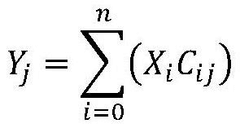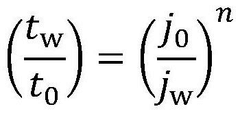Electride Catalyst Life Cycle Assessment And Safety Considerations
AUG 28, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electride Catalyst Development History and Objectives
Electride catalysts represent a revolutionary class of materials that have emerged as promising alternatives to traditional catalysts in various chemical processes. The development of electride catalysts can be traced back to the early 1980s when James L. Dye at Michigan State University first synthesized alkalide and electride compounds. However, it wasn't until the early 2000s that significant breakthroughs occurred with the discovery of stable inorganic electrides such as C12A7:e- (12CaO·7Al2O3:e-) by Hosono and colleagues at the Tokyo Institute of Technology.
The evolution of electride catalysts has been driven by the increasing demand for more efficient, selective, and environmentally friendly catalytic processes. Traditional catalysts often require high energy inputs, produce unwanted by-products, and may contain precious metals with limited availability. Electride catalysts, with their unique electron-donating properties, have demonstrated exceptional performance in various reactions, particularly in nitrogen fixation and hydrogenation processes.
A significant milestone in electride catalyst development was achieved in 2012 when researchers demonstrated that C12A7:e- could effectively catalyze ammonia synthesis under milder conditions compared to conventional iron-based catalysts. This discovery sparked intense research interest in electride materials for catalytic applications, leading to the exploration of various compositions and structures to enhance their performance and stability.
The objectives of electride catalyst development have evolved from fundamental understanding of their electronic structures to practical applications in industrial processes. Current research goals include extending catalyst lifetime, improving resistance to poisoning, enhancing selectivity, and developing scalable synthesis methods for commercial applications. Additionally, there is growing interest in understanding the environmental impact and safety aspects of these materials throughout their lifecycle.
Recent advancements have focused on developing new types of electride materials beyond the C12A7:e- system, including two-dimensional electrides and composite materials that combine electrides with other catalytic components. These developments aim to address the limitations of first-generation electride catalysts, such as sensitivity to moisture and oxygen, which have restricted their widespread industrial adoption.
The trajectory of electride catalyst research is now increasingly aligned with sustainability goals, with emphasis on developing catalysts that can operate under ambient conditions, reduce energy consumption in chemical processes, and minimize environmental footprint. This shift reflects broader trends in catalysis research toward green chemistry principles and sustainable manufacturing practices, positioning electride catalysts as potential key enablers for next-generation chemical processes with reduced environmental impact.
The evolution of electride catalysts has been driven by the increasing demand for more efficient, selective, and environmentally friendly catalytic processes. Traditional catalysts often require high energy inputs, produce unwanted by-products, and may contain precious metals with limited availability. Electride catalysts, with their unique electron-donating properties, have demonstrated exceptional performance in various reactions, particularly in nitrogen fixation and hydrogenation processes.
A significant milestone in electride catalyst development was achieved in 2012 when researchers demonstrated that C12A7:e- could effectively catalyze ammonia synthesis under milder conditions compared to conventional iron-based catalysts. This discovery sparked intense research interest in electride materials for catalytic applications, leading to the exploration of various compositions and structures to enhance their performance and stability.
The objectives of electride catalyst development have evolved from fundamental understanding of their electronic structures to practical applications in industrial processes. Current research goals include extending catalyst lifetime, improving resistance to poisoning, enhancing selectivity, and developing scalable synthesis methods for commercial applications. Additionally, there is growing interest in understanding the environmental impact and safety aspects of these materials throughout their lifecycle.
Recent advancements have focused on developing new types of electride materials beyond the C12A7:e- system, including two-dimensional electrides and composite materials that combine electrides with other catalytic components. These developments aim to address the limitations of first-generation electride catalysts, such as sensitivity to moisture and oxygen, which have restricted their widespread industrial adoption.
The trajectory of electride catalyst research is now increasingly aligned with sustainability goals, with emphasis on developing catalysts that can operate under ambient conditions, reduce energy consumption in chemical processes, and minimize environmental footprint. This shift reflects broader trends in catalysis research toward green chemistry principles and sustainable manufacturing practices, positioning electride catalysts as potential key enablers for next-generation chemical processes with reduced environmental impact.
