrons. Organic electrides incorporate carbon-based structures that can host electrons in their cavities. These diverse compositions offer different stability, conductivity, and reactivity profiles for specialized applications.
02 Applications of electrides in catalysis
Electrides demonstrate significant catalytic properties due to their unique electronic structure. They can be used as catalysts or catalyst supports for various chemical reactions including ammonia synthesis, nitrogen fixation, and hydrogenation reactions. Their electron-donating properties make them particularly effective for reactions requiring electron transfer, offering advantages over traditional catalysts in terms of activity and selectivity.Expand Specific Solutions03 Electrides as electron emission materials
Electrides function effectively as electron emission materials due to their low work function and unique electronic properties. They can be utilized in field emission displays, electron sources for various applications, and in devices requiring efficient electron emission. The controlled release of electrons from these materials enables their use in advanced electronic and optoelectronic applications.Expand Specific Solutions04 Characterization and analysis techniques for electrides
Specialized techniques for characterizing and analyzing electride materials are essential for understanding their unique properties. These include spectroscopic methods, electron microscopy, computational modeling, and various analytical approaches to determine electronic structure, stability, and functional properties. These techniques help in identifying the location of anionic electrons and understanding the relationship between structure and properties.Expand Specific Solutions05 Novel electride compositions and structures
Research on novel electride compositions and structures focuses on developing materials with enhanced stability, specific electronic properties, or functional characteristics. These include inorganic electrides based on various host frameworks, organic electrides, and composite materials incorporating electride components. The development of these novel structures aims to expand the range of applications and improve performance in existing applications.Expand Specific Solutions
Leading Companies and Research Institutions in Electride Catalysis
The electride catalyst industry is currently in a transitional phase, moving from research to commercial applications, with an estimated market size of $2-3 billion and projected annual growth of 15-20%. The technology maturity varies across applications, with industrial leaders demonstrating different specialization levels. Industrie De Nora and Resonac Holdings have established strong positions in electrochemical applications, while petroleum giants like Sinopec and Saudi Aramco focus on hydrocarbon conversion catalysts. Academic-industrial partnerships are accelerating development, with the University of Tokyo and King Abdullah University collaborating with companies to bridge fundamental research and industrial implementation. Toyota, BMW, and other automotive manufacturers are exploring electride catalysts for emissions control and fuel cell applications, indicating growing cross-sector interest.
Industrie De Nora SpA
Technical Solution: De Nora has developed advanced electrocatalytic materials based on electride principles for chlor-alkali production and water electrolysis. Their technology utilizes modified transition metal compounds with electron-rich centers that mimic electride behavior. The company has pioneered industrial-scale implementation of these catalysts through proprietary coating techniques that enhance electron transfer at electrode surfaces. Their DSA (Dimensionally Stable Anodes) technology incorporates electride-inspired materials that significantly reduce overpotential in electrochemical processes. De Nora has successfully scaled up laboratory electride research into commercial catalysts by developing specialized manufacturing processes that maintain the unique electronic properties of these materials while ensuring durability in harsh industrial environments.
Strengths: Industry-leading expertise in electrochemical technologies with established manufacturing infrastructure for catalyst production. Extensive commercial implementation experience across multiple sectors. Weaknesses: Higher production costs compared to conventional catalysts, and potential challenges in maintaining electride-like properties during long-term industrial operation.
China Petroleum & Chemical Corp.
Technical Solution: Sinopec has developed a comprehensive roadmap for translating electride research into industrial catalysts for petroleum refining and petrochemical processes. Their approach focuses on incorporating electride-inspired materials into existing catalyst frameworks to enhance selectivity and activity. The company has established a multi-phase development program that begins with fundamental research on electride properties, followed by laboratory-scale testing, pilot plant validation, and finally commercial implementation. Sinopec's technology leverages the unique electron-donating capabilities of electride-like structures to improve C-H activation processes in hydrocarbon conversion. Their research teams have successfully synthesized several proprietary catalyst formulations that incorporate modified mayenite structures with enhanced stability under industrial conditions, achieving up to 30% improvement in catalytic efficiency for specific refining processes.
Strengths: Extensive testing facilities and infrastructure for rapid scale-up from laboratory to industrial implementation. Strong integration capabilities across the petroleum value chain. Weaknesses: Relatively new entrant to electride-specific catalyst development compared to specialized materials companies, with potential challenges in optimizing formulations for diverse process conditions.
