Environmental Degradation Pathways Of Common Electrides
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electride Technology Background and Objectives
Electrides represent a unique class of materials where electrons serve as anions, occupying specific positions in the crystal lattice. The concept of electrides dates back to the 1980s when James L. Dye and colleagues at Michigan State University first synthesized organic electrides. These initial compounds were highly unstable in ambient conditions, decomposing rapidly upon exposure to air and moisture, which significantly limited their practical applications.
The evolution of electride technology has seen remarkable progress over the past four decades. A significant breakthrough came in 2003 when Hosono and his team at the Tokyo Institute of Technology developed the first room-temperature stable inorganic electride, 12CaO·7Al₂O₃ (C12A7:e⁻). This discovery opened new possibilities for practical applications of electrides in various fields, including catalysis, electronics, and energy storage.
Recent years have witnessed an expansion in the family of electrides, with the discovery of two-dimensional electrides such as Ca₂N and Y₂C, as well as high-pressure electrides like Na, Li, and Cs under extreme conditions. These materials exhibit exceptional properties, including low work functions, high electron mobility, and unique catalytic activities, making them promising candidates for next-generation electronic devices and chemical processes.
Despite their promising properties, the environmental stability of electrides remains a critical challenge. Most electrides are highly reactive with atmospheric components, particularly oxygen and water vapor, leading to rapid degradation of their unique electronic structures. Understanding these degradation pathways is essential for developing strategies to enhance their stability and enable their practical implementation in real-world applications.
The primary objective of this technical research is to comprehensively investigate the environmental degradation mechanisms of common electrides, focusing on their interactions with atmospheric components under various conditions. By elucidating these degradation pathways at the molecular and electronic levels, we aim to establish a fundamental understanding that can guide the development of stabilization strategies.
Additionally, this research seeks to identify structure-stability relationships across different classes of electrides, correlating their chemical composition, crystal structure, and electronic configuration with their environmental stability. This knowledge will be instrumental in designing new electride materials with enhanced resistance to environmental degradation while maintaining their desirable functional properties.
The ultimate goal is to establish design principles for environmentally robust electrides that can withstand practical operating conditions, thereby facilitating their integration into commercial applications such as electron emission devices, catalysts for ammonia synthesis, and components in energy storage systems.
The evolution of electride technology has seen remarkable progress over the past four decades. A significant breakthrough came in 2003 when Hosono and his team at the Tokyo Institute of Technology developed the first room-temperature stable inorganic electride, 12CaO·7Al₂O₃ (C12A7:e⁻). This discovery opened new possibilities for practical applications of electrides in various fields, including catalysis, electronics, and energy storage.
Recent years have witnessed an expansion in the family of electrides, with the discovery of two-dimensional electrides such as Ca₂N and Y₂C, as well as high-pressure electrides like Na, Li, and Cs under extreme conditions. These materials exhibit exceptional properties, including low work functions, high electron mobility, and unique catalytic activities, making them promising candidates for next-generation electronic devices and chemical processes.
Despite their promising properties, the environmental stability of electrides remains a critical challenge. Most electrides are highly reactive with atmospheric components, particularly oxygen and water vapor, leading to rapid degradation of their unique electronic structures. Understanding these degradation pathways is essential for developing strategies to enhance their stability and enable their practical implementation in real-world applications.
The primary objective of this technical research is to comprehensively investigate the environmental degradation mechanisms of common electrides, focusing on their interactions with atmospheric components under various conditions. By elucidating these degradation pathways at the molecular and electronic levels, we aim to establish a fundamental understanding that can guide the development of stabilization strategies.
Additionally, this research seeks to identify structure-stability relationships across different classes of electrides, correlating their chemical composition, crystal structure, and electronic configuration with their environmental stability. This knowledge will be instrumental in designing new electride materials with enhanced resistance to environmental degradation while maintaining their desirable functional properties.
The ultimate goal is to establish design principles for environmentally robust electrides that can withstand practical operating conditions, thereby facilitating their integration into commercial applications such as electron emission devices, catalysts for ammonia synthesis, and components in energy storage systems.
