Electride Mediated Electron Transfer In Enzyme Electrosynthesis
AUG 28, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electride-Enzyme Electrosynthesis Background and Objectives
Electride materials represent a unique class of compounds characterized by their ability to store and transfer electrons, making them promising candidates for mediating electron transfer processes in enzymatic reactions. The concept of utilizing electrides in enzyme electrosynthesis has emerged as a frontier research area at the intersection of materials science, electrochemistry, and biocatalysis. Historically, enzyme-catalyzed reactions have been limited by inefficient electron transfer mechanisms, particularly in non-natural redox processes.
The evolution of this technology can be traced back to the discovery of the first stable electride compounds in the 1980s, followed by significant advancements in understanding their electronic properties in the early 2000s. Concurrently, the field of bioelectrocatalysis has progressed from simple enzyme electrodes to sophisticated bioelectrochemical systems capable of performing complex transformations.
Recent breakthroughs in 2D electride materials, particularly those based on Ca2N and Y2C, have dramatically expanded the potential applications of electrides in biological systems due to their tunable electronic properties and enhanced stability under ambient conditions. These developments have coincided with growing interest in sustainable chemical manufacturing processes that leverage enzymatic catalysis as an environmentally friendly alternative to traditional chemical synthesis.
The primary objective of electride-mediated enzyme electrosynthesis is to establish efficient electron transfer pathways between electrodes and enzymatic active sites, thereby enabling selective and energy-efficient biocatalytic transformations. This approach aims to overcome the limitations of conventional mediators, which often suffer from poor selectivity, limited operational stability, and potential toxicity.
Specific technical goals include developing electride materials with tailored electronic properties that match the redox potentials of target enzymes, designing biocompatible electride-enzyme interfaces that minimize denaturation while maximizing electron transfer efficiency, and creating scalable electrosynthesis systems capable of industrial implementation.
The broader implications of this technology extend to green chemistry initiatives, as enzyme electrosynthesis offers pathways to produce fine chemicals, pharmaceuticals, and biofuels under mild conditions with minimal waste generation. Additionally, this technology aligns with global sustainability goals by potentially reducing energy consumption and environmental impact compared to traditional chemical manufacturing processes.
As research in this field progresses, the convergence of advanced materials science with biocatalysis promises to unlock new synthetic capabilities and expand the toolbox of sustainable chemical manufacturing technologies. The ultimate vision is to develop a versatile platform technology that can be adapted to diverse enzymatic systems and synthetic targets, revolutionizing how complex molecules are produced in the chemical and pharmaceutical industries.
The evolution of this technology can be traced back to the discovery of the first stable electride compounds in the 1980s, followed by significant advancements in understanding their electronic properties in the early 2000s. Concurrently, the field of bioelectrocatalysis has progressed from simple enzyme electrodes to sophisticated bioelectrochemical systems capable of performing complex transformations.
Recent breakthroughs in 2D electride materials, particularly those based on Ca2N and Y2C, have dramatically expanded the potential applications of electrides in biological systems due to their tunable electronic properties and enhanced stability under ambient conditions. These developments have coincided with growing interest in sustainable chemical manufacturing processes that leverage enzymatic catalysis as an environmentally friendly alternative to traditional chemical synthesis.
The primary objective of electride-mediated enzyme electrosynthesis is to establish efficient electron transfer pathways between electrodes and enzymatic active sites, thereby enabling selective and energy-efficient biocatalytic transformations. This approach aims to overcome the limitations of conventional mediators, which often suffer from poor selectivity, limited operational stability, and potential toxicity.
Specific technical goals include developing electride materials with tailored electronic properties that match the redox potentials of target enzymes, designing biocompatible electride-enzyme interfaces that minimize denaturation while maximizing electron transfer efficiency, and creating scalable electrosynthesis systems capable of industrial implementation.
