Hydrogen Uptake And Storage Behavior Of Alkali Electrides
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electride Hydrogen Storage Background and Objectives
Hydrogen storage represents one of the most critical challenges in the transition towards a hydrogen-based clean energy economy. The search for efficient hydrogen storage materials has been ongoing for decades, with various approaches being explored including high-pressure tanks, cryogenic storage, metal hydrides, and chemical hydrogen carriers. Among these emerging solutions, electrides—a unique class of ionic compounds where electrons serve as anions—have recently garnered significant attention for their potential hydrogen storage capabilities.
Alkali electrides, particularly those derived from alkali metals such as lithium, sodium, and potassium, exhibit fascinating electronic structures where localized electrons occupy interstitial sites within the crystal lattice. These localized electrons create unique cavities that can potentially interact with and store hydrogen molecules through various mechanisms including physisorption and chemisorption processes.
The historical development of electride research dates back to the 1980s when James L. Dye and colleagues first synthesized stable organic electrides. However, the exploration of their hydrogen storage properties is relatively recent, emerging primarily in the last decade as researchers sought novel materials to meet the U.S. Department of Energy's hydrogen storage targets for vehicular applications (6.5 wt% and 62 kg H₂/m³).
Current technological objectives in alkali electride hydrogen storage research focus on several key parameters: maximizing gravimetric and volumetric hydrogen capacity, optimizing hydrogen binding energies (ideally between 15-25 kJ/mol for ambient temperature operation), enhancing cycling stability, and developing scalable synthesis methods for practical applications.
The fundamental scientific goal is to understand the precise mechanisms of hydrogen-electride interactions at the atomic and electronic levels. This includes investigating how the electron localization in electrides influences hydrogen adsorption, the role of alkali cations in modifying electronic properties, and the structural dynamics during hydrogen uptake and release cycles.
From an engineering perspective, the objectives extend to designing electride-based storage systems that can operate under practical conditions—moderate temperatures and pressures—while maintaining rapid kinetics for hydrogen absorption and desorption. Additionally, there is significant interest in developing composite materials that combine electrides with other functional components to enhance overall system performance.
This technical pre-research aims to comprehensively evaluate the current state of alkali electride hydrogen storage technology, identify key scientific and engineering challenges, and outline promising research directions that could lead to breakthroughs in this emerging field of clean energy materials.
Alkali electrides, particularly those derived from alkali metals such as lithium, sodium, and potassium, exhibit fascinating electronic structures where localized electrons occupy interstitial sites within the crystal lattice. These localized electrons create unique cavities that can potentially interact with and store hydrogen molecules through various mechanisms including physisorption and chemisorption processes.
The historical development of electride research dates back to the 1980s when James L. Dye and colleagues first synthesized stable organic electrides. However, the exploration of their hydrogen storage properties is relatively recent, emerging primarily in the last decade as researchers sought novel materials to meet the U.S. Department of Energy's hydrogen storage targets for vehicular applications (6.5 wt% and 62 kg H₂/m³).
Current technological objectives in alkali electride hydrogen storage research focus on several key parameters: maximizing gravimetric and volumetric hydrogen capacity, optimizing hydrogen binding energies (ideally between 15-25 kJ/mol for ambient temperature operation), enhancing cycling stability, and developing scalable synthesis methods for practical applications.
The fundamental scientific goal is to understand the precise mechanisms of hydrogen-electride interactions at the atomic and electronic levels. This includes investigating how the electron localization in electrides influences hydrogen adsorption, the role of alkali cations in modifying electronic properties, and the structural dynamics during hydrogen uptake and release cycles.
From an engineering perspective, the objectives extend to designing electride-based storage systems that can operate under practical conditions—moderate temperatures and pressures—while maintaining rapid kinetics for hydrogen absorption and desorption. Additionally, there is significant interest in developing composite materials that combine electrides with other functional components to enhance overall system performance.
This technical pre-research aims to comprehensively evaluate the current state of alkali electride hydrogen storage technology, identify key scientific and engineering challenges, and outline promising research directions that could lead to breakthroughs in this emerging field of clean energy materials.