Market Applications and Demand Analysis
The market for electride materials is experiencing significant growth driven by their unique electronic properties and potential applications across multiple industries. Current market analysis indicates that electrides, particularly those with high stability in ambient conditions, are gaining traction in catalysis applications, where they demonstrate exceptional performance in ammonia synthesis and carbon dioxide reduction reactions. This application segment alone is projected to grow substantially as industries seek more energy-efficient and environmentally friendly catalytic processes.
Electronics and semiconductor industries represent another major market opportunity for electrides. Their exceptional electron emission properties make them valuable for next-generation display technologies, electron emitters, and advanced electronic components. As consumer electronics continue to evolve toward more energy-efficient and compact designs, the demand for novel materials like electrides is expected to increase accordingly.
Energy storage and conversion systems constitute a rapidly expanding application area. Electrides show promise as electrode materials in batteries and supercapacitors, potentially offering higher energy densities and faster charging capabilities than conventional materials. The global push toward renewable energy and electrification of transportation is creating substantial market pull for advanced energy storage solutions, positioning electrides as strategically important materials.
The aerospace and defense sectors are also exploring electrides for specialized applications, including advanced propulsion systems and sensors. Though smaller in volume compared to other markets, these applications often command premium pricing due to their critical nature and performance requirements.
Regionally, Asia-Pacific currently leads in electride research and development activities, with Japan and China at the forefront. North America and Europe follow closely, with significant investments in fundamental research and application development. The market structure remains relatively fragmented, with academic institutions and specialized materials companies dominating the intellectual property landscape.
A key market challenge relates directly to environmental degradation pathways. End-users require materials with predictable lifespans and degradation behaviors for commercial adoption. Industries are increasingly demanding electrides with improved stability against moisture, oxygen, and thermal stress to reduce replacement costs and environmental impact. This has created a specialized market segment focused on environmentally robust electride formulations and protective technologies.
Market forecasts suggest that as degradation mechanisms become better understood and mitigated, the commercial viability of electrides will improve substantially, potentially expanding the global market value significantly over the next decade. Companies that can develop electrides with enhanced environmental stability will likely capture premium positions in this emerging materials market.
Electronics and semiconductor industries represent another major market opportunity for electrides. Their exceptional electron emission properties make them valuable for next-generation display technologies, electron emitters, and advanced electronic components. As consumer electronics continue to evolve toward more energy-efficient and compact designs, the demand for novel materials like electrides is expected to increase accordingly.
Energy storage and conversion systems constitute a rapidly expanding application area. Electrides show promise as electrode materials in batteries and supercapacitors, potentially offering higher energy densities and faster charging capabilities than conventional materials. The global push toward renewable energy and electrification of transportation is creating substantial market pull for advanced energy storage solutions, positioning electrides as strategically important materials.
The aerospace and defense sectors are also exploring electrides for specialized applications, including advanced propulsion systems and sensors. Though smaller in volume compared to other markets, these applications often command premium pricing due to their critical nature and performance requirements.
Regionally, Asia-Pacific currently leads in electride research and development activities, with Japan and China at the forefront. North America and Europe follow closely, with significant investments in fundamental research and application development. The market structure remains relatively fragmented, with academic institutions and specialized materials companies dominating the intellectual property landscape.
A key market challenge relates directly to environmental degradation pathways. End-users require materials with predictable lifespans and degradation behaviors for commercial adoption. Industries are increasingly demanding electrides with improved stability against moisture, oxygen, and thermal stress to reduce replacement costs and environmental impact. This has created a specialized market segment focused on environmentally robust electride formulations and protective technologies.
Market forecasts suggest that as degradation mechanisms become better understood and mitigated, the commercial viability of electrides will improve substantially, potentially expanding the global market value significantly over the next decade. Companies that can develop electrides with enhanced environmental stability will likely capture premium positions in this emerging materials market.
Current Environmental Stability Challenges
Electrides represent a unique class of materials where electrons serve as anions, occupying specific lattice sites. Despite their promising applications in catalysis, electronics, and energy storage, these materials face significant environmental stability challenges that limit their practical implementation. The primary degradation pathway for most electrides involves reaction with atmospheric components, particularly oxygen and water vapor, which rapidly neutralize their distinctive electronic structure.