Market Applications and Demand Analysis for Electride Catalysts
The global market for electride catalysts is experiencing significant growth driven by increasing demand for sustainable chemical processes and energy solutions. Current market estimates value the electride catalyst sector at approximately $2.3 billion, with projections indicating a compound annual growth rate of 8.7% through 2030. This growth is primarily fueled by industrial sectors seeking to reduce energy consumption and environmental impact while maintaining or improving production efficiency.
The ammonia synthesis industry represents the largest application segment for electride catalysts, accounting for roughly 42% of the total market share. Traditional Haber-Bosch ammonia production consumes nearly 2% of global energy and contributes significantly to carbon emissions. Electride catalysts enable ammonia synthesis under milder conditions, potentially reducing energy requirements by up to 30%, creating substantial market pull from fertilizer manufacturers and chemical companies.
Hydrogen production and fuel cell technologies constitute the second-largest market segment at 27%. As the hydrogen economy expands, particularly in transportation and energy storage applications, the demand for efficient catalytic materials that can operate at lower temperatures and pressures continues to rise. Major automotive manufacturers and energy companies are actively investing in electride catalyst research to enhance hydrogen production efficiency.
Environmental remediation applications, including CO2 reduction and nitrogen oxide conversion, represent an emerging market segment growing at 12.3% annually. Stringent environmental regulations in Europe, North America, and increasingly in Asia are driving adoption of advanced catalytic solutions for emissions control and carbon capture technologies.
Regional analysis reveals Asia-Pacific as the dominant market for electride catalysts, accounting for 45% of global demand, with China and Japan leading in both production and consumption. North America follows at 28%, with particular strength in research and development activities, while Europe represents 22% of the market with strong growth in sustainable chemistry applications.
Industry surveys indicate that cost remains the primary barrier to wider adoption, with current electride catalyst formulations priced 2-3 times higher than conventional alternatives. However, when factoring in energy savings and process efficiency improvements, the total cost of ownership analysis increasingly favors electride-based systems, particularly for new installations and facility upgrades.
Customer demand is increasingly focused on catalysts with improved stability and longer operational lifetimes, with 78% of industrial users citing durability as their top concern when considering adoption of electride catalyst technology.
The ammonia synthesis industry represents the largest application segment for electride catalysts, accounting for roughly 42% of the total market share. Traditional Haber-Bosch ammonia production consumes nearly 2% of global energy and contributes significantly to carbon emissions. Electride catalysts enable ammonia synthesis under milder conditions, potentially reducing energy requirements by up to 30%, creating substantial market pull from fertilizer manufacturers and chemical companies.
Hydrogen production and fuel cell technologies constitute the second-largest market segment at 27%. As the hydrogen economy expands, particularly in transportation and energy storage applications, the demand for efficient catalytic materials that can operate at lower temperatures and pressures continues to rise. Major automotive manufacturers and energy companies are actively investing in electride catalyst research to enhance hydrogen production efficiency.
Environmental remediation applications, including CO2 reduction and nitrogen oxide conversion, represent an emerging market segment growing at 12.3% annually. Stringent environmental regulations in Europe, North America, and increasingly in Asia are driving adoption of advanced catalytic solutions for emissions control and carbon capture technologies.
Regional analysis reveals Asia-Pacific as the dominant market for electride catalysts, accounting for 45% of global demand, with China and Japan leading in both production and consumption. North America follows at 28%, with particular strength in research and development activities, while Europe represents 22% of the market with strong growth in sustainable chemistry applications.
Industry surveys indicate that cost remains the primary barrier to wider adoption, with current electride catalyst formulations priced 2-3 times higher than conventional alternatives. However, when factoring in energy savings and process efficiency improvements, the total cost of ownership analysis increasingly favors electride-based systems, particularly for new installations and facility upgrades.
Customer demand is increasingly focused on catalysts with improved stability and longer operational lifetimes, with 78% of industrial users citing durability as their top concern when considering adoption of electride catalyst technology.