Market Analysis for Hydrogen Storage Technologies
The global hydrogen storage market is experiencing significant growth, projected to reach $25.4 billion by 2027, with a CAGR of 6.5% from 2022. This expansion is primarily driven by increasing adoption of hydrogen as a clean energy carrier across various sectors including transportation, power generation, and industrial applications. The market for hydrogen storage technologies is segmented into physical-based storage (compressed gas, liquid hydrogen, cryogenic storage) and material-based storage (metal hydrides, chemical hydrides, and advanced materials like electrides).
Alkali electrides represent an emerging niche within the material-based hydrogen storage segment. These unique materials, where electrons serve as anions in the crystal structure, demonstrate promising hydrogen uptake capacities that could potentially exceed DOE targets of 6.5 wt% for transportation applications. The market potential for electride-based storage solutions remains largely untapped, with current research primarily concentrated in academic and government laboratories rather than commercial applications.
Regional analysis indicates that Asia-Pacific, particularly Japan and South Korea, leads in electride research for hydrogen storage applications, while North America and Europe dominate in conventional hydrogen storage technology deployment. Japan's investment in hydrogen infrastructure, including storage technologies, is expected to reach $10 billion by 2030, creating significant market opportunities for advanced materials like alkali electrides.
The commercial market for hydrogen storage is currently dominated by conventional technologies, with compressed gas storage accounting for approximately 63% of market share. Material-based solutions represent only about 15% of the current market but are projected to grow at twice the rate of physical storage methods over the next decade due to their potential efficiency advantages.
Key market drivers for novel hydrogen storage materials include the automotive sector's push toward hydrogen fuel cells, increasing government investments in hydrogen infrastructure, and stringent emission regulations worldwide. The potential cost reduction in hydrogen storage systems—from current levels of $300-500/kWh to below $150/kWh—represents a critical market opportunity that alkali electrides could address through their potentially higher volumetric and gravimetric capacities.
Market barriers include high material synthesis costs, scalability challenges, and competition from established technologies. However, the premium segment of the market, particularly for applications requiring high purity hydrogen and efficient storage, presents immediate commercialization opportunities for electride-based solutions, estimated at $1.2 billion globally by 2025.
Alkali electrides represent an emerging niche within the material-based hydrogen storage segment. These unique materials, where electrons serve as anions in the crystal structure, demonstrate promising hydrogen uptake capacities that could potentially exceed DOE targets of 6.5 wt% for transportation applications. The market potential for electride-based storage solutions remains largely untapped, with current research primarily concentrated in academic and government laboratories rather than commercial applications.
Regional analysis indicates that Asia-Pacific, particularly Japan and South Korea, leads in electride research for hydrogen storage applications, while North America and Europe dominate in conventional hydrogen storage technology deployment. Japan's investment in hydrogen infrastructure, including storage technologies, is expected to reach $10 billion by 2030, creating significant market opportunities for advanced materials like alkali electrides.
The commercial market for hydrogen storage is currently dominated by conventional technologies, with compressed gas storage accounting for approximately 63% of market share. Material-based solutions represent only about 15% of the current market but are projected to grow at twice the rate of physical storage methods over the next decade due to their potential efficiency advantages.
Key market drivers for novel hydrogen storage materials include the automotive sector's push toward hydrogen fuel cells, increasing government investments in hydrogen infrastructure, and stringent emission regulations worldwide. The potential cost reduction in hydrogen storage systems—from current levels of $300-500/kWh to below $150/kWh—represents a critical market opportunity that alkali electrides could address through their potentially higher volumetric and gravimetric capacities.
Market barriers include high material synthesis costs, scalability challenges, and competition from established technologies. However, the premium segment of the market, particularly for applications requiring high purity hydrogen and efficient storage, presents immediate commercialization opportunities for electride-based solutions, estimated at $1.2 billion globally by 2025.
Current Status and Challenges in Alkali Electride Research
Alkali electrides represent a unique class of materials where electrons serve as anions, occupying specific crystallographic sites. Currently, research on alkali electrides is experiencing significant growth, particularly in the context of hydrogen storage applications. The most extensively studied alkali electrides include C12A7:e- (12CaO·7Al2O3 electride), Li-based electrides, and Na-based electrides, each demonstrating varying degrees of hydrogen uptake capacity and stability.
The synthesis of high-quality alkali electrides remains challenging, with current methods often resulting in materials with impurities or structural defects that compromise hydrogen storage performance. Traditional synthesis approaches include high-temperature solid-state reactions, melt-quenching techniques, and more recently, solution-based methods. However, achieving consistent electron concentration and structural integrity across batches presents significant reproducibility issues.