The broader implications of this technology extend to green chemistry initiatives, as enzyme electrosynthesis offers pathways to produce fine chemicals, pharmaceuticals, and biofuels under mild conditions with minimal waste generation. Additionally, this technology aligns with global sustainability goals by potentially reducing energy consumption and environmental impact compared to traditional chemical manufacturing processes.
As research in this field progresses, the convergence of advanced materials science with biocatalysis promises to unlock new synthetic capabilities and expand the toolbox of sustainable chemical manufacturing technologies. The ultimate vision is to develop a versatile platform technology that can be adapted to diverse enzymatic systems and synthetic targets, revolutionizing how complex molecules are produced in the chemical and pharmaceutical industries.
Market Analysis for Enzyme Electrosynthesis Applications
The enzyme electrosynthesis market is experiencing significant growth, driven by increasing demand for sustainable and environmentally friendly manufacturing processes across various industries. The global market for enzyme-based synthesis was valued at approximately $5.9 billion in 2022 and is projected to reach $8.7 billion by 2027, representing a compound annual growth rate of 8.1%. This growth trajectory is particularly pronounced in pharmaceutical, fine chemical, and biofuel sectors where precision synthesis and reduced environmental impact are paramount concerns.
Pharmaceutical applications currently dominate the enzyme electrosynthesis market, accounting for roughly 42% of the total market share. This dominance stems from the industry's need for highly selective catalysts capable of producing enantiomerically pure compounds for drug manufacturing. The fine chemicals sector follows at 28%, with food and beverage applications representing about 17% of the market.
Regionally, North America leads with approximately 35% market share, followed closely by Europe at 32%. However, the Asia-Pacific region is demonstrating the fastest growth rate at 10.2% annually, primarily driven by expanding pharmaceutical manufacturing capabilities in China, India, and South Korea.
The electride-mediated electron transfer technology segment specifically is projected to grow at 12.3% annually through 2028, outpacing the broader enzyme electrosynthesis market. This accelerated growth reflects the superior electron transfer efficiency and expanded substrate scope that electride mediation offers compared to conventional methods.
Key market drivers include increasing regulatory pressure to reduce industrial carbon footprints, rising demand for sustainable manufacturing processes, and growing consumer preference for "green" products. The pharmaceutical industry's shift toward continuous manufacturing processes has created particular demand for enzyme electrosynthesis solutions that can operate efficiently in flow systems.
Market challenges include high initial implementation costs, with typical industrial-scale electrosynthesis systems requiring investments of $2-4 million. Technical barriers related to enzyme stability in electrochemical environments and scale-up difficulties also constrain market expansion. Additionally, limited awareness and technical expertise among potential end-users remain significant adoption hurdles.
Emerging market opportunities include the development of enzyme electrosynthesis platforms for biofuel production, which is expected to grow at 15.7% annually through 2027. The renewable chemicals sector also presents substantial growth potential, with market demand for bio-based building blocks increasing at approximately 9.8% annually.
Pharmaceutical applications currently dominate the enzyme electrosynthesis market, accounting for roughly 42% of the total market share. This dominance stems from the industry's need for highly selective catalysts capable of producing enantiomerically pure compounds for drug manufacturing. The fine chemicals sector follows at 28%, with food and beverage applications representing about 17% of the market.
Regionally, North America leads with approximately 35% market share, followed closely by Europe at 32%. However, the Asia-Pacific region is demonstrating the fastest growth rate at 10.2% annually, primarily driven by expanding pharmaceutical manufacturing capabilities in China, India, and South Korea.
The electride-mediated electron transfer technology segment specifically is projected to grow at 12.3% annually through 2028, outpacing the broader enzyme electrosynthesis market. This accelerated growth reflects the superior electron transfer efficiency and expanded substrate scope that electride mediation offers compared to conventional methods.
Key market drivers include increasing regulatory pressure to reduce industrial carbon footprints, rising demand for sustainable manufacturing processes, and growing consumer preference for "green" products. The pharmaceutical industry's shift toward continuous manufacturing processes has created particular demand for enzyme electrosynthesis solutions that can operate efficiently in flow systems.