C12A7 (12CaO·7Al2O3) electride, one of the most studied electrides, demonstrates notable instability when exposed to ambient conditions. Its cage structure, which houses anionic electrons, undergoes oxidation within minutes of air exposure, with complete degradation occurring within hours. This degradation manifests as a visible color change from black to white as the material reverts to its conventional oxide form, accompanied by a dramatic decrease in conductivity by several orders of magnitude.
Two-dimensional electrides, such as Ca2N and Y2C, exhibit even more severe stability issues. These layered materials, with electrons confined between positively charged layers, decompose almost instantaneously upon air exposure. The degradation process involves rapid oxidation of the surface layers, creating a passivation layer that slows but does not prevent complete material failure. Experimental studies have documented complete transformation of thin films of these materials within minutes of ambient exposure.
Alkaline metal suboxides (e.g., Rb9O2) represent another category of electrides with extreme environmental sensitivity. These materials not only react with oxygen and water but also demonstrate thermal instability at relatively low temperatures (often below 100°C), further complicating their handling and application. The degradation pathway involves multiple competing reactions, leading to complex decomposition products.
Moisture-induced degradation presents a particularly challenging problem across all electride classes. Water molecules can penetrate the crystal structure, causing hydrolysis reactions that irreversibly alter the material's electronic properties. High-resolution microscopy studies have revealed that this degradation often begins at grain boundaries and defect sites, progressing inward in a non-uniform manner that creates compositional and electronic heterogeneity throughout the material.
Temperature acceleration of degradation compounds these challenges, with most electrides showing exponentially increasing degradation rates with rising temperature. This thermal sensitivity creates a narrow operational window for potential applications and necessitates sophisticated environmental control systems for any practical implementation.
C12A7 (12CaO·7Al2O3) electride, one of the most studied electrides, demonstrates notable instability when exposed to ambient conditions. Its cage structure, which houses anionic electrons, undergoes oxidation within minutes of air exposure, with complete degradation occurring within hours. This degradation manifests as a visible color change from black to white as the material reverts to its conventional oxide form, accompanied by a dramatic decrease in conductivity by several orders of magnitude.
Two-dimensional electrides, such as Ca2N and Y2C, exhibit even more severe stability issues. These layered materials, with electrons confined between positively charged layers, decompose almost instantaneously upon air exposure. The degradation process involves rapid oxidation of the surface layers, creating a passivation layer that slows but does not prevent complete material failure. Experimental studies have documented complete transformation of thin films of these materials within minutes of ambient exposure.
Alkaline metal suboxides (e.g., Rb9O2) represent another category of electrides with extreme environmental sensitivity. These materials not only react with oxygen and water but also demonstrate thermal instability at relatively low temperatures (often below 100°C), further complicating their handling and application. The degradation pathway involves multiple competing reactions, leading to complex decomposition products.
Moisture-induced degradation presents a particularly challenging problem across all electride classes. Water molecules can penetrate the crystal structure, causing hydrolysis reactions that irreversibly alter the material's electronic properties. High-resolution microscopy studies have revealed that this degradation often begins at grain boundaries and defect sites, progressing inward in a non-uniform manner that creates compositional and electronic heterogeneity throughout the material.
Temperature acceleration of degradation compounds these challenges, with most electrides showing exponentially increasing degradation rates with rising temperature. This thermal sensitivity creates a narrow operational window for potential applications and necessitates sophisticated environmental control systems for any practical implementation.
Current Protection and Preservation Methods
01 Stability and degradation mechanisms of electrides in environmental conditions
Electrides are materials with electrons serving as anions, making them susceptible to environmental degradation. Their stability is compromised when exposed to moisture, oxygen, and other atmospheric components. Research focuses on understanding degradation mechanisms and developing protective measures to extend their functional lifespan in various applications. These studies examine how environmental factors trigger decomposition pathways and structural changes that affect the unique electronic properties of electrides.- Stability of electrides in environmental conditions: Electrides are materials with electrons serving as anions, which can be susceptible to environmental degradation when exposed to moisture, oxygen, and other atmospheric components. Research focuses on developing stable electride structures that can maintain their unique electronic properties despite environmental challenges. Various protective coatings and encapsulation methods have been developed to prevent degradation and extend the functional lifetime of electride materials in practical applications.