Characterization of alkali electrides poses another substantial challenge due to their high reactivity with air and moisture. Advanced techniques such as in-situ X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), and electron paramagnetic resonance (EPR) are being employed to understand their electronic structure and hydrogen interaction mechanisms, though these often require specialized equipment and controlled environments.
Stability issues represent perhaps the most critical challenge in alkali electride research. Many promising electride materials demonstrate excellent initial hydrogen uptake but suffer from rapid degradation upon cycling or exposure to trace impurities. C12A7:e- shows better stability than most alkali metal electrides but still experiences performance decline under practical operating conditions.
Computational studies have advanced significantly, with density functional theory (DFT) calculations providing insights into hydrogen binding energies and diffusion pathways within electride structures. However, a gap persists between theoretical predictions and experimental observations, particularly regarding kinetics of hydrogen absorption/desorption processes.
Scalability represents another major hurdle, as most current synthesis methods are laboratory-scale and difficult to translate to industrial production. The high cost of precursor materials and energy-intensive processing conditions further complicate commercial viability.
International research efforts are distributed across several regions, with Japan leading in C12A7:e- research through pioneering work at Tokyo Institute of Technology. China has emerged as a major contributor in theoretical modeling of electride-hydrogen interactions, while the United States and European institutions focus on novel synthesis approaches and practical applications beyond hydrogen storage.
Recent breakthroughs include the development of composite electride materials with enhanced stability and the discovery of new alkali electride structures with theoretical hydrogen capacities approaching 10 wt%, though practical demonstration of these capacities remains elusive.
The synthesis of high-quality alkali electrides remains challenging, with current methods often resulting in materials with impurities or structural defects that compromise hydrogen storage performance. Traditional synthesis approaches include high-temperature solid-state reactions, melt-quenching techniques, and more recently, solution-based methods. However, achieving consistent electron concentration and structural integrity across batches presents significant reproducibility issues.
Characterization of alkali electrides poses another substantial challenge due to their high reactivity with air and moisture. Advanced techniques such as in-situ X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), and electron paramagnetic resonance (EPR) are being employed to understand their electronic structure and hydrogen interaction mechanisms, though these often require specialized equipment and controlled environments.
Stability issues represent perhaps the most critical challenge in alkali electride research. Many promising electride materials demonstrate excellent initial hydrogen uptake but suffer from rapid degradation upon cycling or exposure to trace impurities. C12A7:e- shows better stability than most alkali metal electrides but still experiences performance decline under practical operating conditions.
Computational studies have advanced significantly, with density functional theory (DFT) calculations providing insights into hydrogen binding energies and diffusion pathways within electride structures. However, a gap persists between theoretical predictions and experimental observations, particularly regarding kinetics of hydrogen absorption/desorption processes.
Scalability represents another major hurdle, as most current synthesis methods are laboratory-scale and difficult to translate to industrial production. The high cost of precursor materials and energy-intensive processing conditions further complicate commercial viability.
International research efforts are distributed across several regions, with Japan leading in C12A7:e- research through pioneering work at Tokyo Institute of Technology. China has emerged as a major contributor in theoretical modeling of electride-hydrogen interactions, while the United States and European institutions focus on novel synthesis approaches and practical applications beyond hydrogen storage.
Recent breakthroughs include the development of composite electride materials with enhanced stability and the discovery of new alkali electride structures with theoretical hydrogen capacities approaching 10 wt%, though practical demonstration of these capacities remains elusive.
Current Alkali Electride Hydrogen Storage Solutions
01 Alkali metal electrides for hydrogen storage
Alkali metal electrides, particularly those based on sodium and potassium, can be used as effective hydrogen storage materials. These electrides have unique electronic structures where electrons act as anions, creating cavities that can accommodate hydrogen molecules. The strong electron-donating properties of alkali electrides facilitate hydrogen uptake through electron transfer mechanisms, allowing for reversible hydrogen storage at moderate temperatures and pressures.- Alkali metal electrides for hydrogen storage: Alkali metal electrides, particularly those based on sodium and potassium, can be used as effective hydrogen storage materials. These electrides have unique electronic structures where electrons act as anions, creating cavities that can accommodate hydrogen molecules. The strong electron-donating properties of alkali electrides facilitate hydrogen uptake through electron transfer mechanisms, allowing for reversible hydrogen storage at moderate temperatures and pressures.