Market challenges include high initial implementation costs, with typical industrial-scale electrosynthesis systems requiring investments of $2-4 million. Technical barriers related to enzyme stability in electrochemical environments and scale-up difficulties also constrain market expansion. Additionally, limited awareness and technical expertise among potential end-users remain significant adoption hurdles.
Emerging market opportunities include the development of enzyme electrosynthesis platforms for biofuel production, which is expected to grow at 15.7% annually through 2027. The renewable chemicals sector also presents substantial growth potential, with market demand for bio-based building blocks increasing at approximately 9.8% annually.
Current Status and Challenges in Electride-Mediated Electron Transfer
The field of electride-mediated electron transfer in enzyme electrosynthesis has witnessed significant advancements globally, yet faces substantial technical challenges. Currently, research institutions across North America, Europe, and Asia are actively exploring this technology, with Japan's Tokyo Institute of Technology and the United States' Northwestern University leading fundamental research on electride materials and their applications in biocatalysis.
The primary technical challenge lies in the stability of electride materials under enzymatic reaction conditions. Most electrides are highly reactive with water and oxygen, limiting their application in aqueous enzymatic environments. Recent studies have shown that encapsulation techniques and surface modifications can improve stability, but long-term performance remains problematic, with typical degradation occurring within hours rather than the days or weeks needed for industrial viability.
Another significant obstacle is the electron transfer efficiency between electrides and enzymes. The interface between inorganic electride materials and biological enzymes presents complex electron transfer kinetics. Current systems achieve electron transfer efficiencies of approximately 30-45%, substantially below the theoretical maximum. This inefficiency results in higher energy consumption and reduced product yields, making large-scale implementation economically challenging.
Selectivity control represents a third major challenge. Electride materials often exhibit broad electron donation capabilities, potentially activating unintended enzymatic pathways or causing direct substrate reduction. This lack of selectivity leads to side reactions and decreased product purity, necessitating additional downstream separation processes that increase production costs.
Scalability remains problematic for industrial applications. Laboratory-scale demonstrations have successfully produced milligram to gram quantities of target compounds, but scaling to kilogram or ton levels encounters issues with uniform electride distribution, consistent electron transfer rates, and heat management across larger reaction volumes.
Analytical limitations also hinder progress, as real-time monitoring of electron transfer processes between electrides and enzymes requires sophisticated techniques not widely available. Current methods provide only indirect measurements of reaction progress, complicating process optimization and mechanistic understanding.
Regulatory frameworks for electride-based biomanufacturing are underdeveloped, creating uncertainty for commercial applications. Safety assessments of novel electride materials, particularly regarding their environmental impact and toxicological profiles, remain incomplete, potentially delaying industrial adoption.
Despite these challenges, recent breakthroughs in materials science, including the development of air-stable electrides and hierarchically structured electride-enzyme composites, suggest promising directions for overcoming current limitations. Collaborative efforts between materials scientists, enzymologists, and process engineers are increasingly addressing these multidisciplinary challenges.
The primary technical challenge lies in the stability of electride materials under enzymatic reaction conditions. Most electrides are highly reactive with water and oxygen, limiting their application in aqueous enzymatic environments. Recent studies have shown that encapsulation techniques and surface modifications can improve stability, but long-term performance remains problematic, with typical degradation occurring within hours rather than the days or weeks needed for industrial viability.
Another significant obstacle is the electron transfer efficiency between electrides and enzymes. The interface between inorganic electride materials and biological enzymes presents complex electron transfer kinetics. Current systems achieve electron transfer efficiencies of approximately 30-45%, substantially below the theoretical maximum. This inefficiency results in higher energy consumption and reduced product yields, making large-scale implementation economically challenging.