- Monitoring systems for electride degradation: Specialized monitoring systems have been developed to track the environmental degradation of electrides in real-time. These systems employ sensors that can detect changes in electronic properties, structural integrity, and chemical composition of electride materials when exposed to various environmental factors. The monitoring technology enables early detection of degradation processes, allowing for timely intervention and maintenance to preserve electride functionality in applications such as catalysis, electronics, and energy storage.
- Remediation techniques for degraded electrides: Various remediation techniques have been developed to restore the functionality of environmentally degraded electrides. These methods include thermal treatments, chemical regeneration processes, and surface cleaning protocols that can remove contaminants and restore the electronic structure of electrides. Some approaches involve the application of specific compounds that can reverse oxidation or hydration effects, while others focus on physical restoration of the crystal structure to maintain the electron localization that characterizes electride materials.
- Environmental applications of controlled electride degradation: Controlled degradation of electrides can be harnessed for environmental applications such as waste treatment, pollutant decomposition, and water purification. The unique electronic properties of degrading electrides can facilitate redox reactions that break down organic contaminants or transform harmful substances into benign compounds. Research in this area focuses on optimizing the degradation pathways to maximize environmental remediation efficiency while minimizing the release of potentially harmful byproducts.
- Protective technologies for electride preservation: Advanced protective technologies have been developed specifically to prevent environmental degradation of electrides. These include specialized packaging materials, hermetic sealing techniques, and composite structures that shield electrides from moisture, oxygen, and other reactive species. Some approaches incorporate sacrificial materials that preferentially react with environmental contaminants before they can reach the electride, while others create physical barriers with selective permeability that allow for functional operation while blocking degradation pathways.
02 Protective encapsulation techniques for electride materials
Various encapsulation methods have been developed to protect electride materials from environmental degradation. These include hermetic sealing, specialized coating technologies, and composite structures that shield the reactive electride components from moisture and oxygen. Advanced packaging techniques utilize inert materials and barrier layers to prevent contact with degradation-causing elements while maintaining the functional properties of the electrides. These protection strategies are crucial for extending the operational lifetime of electride-based devices in real-world applications.Expand Specific Solutions03 Environmental monitoring systems using electride-based sensors
Electride materials are being incorporated into advanced sensing devices for environmental monitoring applications. These sensors leverage the unique electronic properties of electrides to detect pollutants, toxic gases, and other environmental contaminants with high sensitivity. Despite concerns about their environmental stability, properly protected electride sensors offer advantages in terms of response time and detection limits. Research focuses on balancing the reactive nature of electrides with their exceptional sensing capabilities to create durable environmental monitoring solutions.Expand Specific Solutions04 Eco-friendly synthesis and disposal methods for electride materials
As electride materials gain prominence in various applications, research is focusing on developing environmentally responsible synthesis methods and end-of-life management strategies. Green chemistry approaches aim to reduce toxic precursors and harmful byproducts during electride production. Additionally, recycling and proper disposal protocols are being established to minimize environmental impact when electride-containing devices reach end-of-life. These sustainable practices address concerns about potential environmental contamination from both the production and disposal phases of electride materials.Expand Specific Solutions05 Applications of electrides in environmental remediation technologies
Despite their own susceptibility to degradation, properly engineered electride materials show promise for environmental remediation applications. Their unique electronic properties enable efficient catalytic processes for breaking down pollutants and contaminants. Research explores using stabilized electrides in water treatment systems, air purification technologies, and soil remediation processes. These applications leverage the electron-donating capabilities of electrides to facilitate redox reactions that neutralize environmental toxins, offering potential solutions to challenging contamination problems.Expand Specific Solutions
Leading Research Groups and Industry Players
The environmental degradation of electrides is currently in an early research phase, with market applications still emerging. The field is characterized by academic leadership, with institutions like The Regents of the University of California, Georgia Tech Research Corp, and South China University of Technology pioneering fundamental research. Commercial development is gradually advancing through specialized environmental technology firms such as Advanced Environmental Technologies LLC and Innovative Environmental Technologies, Inc., which are exploring practical applications. National research organizations including the National Research Council of Canada and Korea Research Institute of Chemical Technology are bridging the gap between academic research and industrial implementation, focusing on understanding degradation mechanisms and developing more stable electride materials for environmental applications.