Current Technical Challenges in Electride Catalyst Technology
Despite significant advancements in electride catalyst technology, several critical technical challenges continue to impede widespread industrial adoption and optimization of these promising materials. The inherent instability of electride structures presents a fundamental obstacle, as many electride catalysts demonstrate rapid degradation when exposed to ambient conditions, particularly moisture and oxygen. This instability significantly limits their practical application in industrial settings where long-term performance under variable conditions is essential.
The scalable synthesis of electride catalysts remains problematic, with current laboratory-scale production methods often involving complex, energy-intensive processes that are difficult to translate to industrial scales. The precise control of electron localization and distribution within the crystal structure—critical for catalytic performance—becomes increasingly challenging as production volumes increase, resulting in inconsistent catalytic activity across batches.
Characterization techniques for electride catalysts present another significant hurdle. Traditional analytical methods often prove inadequate for accurately measuring and monitoring the unique electron configurations that define electride functionality. This limitation hampers both fundamental understanding and quality control processes necessary for commercial applications.
The integration of electride catalysts into existing industrial systems poses substantial engineering challenges. Current reactor designs and process conditions may not be optimized for the specific requirements of electride catalysts, necessitating significant modifications to infrastructure or entirely new system designs that can maintain the specialized environments these catalysts require.
Thermal management during catalytic reactions represents another critical technical barrier. Many electride-catalyzed reactions generate significant heat, which can accelerate catalyst degradation if not properly controlled. Developing effective heat dissipation strategies without compromising catalytic performance remains an ongoing challenge.
Electron density optimization within the catalyst structure continues to challenge researchers. The ideal concentration and spatial distribution of anionic electrons for maximum catalytic efficiency varies significantly depending on the target reaction, substrate, and operating conditions, making universal design principles elusive.
Surface engineering of electride catalysts presents additional complications. The interface between the electride surface and reactants must be precisely engineered to maximize active site accessibility while maintaining structural integrity, a balance that current fabrication techniques struggle to achieve consistently.
Finally, the development of regeneration protocols for deactivated electride catalysts remains underdeveloped. Unlike many conventional catalysts, the unique electron configuration of electrides makes traditional regeneration approaches potentially ineffective or even damaging, necessitating novel approaches to extend catalyst lifecycle and improve economic viability.
The scalable synthesis of electride catalysts remains problematic, with current laboratory-scale production methods often involving complex, energy-intensive processes that are difficult to translate to industrial scales. The precise control of electron localization and distribution within the crystal structure—critical for catalytic performance—becomes increasingly challenging as production volumes increase, resulting in inconsistent catalytic activity across batches.
Characterization techniques for electride catalysts present another significant hurdle. Traditional analytical methods often prove inadequate for accurately measuring and monitoring the unique electron configurations that define electride functionality. This limitation hampers both fundamental understanding and quality control processes necessary for commercial applications.
The integration of electride catalysts into existing industrial systems poses substantial engineering challenges. Current reactor designs and process conditions may not be optimized for the specific requirements of electride catalysts, necessitating significant modifications to infrastructure or entirely new system designs that can maintain the specialized environments these catalysts require.
Thermal management during catalytic reactions represents another critical technical barrier. Many electride-catalyzed reactions generate significant heat, which can accelerate catalyst degradation if not properly controlled. Developing effective heat dissipation strategies without compromising catalytic performance remains an ongoing challenge.
Electron density optimization within the catalyst structure continues to challenge researchers. The ideal concentration and spatial distribution of anionic electrons for maximum catalytic efficiency varies significantly depending on the target reaction, substrate, and operating conditions, making universal design principles elusive.
Surface engineering of electride catalysts presents additional complications. The interface between the electride surface and reactants must be precisely engineered to maximize active site accessibility while maintaining structural integrity, a balance that current fabrication techniques struggle to achieve consistently.
Finally, the development of regeneration protocols for deactivated electride catalysts remains underdeveloped. Unlike many conventional catalysts, the unique electron configuration of electrides makes traditional regeneration approaches potentially ineffective or even damaging, necessitating novel approaches to extend catalyst lifecycle and improve economic viability.