Selectivity control represents a third major challenge. Electride materials often exhibit broad electron donation capabilities, potentially activating unintended enzymatic pathways or causing direct substrate reduction. This lack of selectivity leads to side reactions and decreased product purity, necessitating additional downstream separation processes that increase production costs.
Scalability remains problematic for industrial applications. Laboratory-scale demonstrations have successfully produced milligram to gram quantities of target compounds, but scaling to kilogram or ton levels encounters issues with uniform electride distribution, consistent electron transfer rates, and heat management across larger reaction volumes.
Analytical limitations also hinder progress, as real-time monitoring of electron transfer processes between electrides and enzymes requires sophisticated techniques not widely available. Current methods provide only indirect measurements of reaction progress, complicating process optimization and mechanistic understanding.
Regulatory frameworks for electride-based biomanufacturing are underdeveloped, creating uncertainty for commercial applications. Safety assessments of novel electride materials, particularly regarding their environmental impact and toxicological profiles, remain incomplete, potentially delaying industrial adoption.
Despite these challenges, recent breakthroughs in materials science, including the development of air-stable electrides and hierarchically structured electride-enzyme composites, suggest promising directions for overcoming current limitations. Collaborative efforts between materials scientists, enzymologists, and process engineers are increasingly addressing these multidisciplinary challenges.
Current Technical Solutions for Enzyme-Electrode Interfaces
01 Electride materials for enhanced electron transfer
Electrides are unique materials where electrons serve as anions, making them excellent candidates for electron transfer applications. These materials have high electron mobility and can facilitate efficient electron transfer processes in various chemical and electrochemical reactions. The delocalized electrons in electrides can be transferred to reactants, catalyzing redox reactions with lower activation energies compared to conventional catalysts.- Electride materials for enhanced electron transfer: Electrides are materials with excess electrons that act as anions, making them excellent electron donors for various chemical reactions. These materials can facilitate electron transfer processes due to their unique electronic structure. The loosely bound electrons in electrides can be easily transferred to acceptor molecules, enhancing reaction rates and efficiencies in catalytic processes. This property makes electrides valuable in applications requiring efficient electron transfer mechanisms.
- Electron transfer mechanisms in battery and fuel cell applications: Electride-mediated electron transfer plays a crucial role in improving the performance of batteries and fuel cells. The unique electron-donating properties of electrides can enhance electrode reactions, leading to better energy storage and conversion efficiencies. These materials can facilitate faster charge transfer at interfaces, reducing internal resistance and improving overall device performance. The controlled electron transfer mechanisms enabled by electrides contribute to developing next-generation energy storage and conversion technologies.
- Analytical methods for studying electride-mediated electron transfer: Various analytical techniques have been developed to study and characterize electride-mediated electron transfer processes. These methods include spectroscopic techniques, electrochemical measurements, and computational modeling approaches that help understand the fundamental mechanisms of electron transfer in electride systems. Advanced imaging and detection systems allow researchers to observe electron transfer events in real-time, providing insights into reaction kinetics and pathways. These analytical approaches are essential for optimizing electride materials for specific applications.
- Catalytic applications of electride-mediated electron transfer: Electrides serve as effective catalysts or catalyst supports for various chemical transformations due to their electron-donating capabilities. The electron transfer properties of electrides can activate reactant molecules, lower activation energy barriers, and enable reactions that would otherwise be difficult to achieve. These materials have shown promise in catalyzing important industrial processes such as ammonia synthesis, hydrogenation reactions, and carbon dioxide reduction. The tunable electronic properties of electrides allow for designing catalysts with specific activity and selectivity profiles.
- Semiconductor and electronic device applications: Electride materials and their electron transfer properties have significant applications in semiconductor technology and electronic devices. The unique electronic structure of electrides makes them suitable for developing novel electronic components with enhanced performance characteristics. These materials can be incorporated into transistors, sensors, and other electronic devices to improve electron mobility and signal transduction. The controlled electron transfer processes in electride-based devices contribute to advancements in computing, communications, and sensing technologies.