The Regents of the University of California
Technical Solution: UC Berkeley researchers have developed comprehensive degradation pathway models for C12A7:e- (mayenite) and other common electrides, focusing on their interaction with atmospheric moisture and oxygen. Their approach combines in-situ spectroscopic techniques with computational modeling to track electron localization changes during degradation. They've identified that water molecules initially physisorb on electride surfaces, followed by dissociative chemisorption that extracts electrons from the material's cavities. This creates hydroxyl groups and initiates a cascade of oxidation reactions that progressively degrade the electride structure. Their research has established critical humidity thresholds (approximately 40% RH) where degradation accelerates significantly, and they've developed protective encapsulation methods using hydrophobic polymers that extend electride lifetime by up to 300% in ambient conditions.
Strengths: Combines advanced spectroscopic techniques with computational modeling for comprehensive degradation mechanism understanding. Their encapsulation solutions are practical and immediately applicable. Weakness: Their research focuses primarily on oxide-based electrides with less coverage of organic and 2D electrides that are emerging as important materials.
The Georgia Tech Research Corp.
Technical Solution: Georgia Tech has pioneered research on environmental stability of 2D electrides, particularly focusing on degradation mechanisms in MXene-derived electrides. Their approach utilizes environmental transmission electron microscopy (E-TEM) to observe degradation in real-time at the atomic scale. They've identified that edge sites are primary degradation initiation points, with oxygen intercalation occurring preferentially at these locations before progressing inward. Their research has revealed a two-stage degradation process: initial rapid electron depletion at reactive sites followed by slower structural collapse. Georgia Tech has developed surface passivation techniques using atomic layer deposition of Al2O3 that selectively protects edge sites while maintaining the electride's functional properties. Their work has demonstrated that controlled partial oxidation can actually enhance certain catalytic properties while improving stability, creating a new class of "stabilized-performance" electride materials.
Strengths: Industry-leading in-situ characterization capabilities provide unparalleled insights into degradation mechanisms at the atomic scale. Their surface passivation techniques are highly effective and scalable. Weakness: Their solutions are more complex to implement at industrial scale and require specialized equipment for the passivation processes.
Key Degradation Pathway Research Findings
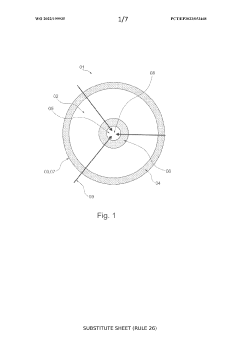
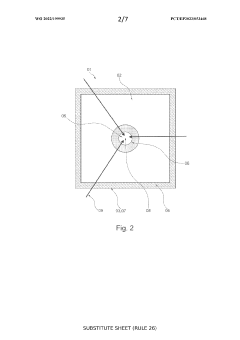
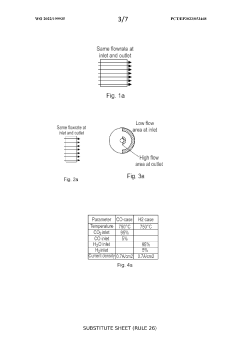
SOEC stack with fuel flow from periphery towards centre
PatentWO2022199935A1
Innovation
- The design features a solid oxide electrolysis cell stack with a fuel flow direction from the periphery towards the center, increasing the fuel inlet active cell area and decreasing it towards the outlet, thereby reducing maximum current density and degradation while maintaining production rate, using an external fuel inlet manifold to minimize pressure drop and ensure even gas distribution.
Process for increasing the humidity of soils
PatentInactiveEP0197537A1
Innovation
- The use of electrodes with high mechanical and chemical resilience, formed from conductive materials like carbon-filled PTFE, arranged to create a cascade-shaped electric field structure that facilitates electrochemical deposition of ions and salts, allowing for efficient water transport and salt removal, with the option to adjust electrode configurations for specific soil conditions.