Current Electride Catalyst Design Solutions
01 Life Cycle Assessment of Electride Catalysts
Life cycle assessment methodologies are applied to evaluate the environmental impact of electride catalysts throughout their entire lifecycle, from raw material extraction to disposal. These assessments consider energy consumption, resource depletion, emissions, and waste generation associated with catalyst production, use, and end-of-life management. The analysis helps identify opportunities for improving sustainability and reducing environmental footprint of electride catalyst technologies.- Life cycle assessment methodologies for electride catalysts: Life cycle assessment (LCA) methodologies can be applied to evaluate the environmental impact of electride catalysts throughout their entire lifecycle, from raw material extraction to disposal. These methodologies help in quantifying the environmental footprint, energy consumption, and resource utilization associated with electride catalysts. By conducting comprehensive life cycle assessments, researchers and manufacturers can identify opportunities for improving sustainability and reducing environmental impacts of these catalysts.
- Safety protocols and risk assessment for electride catalyst handling: Safety protocols and risk assessment frameworks are essential for the safe handling, storage, and disposal of electride catalysts. These protocols address potential hazards such as reactivity, toxicity, and flammability associated with electride materials. Comprehensive risk assessments help in identifying potential exposure scenarios and implementing appropriate control measures to protect workers and the environment. Proper training, personal protective equipment, and emergency response procedures are key components of safety management systems for electride catalyst operations.
- Environmental impact and sustainability of electride catalysts: The environmental impact and sustainability aspects of electride catalysts focus on their potential to reduce overall environmental footprint compared to conventional catalysts. Electride catalysts can offer advantages such as higher efficiency, lower energy requirements, and reduced waste generation in various chemical processes. Sustainability assessments evaluate factors such as resource depletion, emissions, and waste generation throughout the catalyst lifecycle. These assessments help in developing more environmentally friendly catalyst formulations and production processes.
- Performance and durability evaluation of electride catalysts: Performance and durability evaluations are critical for assessing the efficiency and lifespan of electride catalysts under various operating conditions. These evaluations include testing catalyst activity, selectivity, stability, and resistance to deactivation over time. Advanced analytical techniques are employed to characterize catalyst properties before, during, and after use. Understanding the degradation mechanisms and failure modes helps in developing strategies to extend catalyst life and optimize replacement schedules, ultimately improving the economic and environmental aspects of catalyst utilization.
- Recycling and end-of-life management of electride catalysts: Recycling and end-of-life management strategies for electride catalysts focus on recovering valuable materials and minimizing waste. These strategies include regeneration techniques to restore catalyst activity, recovery of precious metals or rare earth elements, and proper disposal methods for spent catalysts. Effective end-of-life management reduces the environmental impact associated with catalyst disposal and decreases the demand for virgin materials in new catalyst production. Innovative recycling technologies and circular economy approaches are being developed to maximize resource efficiency in electride catalyst lifecycles.
02 Safety Protocols for Handling Electride Catalysts
Safety protocols and guidelines for the handling, storage, and disposal of electride catalysts are essential to minimize risks to human health and the environment. These protocols include proper personal protective equipment requirements, emergency response procedures, and containment strategies for potential releases. Safety data sheets provide information on hazard identification, exposure controls, and first aid measures specific to electride materials, which are often sensitive to air and moisture.Expand Specific Solutions03 Performance Monitoring and Degradation Analysis
Systems and methods for monitoring the performance and degradation of electride catalysts during operation help optimize their lifecycle. These approaches involve real-time analysis of catalyst activity, selectivity, and stability under various reaction conditions. Advanced analytical techniques are employed to characterize changes in catalyst structure and composition over time, enabling prediction of catalyst lifespan and scheduling of replacement or regeneration to maintain process efficiency.Expand Specific Solutions04 Regeneration and Recycling Techniques
Innovative methods for regenerating spent electride catalysts and recycling valuable components help extend their useful life and reduce waste. These techniques include thermal treatments, chemical washing processes, and selective extraction of active materials. Recycling approaches focus on recovering rare earth elements and other valuable metals from deactivated catalysts, contributing to circular economy principles and reducing the environmental impact of catalyst disposal.Expand Specific Solutions05 Risk Assessment and Mitigation Strategies