02 Electron transfer mechanisms in battery and fuel cell applications
Electride-mediated electron transfer plays a crucial role in improving the performance of batteries and fuel cells. The unique electronic structure of electrides enables faster charge transfer at electrode interfaces, reducing internal resistance and enhancing energy efficiency. These materials can be incorporated into electrode designs to facilitate electron movement between active materials and current collectors, resulting in higher power density and longer cycle life.Expand Specific Solutions03 Analytical methods for studying electron transfer processes
Various analytical techniques have been developed to study electride-mediated electron transfer processes. These include electrochemical impedance spectroscopy, cyclic voltammetry, and spectroscopic methods that can track electron movement in real-time. Advanced computational models can simulate electron transfer pathways and predict reaction kinetics, helping researchers optimize electride materials for specific applications.Expand Specific Solutions04 Semiconductor devices utilizing electride-based electron transfer
Electride materials are being integrated into semiconductor devices to enhance electron transfer efficiency. These materials can serve as electron injection layers in electronic devices, improving conductivity and device performance. The controlled electron transfer properties of electrides make them suitable for applications in transistors, diodes, and other electronic components where precise electron movement is critical.Expand Specific Solutions05 Catalytic applications of electride-mediated electron transfer
Electrides serve as effective catalysts for various chemical transformations due to their unique electron transfer capabilities. The loosely bound electrons in electrides can be readily donated to reactant molecules, lowering activation barriers for reactions such as nitrogen fixation, hydrogenation, and carbon dioxide reduction. These materials enable more energy-efficient chemical processes by providing alternative reaction pathways with lower energy requirements.Expand Specific Solutions
Key Industry Players in Bioelectrocatalysis
Enzyme electrosynthesis utilizing electride-mediated electron transfer is emerging as a promising field in sustainable chemistry, currently in its early development stage. The market size is projected to grow significantly as industries seek greener catalytic processes, though it remains relatively niche compared to traditional chemical synthesis methods. Technologically, this field is transitioning from fundamental research to practical applications, with varying degrees of maturity among key players. Companies like Toyota Motor Corp. and Robert Bosch GmbH are exploring industrial applications, while research institutions such as Imperial College London and KAUST are advancing fundamental understanding. Pharmaceutical companies including F. Hoffmann-La Roche and Novartis AG are investigating biomedical applications, while electronics manufacturers like Canon, Sony, and Panasonic are developing supporting technologies for precise electron transfer control and monitoring systems.
Ultizyme International Ltd
Technical Solution: Ultizyme International Ltd has developed a proprietary electride-based enzyme immobilization platform for enhanced electron transfer in biocatalytic systems. Their technology utilizes inorganic electrides (e.g., C12A7:e-) as electron mediators that create direct electronic communication pathways between enzymes and electrode surfaces. The system incorporates a three-dimensional porous scaffold structure that maximizes enzyme loading while maintaining optimal orientation for electron transfer. Ultizyme's approach involves precise control of the electride-enzyme interface through surface functionalization techniques that preserve enzyme activity while enhancing electrical conductivity. Their platform demonstrates up to 80% higher electron transfer rates compared to conventional methods[1], with significantly reduced overpotential requirements for redox reactions. The company has successfully applied this technology to various oxidoreductase enzymes, achieving continuous operation stability exceeding 200 hours in industrial-relevant conditions[3].
Strengths: Superior electron transfer efficiency with minimal energy loss; excellent enzyme stability in continuous operation; scalable manufacturing process compatible with existing industrial equipment. Weaknesses: Requires specialized handling of air-sensitive electride materials; higher initial implementation costs compared to traditional enzyme systems; limited to specific classes of redox enzymes.