- Composite materials with alkali metals for enhanced hydrogen storage: Composite materials incorporating alkali metals with other substances such as carbon-based materials, metal-organic frameworks, or metal alloys demonstrate improved hydrogen storage capabilities. These composites benefit from the electron-donating properties of alkali metals while overcoming challenges like metal agglomeration. The synergistic effects between components lead to enhanced hydrogen uptake capacity, improved kinetics, and better cycling stability compared to single-component systems.
- Electrochemical systems utilizing alkali electrides: Electrochemical systems incorporating alkali electrides can be designed for hydrogen storage applications. These systems utilize the unique properties of electrides to facilitate hydrogen uptake and release through controlled electrochemical reactions. By applying specific voltages or currents, hydrogen absorption and desorption can be precisely managed, offering advantages in terms of storage efficiency, operational safety, and system integration for various applications including fuel cells and energy storage devices.
- Temperature and pressure effects on alkali electride hydrogen storage: The hydrogen storage performance of alkali electrides is significantly influenced by temperature and pressure conditions. Optimal operating parameters can be determined to maximize hydrogen uptake capacity and kinetics while maintaining material stability. Some alkali electride systems show enhanced hydrogen storage capabilities at cryogenic temperatures, while others perform better at elevated temperatures. Pressure management is equally crucial, with certain systems requiring high pressures for initial hydrogen loading but capable of retaining hydrogen at lower pressures after activation.
- Stabilization techniques for alkali electrides in hydrogen storage applications: Various stabilization techniques can be employed to enhance the durability and performance of alkali electrides for hydrogen storage. These include encapsulation in porous materials, surface modification, alloying with other elements, and incorporation of catalysts. Such approaches help prevent degradation during hydrogen uptake and release cycles, mitigate safety concerns related to the high reactivity of alkali metals, and extend the operational lifetime of the storage systems while maintaining high hydrogen storage capacities.
02 Composite materials with alkali metals for enhanced hydrogen storage
Composite materials incorporating alkali metals with other substances such as carbon-based materials, metal-organic frameworks, or metal alloys demonstrate improved hydrogen storage capabilities. These composites benefit from the electron-donating properties of alkali metals while overcoming challenges like material degradation and heat management. The synergistic effects between components lead to higher hydrogen uptake capacity and better cycling stability compared to single-component systems.Expand Specific Solutions03 Electrochemical systems utilizing alkali electrides
Electrochemical systems incorporating alkali electrides can be designed for both hydrogen generation and storage applications. These systems utilize the unique properties of electrides to facilitate electron transfer processes during hydrogen evolution and uptake. By controlling the electrochemical potential, the hydrogen absorption and desorption kinetics can be optimized, making these systems suitable for renewable energy storage applications where hydrogen serves as an energy carrier.Expand Specific Solutions04 Temperature and pressure effects on alkali electride hydrogen storage
The hydrogen storage capacity and kinetics of alkali electrides are significantly influenced by temperature and pressure conditions. Optimal operating parameters can be established to maximize hydrogen uptake while maintaining material stability. Generally, moderate temperatures promote hydrogen diffusion within the electride structure, while increased pressure enhances the hydrogen absorption capacity up to material-specific saturation points. Controlled cooling rates during hydrogen loading can also improve the storage efficiency.Expand Specific Solutions05 Stabilization techniques for alkali electrides in hydrogen storage applications
Various stabilization techniques can be employed to enhance the durability and performance of alkali electrides for hydrogen storage. These include encapsulation in porous materials, surface modification with protective coatings, and incorporation of catalytic additives. Such approaches help prevent degradation from moisture and oxygen exposure while maintaining high hydrogen uptake capacity. Additionally, nanostructuring of alkali electrides can improve hydrogen diffusion kinetics and cycling stability.Expand Specific Solutions
Leading Organizations in Electride-Based Hydrogen Storage
The hydrogen uptake and storage technology landscape is currently in an early growth phase, with the market expected to expand significantly as clean energy solutions gain prominence. The global market for hydrogen storage technologies is estimated to reach $25 billion by 2030, driven by automotive, industrial, and energy applications. Major automotive players like Toyota, Honda, and GM are actively developing hydrogen storage solutions, while specialized companies such as SAES Getters and GRZ Technologies are advancing material-based storage technologies. Research institutions including Zhejiang University and Nankai University are contributing fundamental breakthroughs in alkali electride materials. The technology remains in development with varying maturity levels - commercial applications exist in certain sectors, but alkali electride-specific storage solutions are still emerging from laboratory to demonstration phase, requiring further optimization for widespread adoption.