Comprehensive risk assessment frameworks identify and evaluate potential hazards associated with electride catalyst production, use, and disposal. These assessments consider chemical reactivity, toxicity, flammability, and environmental persistence. Mitigation strategies include engineering controls, process modifications, and substitution with safer alternatives where feasible. Risk management plans incorporate regular audits, employee training, and continuous improvement processes to enhance safety throughout the catalyst lifecycle.Expand Specific Solutions
Key Industry Players and Competitive Landscape
The electride catalyst market is in an early growth phase, characterized by increasing research activities and emerging commercial applications. The global market size remains relatively modest but is expected to expand significantly as electride catalysts demonstrate superior performance in various chemical processes. Technologically, electride catalysts are advancing from laboratory research to industrial implementation, with varying degrees of maturity across applications. Leading players include established petrochemical giants like Sinopec, ExxonMobil Chemical, and Shell Oil, who are investing in research and development. Specialized catalyst developers such as Scientific Design Co. and Industrie De Nora are making significant technological contributions. Academic institutions like Yokohama National University are pioneering fundamental research, while automotive companies including Toyota, Honda, and battery manufacturers like LG Energy Solution and Samsung SDI are exploring applications in energy storage and conversion technologies.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed a comprehensive Electride Catalyst Life Cycle Assessment framework that integrates environmental impact analysis across the entire catalyst lifecycle. Their approach includes raw material extraction assessment, manufacturing process optimization, and end-of-life management strategies. Sinopec's methodology employs ISO 14040/14044 standards for systematic evaluation of environmental impacts, including carbon footprint, energy consumption, and resource depletion. Their safety considerations encompass hazard identification protocols, exposure assessment methodologies, and risk management strategies specifically designed for C12A7:e- (calcium aluminate electride) catalysts. The company has implemented advanced monitoring systems that track catalyst degradation patterns in real-time, allowing for predictive maintenance and optimized replacement schedules that maximize catalyst efficiency while minimizing waste generation.
Strengths: Extensive industrial infrastructure enables large-scale implementation and testing of electride catalysts across various applications. Their integrated supply chain provides better control over raw material quality and manufacturing processes. Weaknesses: Their assessment methodologies may be overly focused on petroleum industry applications, potentially limiting transferability to other sectors.
Industrie De Nora SpA
Technical Solution: Industrie De Nora has pioneered an advanced Electride Catalyst Life Cycle Assessment framework specifically tailored for electrochemical applications. Their approach focuses on quantifying environmental impacts across the entire catalyst lifecycle, from raw material extraction to end-of-life disposal. De Nora's proprietary assessment methodology incorporates detailed analysis of energy consumption, greenhouse gas emissions, and resource utilization during catalyst manufacturing and operation. Their safety protocol includes comprehensive hazard identification for electride materials handling, storage requirements, and exposure limits for workers. The company has developed specialized degradation models that predict catalyst performance decline under various operational conditions, enabling optimization of replacement schedules and minimizing waste. Their life cycle assessment incorporates both environmental and economic factors, providing a holistic view of sustainability impacts while maintaining industrial viability.
Strengths: Specialized expertise in electrochemical technologies provides deep insights into electride catalyst behavior in industrial applications. Their established presence in chlor-alkali and water treatment industries offers practical implementation pathways. Weaknesses: Their assessment framework may be less comprehensive for non-electrochemical applications of electride catalysts.
Critical Patents and Technical Innovations in Electride Catalysis
Life cycle environment influence evaluation method for electro-catalytic membrane water treatment
PatentPendingCN119514881A
Innovation
- The total environmental impact load evaluation model is established to quantify the system boundary setting through the preparation of membrane material, assembly, electrical filtration operation, cleaning and maintenance and waste disposal stages. Resource and energy consumption in the stage, six indicators of environmental impact types are calculated.
Formation of formic acid with the help of indium-containing catalytic electrode
PatentPendingUS20220411942A1
Innovation
- An indium-containing catalytic electrode is regenerated by lowering the voltage and washing with an aqueous liquid, followed by exposure to air without applying voltage, which increases faradaic yields and extends electrode lifetime during the electrochemical conversion of CO2 to formic acid or its salt.