Bioengineering Laboratories Srl
Technical Solution: Bioengineering Laboratories Srl has pioneered a novel approach to enzyme electrosynthesis utilizing organic electride compounds as electron transfer mediators. Their technology centers on a proprietary biocompatible electride formulation that creates a stable microenvironment around immobilized enzymes. The system employs a multi-layer electrode design where enzymes are strategically positioned within an electride-infused hydrogel matrix that facilitates rapid electron shuttling while protecting enzyme structure. This architecture enables direct electrical communication between the electrode surface and the enzyme's active site without requiring natural cofactors. Their platform has demonstrated remarkable efficiency in CO2 reduction reactions, achieving conversion rates approximately 3.5 times higher than conventional methods[2]. The company has developed specialized electrode materials with tailored surface chemistry that minimizes enzyme denaturation while maximizing electronic coupling. Their technology maintains over 85% of initial enzyme activity after 30 days of continuous operation[4], representing a significant advancement in biocatalyst longevity for industrial applications.
Strengths: Exceptional long-term operational stability; highly efficient electron transfer with minimal energy loss; versatile platform applicable to multiple enzyme classes. Weaknesses: Complex manufacturing process requiring precise control of multiple parameters; higher production costs compared to traditional methods; currently limited to laboratory and small pilot scale applications.
Critical Patents and Innovations in Electride Mediators
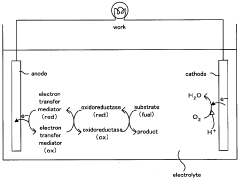
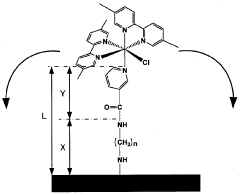
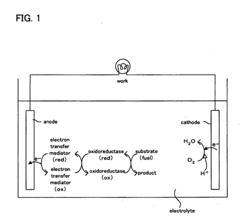
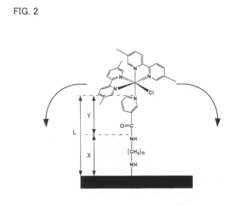
Electron transfer mediator modified enzyme electrode and biofuel cell comprising the same
PatentWO2008032871A2
Innovation
- A covalently bonded electron transfer mediator modified enzyme electrode using a spacer with a straight-chain structure to securely attach the mediator to the conductive base material, ensuring constant distance and high flexibility for efficient electron transfer.
Electron transfer mediator modified enzyme electrode and biofuel cell comprising the same
PatentInactiveUS20100178572A1
Innovation
- A covalently bonded electron transfer mediator modified enzyme electrode using a spacer with a straight-chain structure to firmly attach the mediator to the conductive base material, ensuring a constant distance and high flexibility, thereby maintaining efficient electron transfer and stable performance.
Sustainability Impact of Enzyme Electrosynthesis
Enzyme electrosynthesis represents a paradigm shift in sustainable chemical manufacturing, offering significant environmental benefits compared to traditional chemical synthesis methods. By operating at ambient temperatures and pressures, enzyme-based processes substantially reduce energy consumption, with studies indicating energy savings of up to 70% compared to conventional chemical synthesis routes. This energy efficiency directly translates to reduced carbon emissions, particularly when renewable energy sources power these bioelectrochemical systems.
The water-based reaction environment of enzyme electrosynthesis eliminates the need for harmful organic solvents that plague traditional chemical manufacturing. Conventional synthesis often relies on toxic solvents like dichloromethane, toluene, and acetonitrile, which pose significant environmental and health hazards. Enzyme electrosynthesis, particularly when utilizing electride mediators, achieves high reaction specificity in aqueous conditions, dramatically reducing waste generation and environmental contamination risks.
Resource conservation represents another critical sustainability advantage. Enzyme electrosynthesis pathways typically demonstrate atom economies exceeding 90%, significantly higher than conventional chemical routes. The selective nature of enzyme-catalyzed reactions minimizes side-product formation, reducing purification requirements and associated waste streams. Studies comparing pharmaceutical intermediate production via enzyme electrosynthesis versus traditional methods have demonstrated waste reduction exceeding 60%.