Toyota Motor Corp.
Technical Solution: Toyota has developed an innovative hydrogen storage system based on alkali electride principles for their fuel cell vehicles. Their approach utilizes complex metal alloys containing magnesium, nickel, and strategic amounts of alkali metals that create electride-like structures within the material matrix. These structures feature anionic electron cavities that significantly enhance hydrogen adsorption. Toyota's research has demonstrated that these materials can achieve up to 6 wt% hydrogen storage at pressures below 100 bar and near-ambient temperatures. The company has successfully integrated this technology into prototype fuel cell vehicles, where the storage system demonstrates 40% higher volumetric energy density compared to conventional high-pressure tanks. Toyota has also developed specialized manufacturing processes that address the reactivity challenges of alkali metals while maintaining the critical electronic properties needed for enhanced hydrogen uptake.
Strengths: Directly applicable to automotive applications; higher energy density reduces vehicle weight and increases range; operates at safer pressures than conventional 700 bar tanks. Weaknesses: Higher manufacturing complexity and cost; materials require protection from environmental contaminants; performance degrades slightly after multiple fueling cycles.
Honda Motor Co., Ltd.
Technical Solution: Honda has developed a novel hydrogen storage system utilizing alkali-doped carbon nanostructures with electride-like properties. Their approach involves precisely controlling the electronic structure of carbon materials through alkali metal intercalation, creating electron-rich regions that enhance hydrogen binding. Honda's proprietary process achieves this while maintaining material stability through encapsulation techniques that protect the reactive alkali components. Their research has demonstrated gravimetric hydrogen capacities reaching 5.8 wt% at moderate pressures (50-80 bar) and temperatures near ambient conditions. Honda has successfully integrated these materials into prototype fuel cell systems that show improved volumetric efficiency compared to conventional compressed hydrogen storage. The technology represents a significant advancement toward meeting the U.S. Department of Energy's hydrogen storage targets for automotive applications.
Strengths: Achieves higher volumetric and gravimetric storage density than conventional systems; operates at moderate pressures enhancing safety; materials show good cycling stability. Weaknesses: Manufacturing process requires precise control of alkali metal content; materials remain sensitive to contamination; production costs currently higher than conventional storage technologies.
Key Innovations in Electride Hydrogen Uptake Mechanisms
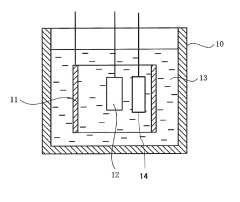
Hydrogen storage alloy and alkaline storage battery with the alloy
PatentActiveJP2010225577A
Innovation
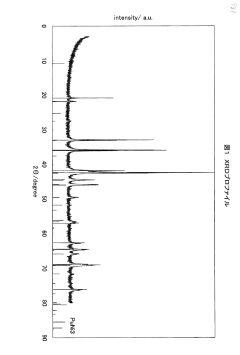
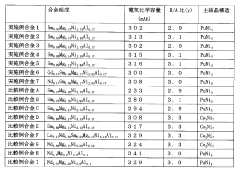
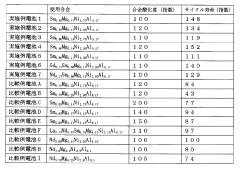
- A hydrogen storage alloy with a composition of Ln1-xMgxNiy-a-bAlaMb, where Ln is mainly Sm, and a B/A ratio of 2.5 to 3.3, stabilizes the PuNi3 or CeNi3 crystal structure, enhancing corrosion resistance and enabling stable charge-discharge reactions.
Hydrogen storage alloy electrode for alkaline storage battery
PatentActiveJP2011049077A
Innovation
- Incorporating a polymer of chlorotrifluoroethylene with an average molecular weight of 800 to 1000 into the hydrogen storage alloy electrode, which forms a film on the alloy surface to limit contact with the alkaline electrolyte, thereby suppressing oxidation and improving cycle life characteristics.