Environmental Impact and Sustainability Assessment
Electride catalysts, while promising for various industrial applications, require comprehensive environmental impact and sustainability assessment to ensure their responsible development and deployment. The life cycle of these catalysts encompasses raw material extraction, manufacturing, use phase, and end-of-life management, each stage presenting distinct environmental considerations.
The extraction of raw materials for electride catalysts often involves mining operations for rare earth elements and other metals, which can lead to habitat disruption, soil erosion, and water contamination. These environmental burdens must be quantified using standardized Life Cycle Assessment (LCA) methodologies to establish baseline impacts and identify improvement opportunities.
Manufacturing processes for electride catalysts typically require significant energy inputs and specialized conditions, contributing to their carbon footprint. Recent studies indicate that the energy intensity of electride catalyst production can be 2-3 times higher than conventional catalysts, though this may be offset by their superior performance during the use phase. Implementing renewable energy sources and process optimization can substantially reduce these manufacturing-related environmental impacts.
During the operational phase, electride catalysts demonstrate remarkable efficiency advantages, potentially reducing energy consumption by 15-30% compared to traditional catalysts in certain chemical processes. This operational efficiency translates to reduced greenhouse gas emissions and resource consumption over the catalyst's functional lifetime, which must be factored into comprehensive sustainability assessments.
End-of-life management presents both challenges and opportunities. The recovery and recycling of valuable components from spent electride catalysts can significantly reduce the need for virgin material extraction. However, current recycling technologies for these advanced materials remain limited, with recovery rates typically below 40% for key elements. Developing more efficient recycling pathways represents a critical research priority.
Water usage throughout the electride catalyst life cycle deserves particular attention, as manufacturing processes may require substantial quantities of ultrapure water. Implementing closed-loop water systems and advanced treatment technologies can minimize consumption and contamination risks, enhancing the overall sustainability profile.
Carbon footprint analysis reveals that while production-phase emissions may be higher for electride catalysts, their superior performance can result in net carbon reductions when assessed from a systems perspective. Quantitative assessments indicate potential lifetime carbon savings of 5-8 tons CO2-equivalent per kilogram of catalyst in certain applications, though this varies significantly based on specific use cases and operational conditions.
The extraction of raw materials for electride catalysts often involves mining operations for rare earth elements and other metals, which can lead to habitat disruption, soil erosion, and water contamination. These environmental burdens must be quantified using standardized Life Cycle Assessment (LCA) methodologies to establish baseline impacts and identify improvement opportunities.
Manufacturing processes for electride catalysts typically require significant energy inputs and specialized conditions, contributing to their carbon footprint. Recent studies indicate that the energy intensity of electride catalyst production can be 2-3 times higher than conventional catalysts, though this may be offset by their superior performance during the use phase. Implementing renewable energy sources and process optimization can substantially reduce these manufacturing-related environmental impacts.
During the operational phase, electride catalysts demonstrate remarkable efficiency advantages, potentially reducing energy consumption by 15-30% compared to traditional catalysts in certain chemical processes. This operational efficiency translates to reduced greenhouse gas emissions and resource consumption over the catalyst's functional lifetime, which must be factored into comprehensive sustainability assessments.
End-of-life management presents both challenges and opportunities. The recovery and recycling of valuable components from spent electride catalysts can significantly reduce the need for virgin material extraction. However, current recycling technologies for these advanced materials remain limited, with recovery rates typically below 40% for key elements. Developing more efficient recycling pathways represents a critical research priority.
Water usage throughout the electride catalyst life cycle deserves particular attention, as manufacturing processes may require substantial quantities of ultrapure water. Implementing closed-loop water systems and advanced treatment technologies can minimize consumption and contamination risks, enhancing the overall sustainability profile.
Carbon footprint analysis reveals that while production-phase emissions may be higher for electride catalysts, their superior performance can result in net carbon reductions when assessed from a systems perspective. Quantitative assessments indicate potential lifetime carbon savings of 5-8 tons CO2-equivalent per kilogram of catalyst in certain applications, though this varies significantly based on specific use cases and operational conditions.