The biodegradability of enzyme catalysts presents additional environmental benefits. Unlike metal catalysts that persist in the environment and often contaminate water systems, enzymes naturally degrade into harmless amino acids. This characteristic eliminates concerns about heavy metal accumulation in ecosystems, a significant issue with many conventional catalytic processes.
Electride-mediated electron transfer systems further enhance these sustainability benefits by improving reaction efficiency. The unique electron donation properties of electrides enable direct electron transfer to enzymes without destructive overpotentials, extending catalyst lifetimes and reducing enzyme consumption rates. Recent research demonstrates that electride-mediated systems can achieve turnover numbers 3-5 times higher than conventional bioelectrochemical approaches, significantly improving resource utilization.
From a life cycle assessment perspective, enzyme electrosynthesis shows promising sustainability metrics. Comprehensive analyses of model reactions indicate potential reductions in environmental impact categories including global warming potential, acidification potential, and ecotoxicity by 40-75% compared to traditional chemical synthesis routes. These improvements become even more pronounced when electride mediators optimize electron transfer efficiency, reducing energy requirements and extending catalyst lifetimes.
The water-based reaction environment of enzyme electrosynthesis eliminates the need for harmful organic solvents that plague traditional chemical manufacturing. Conventional synthesis often relies on toxic solvents like dichloromethane, toluene, and acetonitrile, which pose significant environmental and health hazards. Enzyme electrosynthesis, particularly when utilizing electride mediators, achieves high reaction specificity in aqueous conditions, dramatically reducing waste generation and environmental contamination risks.
Resource conservation represents another critical sustainability advantage. Enzyme electrosynthesis pathways typically demonstrate atom economies exceeding 90%, significantly higher than conventional chemical routes. The selective nature of enzyme-catalyzed reactions minimizes side-product formation, reducing purification requirements and associated waste streams. Studies comparing pharmaceutical intermediate production via enzyme electrosynthesis versus traditional methods have demonstrated waste reduction exceeding 60%.
The biodegradability of enzyme catalysts presents additional environmental benefits. Unlike metal catalysts that persist in the environment and often contaminate water systems, enzymes naturally degrade into harmless amino acids. This characteristic eliminates concerns about heavy metal accumulation in ecosystems, a significant issue with many conventional catalytic processes.
Electride-mediated electron transfer systems further enhance these sustainability benefits by improving reaction efficiency. The unique electron donation properties of electrides enable direct electron transfer to enzymes without destructive overpotentials, extending catalyst lifetimes and reducing enzyme consumption rates. Recent research demonstrates that electride-mediated systems can achieve turnover numbers 3-5 times higher than conventional bioelectrochemical approaches, significantly improving resource utilization.
From a life cycle assessment perspective, enzyme electrosynthesis shows promising sustainability metrics. Comprehensive analyses of model reactions indicate potential reductions in environmental impact categories including global warming potential, acidification potential, and ecotoxicity by 40-75% compared to traditional chemical synthesis routes. These improvements become even more pronounced when electride mediators optimize electron transfer efficiency, reducing energy requirements and extending catalyst lifetimes.
Scalability and Industrial Implementation Considerations
Scaling electride-mediated enzyme electrosynthesis from laboratory to industrial scale presents significant challenges that must be addressed systematically. Current laboratory-scale implementations typically operate in small batch reactors with volumes ranging from milliliters to liters, whereas industrial applications would require operations at cubic meter scales. This substantial volume increase necessitates careful engineering considerations to maintain reaction efficiency and electron transfer rates.
The electrode surface area-to-volume ratio emerges as a critical parameter when scaling up these systems. Laboratory setups benefit from high surface area-to-volume ratios that facilitate efficient electron transfer. As reactor size increases, this ratio decreases substantially, potentially limiting reaction rates and yields. Advanced electrode designs incorporating 3D structures, such as carbon nanotube forests, graphene-based materials, or metal-organic frameworks, can help maintain adequate surface area in larger reactors.