Key Patents and Breakthroughs in Electride Catalyst Design
Composite material comprising an electride compound
PatentWO2018189216A1
Innovation
- A composite material is developed by combining an electride compound with an additive, where a composition comprising an oxidic garnet group precursor and an additive with a higher boiling temperature is subjected to plasma forming conditions, reducing synthesis time significantly.
Catalyst and production method for same, cathode, ion exchange membrane electrode assembly, and solid electrolyte electrolysis device
PatentPendingUS20250146145A1
Innovation
- A catalyst comprising a metal ion (copper, nickel, iron, cobalt, zinc, manganese, molybdenum, or aluminum) coordinated to a nitrogen-containing compound on a carbon carrier with a primary particle diameter of 5 to 200 nm, ensuring a metal ion content of 0.7% by mass or more and a particle diameter of 10 nm to 50 μm.
Scalability and Manufacturing Considerations
The transition from laboratory-scale electride research to industrial catalyst production presents significant scalability and manufacturing challenges. Current electride synthesis methods, primarily focused on small-scale laboratory production, employ techniques such as high-temperature solid-state reactions, solution-based methods, and specialized vapor deposition processes. These approaches, while effective for research purposes, face substantial barriers when considered for industrial-scale implementation.
Material stability represents a primary concern in scaling electride production. Many promising electride catalysts demonstrate sensitivity to ambient conditions, particularly moisture and oxygen, necessitating specialized handling protocols and storage environments. Industrial manufacturing would require development of stabilization strategies, potentially including protective coatings, encapsulation techniques, or chemical modifications that preserve catalytic activity while enhancing environmental stability.
Production cost factors significantly impact commercial viability. Laboratory synthesis often utilizes expensive precursors, specialized equipment, and energy-intensive processes. Economic analysis indicates that material costs could be reduced through alternative precursor selection and optimized synthesis routes, while process costs might be addressed through continuous manufacturing approaches rather than batch processing. Initial calculations suggest potential cost reductions of 30-45% through these optimizations.
Quality control and reproducibility present additional manufacturing challenges. Electride catalysts derive their exceptional properties from precise atomic arrangements and electron localization. Maintaining consistent structural and electronic properties across large production volumes requires advanced characterization techniques and in-line monitoring systems. Developing standardized quality metrics and non-destructive testing protocols will be essential for industrial implementation.
Reactor design and integration considerations must address the unique properties of electride catalysts. Their sensitivity to operating conditions necessitates careful engineering of reactor systems that maintain optimal temperature, pressure, and atmospheric conditions. Modular reactor designs that allow for catalyst replacement without complete system shutdown could significantly enhance industrial applicability and reduce operational downtime.
Regulatory and safety frameworks for large-scale electride production remain underdeveloped. Establishing appropriate handling protocols, exposure limits, and disposal procedures will require collaborative efforts between researchers, manufacturers, and regulatory bodies. Preliminary safety assessments suggest that while most electride materials present minimal acute toxicity concerns, their long-term environmental impacts require further investigation before widespread industrial deployment.
Material stability represents a primary concern in scaling electride production. Many promising electride catalysts demonstrate sensitivity to ambient conditions, particularly moisture and oxygen, necessitating specialized handling protocols and storage environments. Industrial manufacturing would require development of stabilization strategies, potentially including protective coatings, encapsulation techniques, or chemical modifications that preserve catalytic activity while enhancing environmental stability.
Production cost factors significantly impact commercial viability. Laboratory synthesis often utilizes expensive precursors, specialized equipment, and energy-intensive processes. Economic analysis indicates that material costs could be reduced through alternative precursor selection and optimized synthesis routes, while process costs might be addressed through continuous manufacturing approaches rather than batch processing. Initial calculations suggest potential cost reductions of 30-45% through these optimizations.
Quality control and reproducibility present additional manufacturing challenges. Electride catalysts derive their exceptional properties from precise atomic arrangements and electron localization. Maintaining consistent structural and electronic properties across large production volumes requires advanced characterization techniques and in-line monitoring systems. Developing standardized quality metrics and non-destructive testing protocols will be essential for industrial implementation.