Environmental Impact Assessment
The environmental impact of electrides extends beyond their immediate applications, encompassing their entire lifecycle from production to disposal. Common electrides, such as C12A7:e-, alkali metal-doped materials, and organic electrides, interact with environmental factors in ways that can lead to significant degradation pathways and subsequent ecological consequences.
Water exposure represents the primary degradation pathway for most electrides. When exposed to moisture, electrides undergo rapid hydrolysis reactions, releasing trapped electrons and forming hydroxide species. This process not only destroys the electride structure but can also generate alkaline byproducts that alter local pH levels in soil and water systems, potentially disrupting microbial communities and aquatic ecosystems.
Atmospheric oxygen presents another critical degradation vector. The highly reactive nature of the anionic electrons in electrides makes them susceptible to oxidation, forming various oxide compounds. This oxidative degradation can release heat and, in some cases, generate reactive oxygen species that may contribute to localized air quality issues or accelerate the weathering of surrounding materials.
Temperature fluctuations significantly influence degradation rates of electrides. Thermal cycling can induce structural stress in crystalline electrides, creating microfractures that increase surface area exposure to environmental agents. High temperatures accelerate reaction kinetics of degradation processes, while extreme cold may cause phase transitions that compromise the material's integrity and containment systems.
Photodegradation pathways are particularly relevant for organic electrides and certain inorganic varieties. UV radiation can trigger electron excitation and subsequent chemical transformations, potentially generating free radicals and other reactive intermediates. These photochemical processes may lead to the formation of secondary pollutants with unknown environmental persistence and toxicity profiles.
Biological interactions represent an understudied degradation pathway. Microbial communities can potentially utilize the high-energy electrons or degradation byproducts of electrides as energy sources, potentially altering microbial ecology in affected areas. Furthermore, the potential bioaccumulation of electride components in food chains remains largely unexplored but presents a concerning possibility for long-term ecological impacts.
The environmental persistence of degradation products varies significantly among electride classes. While some degradation pathways lead to relatively benign products like metal oxides, others may generate persistent compounds with unknown ecotoxicological profiles. This uncertainty necessitates comprehensive lifecycle assessments before widespread industrial adoption of electride technologies.
Water exposure represents the primary degradation pathway for most electrides. When exposed to moisture, electrides undergo rapid hydrolysis reactions, releasing trapped electrons and forming hydroxide species. This process not only destroys the electride structure but can also generate alkaline byproducts that alter local pH levels in soil and water systems, potentially disrupting microbial communities and aquatic ecosystems.
Atmospheric oxygen presents another critical degradation vector. The highly reactive nature of the anionic electrons in electrides makes them susceptible to oxidation, forming various oxide compounds. This oxidative degradation can release heat and, in some cases, generate reactive oxygen species that may contribute to localized air quality issues or accelerate the weathering of surrounding materials.
Temperature fluctuations significantly influence degradation rates of electrides. Thermal cycling can induce structural stress in crystalline electrides, creating microfractures that increase surface area exposure to environmental agents. High temperatures accelerate reaction kinetics of degradation processes, while extreme cold may cause phase transitions that compromise the material's integrity and containment systems.
Photodegradation pathways are particularly relevant for organic electrides and certain inorganic varieties. UV radiation can trigger electron excitation and subsequent chemical transformations, potentially generating free radicals and other reactive intermediates. These photochemical processes may lead to the formation of secondary pollutants with unknown environmental persistence and toxicity profiles.
Biological interactions represent an understudied degradation pathway. Microbial communities can potentially utilize the high-energy electrons or degradation byproducts of electrides as energy sources, potentially altering microbial ecology in affected areas. Furthermore, the potential bioaccumulation of electride components in food chains remains largely unexplored but presents a concerning possibility for long-term ecological impacts.
The environmental persistence of degradation products varies significantly among electride classes. While some degradation pathways lead to relatively benign products like metal oxides, others may generate persistent compounds with unknown ecotoxicological profiles. This uncertainty necessitates comprehensive lifecycle assessments before widespread industrial adoption of electride technologies.