Mass transfer limitations represent another significant hurdle in scaled implementations. In larger reactors, ensuring uniform distribution of electrides, substrates, and enzymes becomes increasingly difficult. Computational fluid dynamics modeling can optimize reactor design to enhance mixing while minimizing shear stress that might damage enzymatic structures. Novel reactor configurations such as flow-through electrodes or fluidized bed electrochemical reactors may offer solutions to these mass transfer challenges.
Energy efficiency considerations become paramount at industrial scale. The electrical power requirements for large-scale electrosynthesis can be substantial, necessitating careful optimization of applied potentials and current densities. Integration with renewable energy sources presents an opportunity to improve sustainability metrics while potentially reducing operational costs, though this introduces additional complexities related to managing intermittent power supplies.
Process stability and enzyme longevity represent key economic factors for industrial implementation. Immobilization strategies that enhance enzyme stability while maintaining accessibility to electrides must be refined for industrial conditions. Continuous operation models, rather than batch processing, may prove more economically viable but require robust enzyme systems capable of maintaining activity over extended periods.
Regulatory considerations and safety protocols for industrial-scale electride handling must be established, as some electride materials may present unique hazards when used in large quantities. Standardized safety procedures and containment strategies specific to electride-mediated processes will need development in parallel with the scaling technology.
Cost analysis indicates that while initial capital expenditure for specialized electrochemical equipment is high, the operational efficiency and reduced waste streams compared to traditional chemical synthesis may offer favorable long-term economics. Preliminary models suggest that break-even points could be achieved within 3-5 years for high-value pharmaceutical intermediates, though commodity chemicals may require further technological advances to achieve economic viability.
The electrode surface area-to-volume ratio emerges as a critical parameter when scaling up these systems. Laboratory setups benefit from high surface area-to-volume ratios that facilitate efficient electron transfer. As reactor size increases, this ratio decreases substantially, potentially limiting reaction rates and yields. Advanced electrode designs incorporating 3D structures, such as carbon nanotube forests, graphene-based materials, or metal-organic frameworks, can help maintain adequate surface area in larger reactors.
Mass transfer limitations represent another significant hurdle in scaled implementations. In larger reactors, ensuring uniform distribution of electrides, substrates, and enzymes becomes increasingly difficult. Computational fluid dynamics modeling can optimize reactor design to enhance mixing while minimizing shear stress that might damage enzymatic structures. Novel reactor configurations such as flow-through electrodes or fluidized bed electrochemical reactors may offer solutions to these mass transfer challenges.
Energy efficiency considerations become paramount at industrial scale. The electrical power requirements for large-scale electrosynthesis can be substantial, necessitating careful optimization of applied potentials and current densities. Integration with renewable energy sources presents an opportunity to improve sustainability metrics while potentially reducing operational costs, though this introduces additional complexities related to managing intermittent power supplies.
Process stability and enzyme longevity represent key economic factors for industrial implementation. Immobilization strategies that enhance enzyme stability while maintaining accessibility to electrides must be refined for industrial conditions. Continuous operation models, rather than batch processing, may prove more economically viable but require robust enzyme systems capable of maintaining activity over extended periods.
Regulatory considerations and safety protocols for industrial-scale electride handling must be established, as some electride materials may present unique hazards when used in large quantities. Standardized safety procedures and containment strategies specific to electride-mediated processes will need development in parallel with the scaling technology.
Cost analysis indicates that while initial capital expenditure for specialized electrochemical equipment is high, the operational efficiency and reduced waste streams compared to traditional chemical synthesis may offer favorable long-term economics. Preliminary models suggest that break-even points could be achieved within 3-5 years for high-value pharmaceutical intermediates, though commodity chemicals may require further technological advances to achieve economic viability.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!