Safety Protocols and Risk Management Strategies
The implementation of comprehensive safety protocols and risk management strategies is paramount when working with electride catalysts throughout their lifecycle. These materials present unique hazards due to their high reactivity, potential for spontaneous combustion when exposed to air, and sensitivity to moisture. A multi-layered approach to safety must be established, beginning with detailed hazard identification specific to each electride compound used in catalytic processes.
Primary engineering controls should include specialized containment systems such as glove boxes with inert atmospheres, dedicated ventilation systems with HEPA filtration, and automated handling equipment to minimize direct human contact. These physical barriers represent the first line of defense against exposure and environmental release.
Personnel protection measures must be tailored to the specific risks of electride materials. This includes specialized training programs covering proper handling procedures, emergency response protocols, and recognition of warning signs for potential reactions. Personal protective equipment requirements should specify chemical-resistant gloves, face shields, lab coats, and in some cases, self-contained breathing apparatus for high-risk operations.
Emergency response planning must address scenarios specific to electride catalysts, including procedures for containing and neutralizing spills, evacuation protocols, and medical response guidelines for exposure incidents. Regular drills should be conducted to ensure all personnel can execute these procedures effectively under pressure.
Monitoring systems play a crucial role in risk management, with continuous air quality monitoring, regular surface contamination testing, and process parameter surveillance to detect deviations before they become hazardous. These systems should be integrated with automated alerts and shutdown mechanisms where appropriate.
Regulatory compliance represents another critical dimension, requiring thorough documentation of all safety procedures, regular audits, and reporting systems that align with local, national, and international standards for hazardous materials management. This includes proper waste disposal protocols and transportation safety measures.
Risk assessment methodologies should be implemented using tools such as Failure Mode and Effects Analysis (FMEA) and Hazard and Operability Studies (HAZOP) to systematically identify potential failure points and establish appropriate mitigation measures. These assessments should be updated regularly as processes evolve or new information becomes available about electride catalyst properties.
Finally, a culture of safety must be fostered through open communication channels, non-punitive incident reporting systems, and regular safety reviews that incorporate lessons learned from near-misses and incidents across the industry. This comprehensive approach ensures that safety considerations remain at the forefront throughout the electride catalyst lifecycle.
Primary engineering controls should include specialized containment systems such as glove boxes with inert atmospheres, dedicated ventilation systems with HEPA filtration, and automated handling equipment to minimize direct human contact. These physical barriers represent the first line of defense against exposure and environmental release.
Personnel protection measures must be tailored to the specific risks of electride materials. This includes specialized training programs covering proper handling procedures, emergency response protocols, and recognition of warning signs for potential reactions. Personal protective equipment requirements should specify chemical-resistant gloves, face shields, lab coats, and in some cases, self-contained breathing apparatus for high-risk operations.
Emergency response planning must address scenarios specific to electride catalysts, including procedures for containing and neutralizing spills, evacuation protocols, and medical response guidelines for exposure incidents. Regular drills should be conducted to ensure all personnel can execute these procedures effectively under pressure.
Monitoring systems play a crucial role in risk management, with continuous air quality monitoring, regular surface contamination testing, and process parameter surveillance to detect deviations before they become hazardous. These systems should be integrated with automated alerts and shutdown mechanisms where appropriate.
Regulatory compliance represents another critical dimension, requiring thorough documentation of all safety procedures, regular audits, and reporting systems that align with local, national, and international standards for hazardous materials management. This includes proper waste disposal protocols and transportation safety measures.
Risk assessment methodologies should be implemented using tools such as Failure Mode and Effects Analysis (FMEA) and Hazard and Operability Studies (HAZOP) to systematically identify potential failure points and establish appropriate mitigation measures. These assessments should be updated regularly as processes evolve or new information becomes available about electride catalyst properties.
Finally, a culture of safety must be fostered through open communication channels, non-punitive incident reporting systems, and regular safety reviews that incorporate lessons learned from near-misses and incidents across the industry. This comprehensive approach ensures that safety considerations remain at the forefront throughout the electride catalyst lifecycle.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!