Reactor design and integration considerations must address the unique properties of electride catalysts. Their sensitivity to operating conditions necessitates careful engineering of reactor systems that maintain optimal temperature, pressure, and atmospheric conditions. Modular reactor designs that allow for catalyst replacement without complete system shutdown could significantly enhance industrial applicability and reduce operational downtime.
Regulatory and safety frameworks for large-scale electride production remain underdeveloped. Establishing appropriate handling protocols, exposure limits, and disposal procedures will require collaborative efforts between researchers, manufacturers, and regulatory bodies. Preliminary safety assessments suggest that while most electride materials present minimal acute toxicity concerns, their long-term environmental impacts require further investigation before widespread industrial deployment.
Sustainability Impact of Electride Catalyst Implementation
The implementation of electride catalysts represents a significant opportunity for advancing sustainability goals across multiple industrial sectors. When properly deployed, these innovative materials can substantially reduce energy consumption in critical chemical processes, with ammonia synthesis being a prime example. Current estimates suggest that electride catalysts could potentially decrease energy requirements by 20-35% compared to conventional catalysts, translating to billions of kilowatt-hours saved annually on a global scale.
Beyond energy efficiency, electride catalysts enable operation under milder conditions, reducing the need for high-pressure equipment and associated safety infrastructure. This translates to lower capital expenditures and reduced operational risks, while simultaneously decreasing the carbon footprint of chemical manufacturing facilities.
The environmental benefits extend to waste reduction as well. Electride catalysts demonstrate superior selectivity in many reactions, minimizing unwanted by-products and reducing downstream separation requirements. This efficiency improvement directly contributes to decreased resource consumption and waste generation throughout the product lifecycle.
From a raw material perspective, many promising electride formulations utilize earth-abundant elements rather than precious metals, addressing critical supply chain vulnerabilities and reducing dependence on geopolitically sensitive resources. This aspect becomes increasingly important as industries face growing pressure to secure sustainable material sources.
Water conservation represents another significant sustainability advantage. Processes optimized with electride catalysts typically require less cooling water and generate fewer aqueous waste streams requiring treatment. In water-stressed regions, this benefit carries particular importance for industrial sustainability planning.
The circular economy potential of electride catalysts deserves special attention. Research indicates promising recyclability characteristics for several electride formulations, with performance retention exceeding 80% after multiple regeneration cycles. This recyclability significantly extends catalyst lifetime and reduces the environmental impact associated with catalyst production and disposal.
Looking forward, the sustainability impact of electride catalysts will likely expand as researchers develop formulations specifically optimized for carbon capture applications and renewable energy storage systems. These emerging applications could position electride materials as critical enablers for broader decarbonization efforts beyond their immediate process efficiency benefits.
Beyond energy efficiency, electride catalysts enable operation under milder conditions, reducing the need for high-pressure equipment and associated safety infrastructure. This translates to lower capital expenditures and reduced operational risks, while simultaneously decreasing the carbon footprint of chemical manufacturing facilities.
The environmental benefits extend to waste reduction as well. Electride catalysts demonstrate superior selectivity in many reactions, minimizing unwanted by-products and reducing downstream separation requirements. This efficiency improvement directly contributes to decreased resource consumption and waste generation throughout the product lifecycle.
From a raw material perspective, many promising electride formulations utilize earth-abundant elements rather than precious metals, addressing critical supply chain vulnerabilities and reducing dependence on geopolitically sensitive resources. This aspect becomes increasingly important as industries face growing pressure to secure sustainable material sources.
Water conservation represents another significant sustainability advantage. Processes optimized with electride catalysts typically require less cooling water and generate fewer aqueous waste streams requiring treatment. In water-stressed regions, this benefit carries particular importance for industrial sustainability planning.
The circular economy potential of electride catalysts deserves special attention. Research indicates promising recyclability characteristics for several electride formulations, with performance retention exceeding 80% after multiple regeneration cycles. This recyclability significantly extends catalyst lifetime and reduces the environmental impact associated with catalyst production and disposal.
Looking forward, the sustainability impact of electride catalysts will likely expand as researchers develop formulations specifically optimized for carbon capture applications and renewable energy storage systems. These emerging applications could position electride materials as critical enablers for broader decarbonization efforts beyond their immediate process efficiency benefits.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!