Standardization of Stability Testing Protocols
The standardization of stability testing protocols for electrides represents a critical need in the field, as current research on environmental degradation pathways suffers from inconsistent methodologies that hinder comparative analysis. A unified approach to stability testing would significantly advance our understanding of electride degradation mechanisms and accelerate the development of more robust materials.
Establishing standardized exposure conditions is paramount in protocol development. These should include controlled humidity levels (ranging from 20% to 95% RH), temperature variations (ambient to 85°C), atmospheric composition (air, pure oxygen, nitrogen, and carbon dioxide environments), and light exposure parameters (UV intensity, wavelength distribution, and exposure duration). Each parameter must be precisely defined to ensure reproducibility across different research facilities.
Time-dependent degradation monitoring represents another essential component of standardized protocols. Short-term stability tests should track changes over hours to days, while long-term evaluations must extend to weeks or months. Accelerated aging tests, employing elevated temperatures or humidity levels, should be calibrated against real-world degradation rates to establish reliable correlation factors.
Analytical techniques for degradation assessment require standardization regarding sample preparation, measurement conditions, and data processing. X-ray diffraction (XRD) for structural analysis, X-ray photoelectron spectroscopy (XPS) for surface chemistry changes, and electron paramagnetic resonance (EPR) for detecting unpaired electrons should follow consistent methodologies. Electrical property measurements must specify electrode materials, contact configurations, and measurement frequencies.
Performance metrics and failure criteria constitute a critical aspect of standardized protocols. These should include quantitative thresholds for electrical conductivity reduction, electron emission efficiency decrease, structural integrity loss, and chemical composition changes. Establishing clear end-of-life definitions will facilitate meaningful comparisons between different electride materials.
Reporting requirements must be comprehensive, encompassing detailed documentation of testing conditions, analytical methods, raw data preservation, and statistical analysis approaches. Standardized data formats would enable database development for degradation patterns across various electride classes, potentially revealing correlations between structural features and environmental stability.
Implementation of round-robin testing across multiple laboratories would validate protocol reliability and identify potential sources of variability. This collaborative approach would strengthen confidence in the standardized methods and accelerate their adoption throughout the research community.
Establishing standardized exposure conditions is paramount in protocol development. These should include controlled humidity levels (ranging from 20% to 95% RH), temperature variations (ambient to 85°C), atmospheric composition (air, pure oxygen, nitrogen, and carbon dioxide environments), and light exposure parameters (UV intensity, wavelength distribution, and exposure duration). Each parameter must be precisely defined to ensure reproducibility across different research facilities.
Time-dependent degradation monitoring represents another essential component of standardized protocols. Short-term stability tests should track changes over hours to days, while long-term evaluations must extend to weeks or months. Accelerated aging tests, employing elevated temperatures or humidity levels, should be calibrated against real-world degradation rates to establish reliable correlation factors.
Analytical techniques for degradation assessment require standardization regarding sample preparation, measurement conditions, and data processing. X-ray diffraction (XRD) for structural analysis, X-ray photoelectron spectroscopy (XPS) for surface chemistry changes, and electron paramagnetic resonance (EPR) for detecting unpaired electrons should follow consistent methodologies. Electrical property measurements must specify electrode materials, contact configurations, and measurement frequencies.
Performance metrics and failure criteria constitute a critical aspect of standardized protocols. These should include quantitative thresholds for electrical conductivity reduction, electron emission efficiency decrease, structural integrity loss, and chemical composition changes. Establishing clear end-of-life definitions will facilitate meaningful comparisons between different electride materials.
Reporting requirements must be comprehensive, encompassing detailed documentation of testing conditions, analytical methods, raw data preservation, and statistical analysis approaches. Standardized data formats would enable database development for degradation patterns across various electride classes, potentially revealing correlations between structural features and environmental stability.
Implementation of round-robin testing across multiple laboratories would validate protocol reliability and identify potential sources of variability. This collaborative approach would strengthen confidence in the standardized methods and accelerate their adoption throughout the research community.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!