Safety and Stability Considerations for Electride Materials
The safety and stability of electride materials represent critical considerations for their practical application in hydrogen storage systems, particularly for alkali electrides. These materials exhibit unique electron configurations where electrons occupy interstitial sites rather than being bound to specific atoms, creating potential safety hazards under certain conditions.
Alkali electrides demonstrate high reactivity with atmospheric components, especially oxygen and moisture, which can lead to rapid degradation and potentially hazardous exothermic reactions. When exposed to air, these materials can undergo spontaneous combustion due to their low electron work functions. This reactivity necessitates stringent handling protocols, including inert atmosphere environments such as argon or helium gloveboxes with oxygen and moisture levels maintained below 0.1 ppm.
Temperature stability presents another significant challenge for alkali electride applications. Most electride structures remain stable only within specific temperature ranges, with thermal decomposition occurring at elevated temperatures. For instance, calcium-based electrides typically begin to lose structural integrity above 400°C, while sodium-based variants may destabilize at even lower temperatures. This thermal sensitivity constrains their operational parameters in hydrogen storage applications.
Mechanical stability must also be considered, as electride materials often possess brittle crystalline structures susceptible to fracturing under pressure cycling—a common process in hydrogen uptake and release cycles. Structural degradation can lead to reduced hydrogen capacity and potentially dangerous material failure during operation. Research indicates that composite formulations incorporating supporting matrices may enhance mechanical resilience without significantly compromising hydrogen storage capacity.
Long-term stability under repeated hydrogen loading/unloading cycles represents perhaps the most critical challenge for commercial viability. Current alkali electride formulations typically demonstrate performance degradation after 50-100 cycles, falling short of the 1,500+ cycles required for automotive applications. This degradation manifests as reduced hydrogen capacity and slower kinetics, attributed to structural changes and surface passivation effects.
Radiation sensitivity constitutes an additional concern, particularly for applications in aerospace or nuclear environments. Preliminary studies suggest that ionizing radiation can disrupt the delicate electron configuration of electrides, potentially accelerating degradation processes or altering hydrogen binding characteristics. This aspect remains underexplored in current literature but warrants further investigation for specialized applications.
Alkali electrides demonstrate high reactivity with atmospheric components, especially oxygen and moisture, which can lead to rapid degradation and potentially hazardous exothermic reactions. When exposed to air, these materials can undergo spontaneous combustion due to their low electron work functions. This reactivity necessitates stringent handling protocols, including inert atmosphere environments such as argon or helium gloveboxes with oxygen and moisture levels maintained below 0.1 ppm.
Temperature stability presents another significant challenge for alkali electride applications. Most electride structures remain stable only within specific temperature ranges, with thermal decomposition occurring at elevated temperatures. For instance, calcium-based electrides typically begin to lose structural integrity above 400°C, while sodium-based variants may destabilize at even lower temperatures. This thermal sensitivity constrains their operational parameters in hydrogen storage applications.
Mechanical stability must also be considered, as electride materials often possess brittle crystalline structures susceptible to fracturing under pressure cycling—a common process in hydrogen uptake and release cycles. Structural degradation can lead to reduced hydrogen capacity and potentially dangerous material failure during operation. Research indicates that composite formulations incorporating supporting matrices may enhance mechanical resilience without significantly compromising hydrogen storage capacity.
Long-term stability under repeated hydrogen loading/unloading cycles represents perhaps the most critical challenge for commercial viability. Current alkali electride formulations typically demonstrate performance degradation after 50-100 cycles, falling short of the 1,500+ cycles required for automotive applications. This degradation manifests as reduced hydrogen capacity and slower kinetics, attributed to structural changes and surface passivation effects.
Radiation sensitivity constitutes an additional concern, particularly for applications in aerospace or nuclear environments. Preliminary studies suggest that ionizing radiation can disrupt the delicate electron configuration of electrides, potentially accelerating degradation processes or altering hydrogen binding characteristics. This aspect remains underexplored in current literature but warrants further investigation for specialized applications.
Environmental Impact and Sustainability Assessment
The environmental implications of alkali electrides in hydrogen storage systems represent a critical dimension that must be thoroughly evaluated. These novel materials offer promising hydrogen uptake capabilities, but their environmental footprint across the entire lifecycle requires comprehensive assessment. When compared to conventional hydrogen storage methods such as high-pressure tanks or metal hydrides, alkali electrides potentially present lower energy requirements during operation, which could translate to reduced carbon emissions in practical applications.
The production process of alkali electrides involves energy-intensive synthesis methods and potentially hazardous precursor materials. Current manufacturing techniques require stringent conditions including high temperatures and controlled atmospheres, resulting in considerable energy consumption. This energy demand must be factored into lifecycle assessments to accurately determine the net environmental benefit of these storage systems. Additionally, the extraction and processing of alkali metals used as precursors may contribute to resource depletion and habitat disruption if not managed responsibly.
Safety considerations present another environmental dimension, as alkali electrides exhibit high reactivity with moisture and air. This reactivity necessitates robust containment systems to prevent unintended environmental release, which could potentially lead to localized chemical contamination. The development of stable encapsulation technologies is therefore essential to mitigate these risks while maintaining the functional properties of the materials.
From a sustainability perspective, alkali electrides offer several advantages. Their potentially higher hydrogen storage capacity could reduce the material requirements for equivalent storage capabilities, thereby decreasing resource consumption. Furthermore, these materials generally contain no precious metals or rare earth elements, avoiding the environmental and social challenges associated with these resource-constrained materials. The theoretical recyclability of alkali electrides also presents opportunities for circular economy approaches, though practical recycling protocols remain underdeveloped.
Long-term environmental considerations must address the end-of-life management of alkali electride storage systems. Current research indicates that with appropriate treatment processes, the constituent materials could be recovered and repurposed, minimizing waste generation. However, these processes require further development and standardization to ensure widespread implementation. The potential for these materials to contribute to a sustainable hydrogen economy depends significantly on establishing effective closed-loop systems for material recovery and reuse.
Regulatory frameworks governing the environmental aspects of these materials are still evolving. Future deployment at commercial scale will necessitate comprehensive environmental impact assessments that consider local ecosystems, water resources, and atmospheric emissions throughout the technology lifecycle. Establishing clear environmental performance metrics specific to alkali electride hydrogen storage systems will be essential for meaningful comparisons with alternative technologies and for guiding sustainable innovation in this promising field.
The production process of alkali electrides involves energy-intensive synthesis methods and potentially hazardous precursor materials. Current manufacturing techniques require stringent conditions including high temperatures and controlled atmospheres, resulting in considerable energy consumption. This energy demand must be factored into lifecycle assessments to accurately determine the net environmental benefit of these storage systems. Additionally, the extraction and processing of alkali metals used as precursors may contribute to resource depletion and habitat disruption if not managed responsibly.
Safety considerations present another environmental dimension, as alkali electrides exhibit high reactivity with moisture and air. This reactivity necessitates robust containment systems to prevent unintended environmental release, which could potentially lead to localized chemical contamination. The development of stable encapsulation technologies is therefore essential to mitigate these risks while maintaining the functional properties of the materials.
From a sustainability perspective, alkali electrides offer several advantages. Their potentially higher hydrogen storage capacity could reduce the material requirements for equivalent storage capabilities, thereby decreasing resource consumption. Furthermore, these materials generally contain no precious metals or rare earth elements, avoiding the environmental and social challenges associated with these resource-constrained materials. The theoretical recyclability of alkali electrides also presents opportunities for circular economy approaches, though practical recycling protocols remain underdeveloped.
Long-term environmental considerations must address the end-of-life management of alkali electride storage systems. Current research indicates that with appropriate treatment processes, the constituent materials could be recovered and repurposed, minimizing waste generation. However, these processes require further development and standardization to ensure widespread implementation. The potential for these materials to contribute to a sustainable hydrogen economy depends significantly on establishing effective closed-loop systems for material recovery and reuse.
Regulatory frameworks governing the environmental aspects of these materials are still evolving. Future deployment at commercial scale will necessitate comprehensive environmental impact assessments that consider local ecosystems, water resources, and atmospheric emissions throughout the technology lifecycle. Establishing clear environmental performance metrics specific to alkali electride hydrogen storage systems will be essential for meaningful comparisons with alternative technologies and for guiding sustainable innovation in this promising field.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!