Cathodic Protection and Its Impact on Hydrogen Uptake: What Engineers Need to Know
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cathodic Protection Evolution and Objectives
Cathodic protection has evolved significantly since its inception in the early 19th century. Initially developed to protect naval vessels from corrosion, this technology has become a cornerstone in preserving metal structures across various industries. The evolution of cathodic protection has been driven by the need to combat corrosion in increasingly complex environments and structures.
In the early stages, Sir Humphry Davy's experiments with zinc anodes on copper-clad ships marked the beginning of cathodic protection. This rudimentary approach laid the foundation for future advancements. As industrial applications expanded, so did the need for more sophisticated protection methods. The introduction of impressed current cathodic protection (ICCP) in the mid-20th century represented a significant leap forward, allowing for more precise control and broader application.
The objectives of cathodic protection have remained consistent throughout its evolution: to prevent or mitigate corrosion in metal structures exposed to electrolytes. However, the scope and complexity of these objectives have expanded. Modern cathodic protection systems aim not only to extend the lifespan of structures but also to ensure safety, reduce maintenance costs, and comply with increasingly stringent environmental regulations.
Recent technological advancements have focused on improving the efficiency and effectiveness of cathodic protection systems. The integration of remote monitoring and control systems has enabled real-time adjustments and predictive maintenance, significantly enhancing the reliability of protection measures. Additionally, the development of more durable and efficient anode materials has extended the lifespan of cathodic protection systems, reducing the frequency of replacements and interventions.
A critical objective in the current landscape of cathodic protection is understanding and mitigating its impact on hydrogen uptake in protected structures. This aspect has gained prominence due to the potential for hydrogen embrittlement, particularly in high-strength steels used in critical applications. Engineers are now tasked with balancing effective corrosion protection against the risk of hydrogen-induced damage, necessitating a more nuanced approach to cathodic protection design and implementation.
Looking forward, the evolution of cathodic protection is likely to be influenced by advancements in materials science, data analytics, and sustainable energy sources. The integration of artificial intelligence and machine learning algorithms promises to optimize protection strategies, predicting corrosion patterns and adjusting protection parameters dynamically. Furthermore, the development of environmentally friendly anode materials and the use of renewable energy sources for impressed current systems align with global sustainability goals.
In the early stages, Sir Humphry Davy's experiments with zinc anodes on copper-clad ships marked the beginning of cathodic protection. This rudimentary approach laid the foundation for future advancements. As industrial applications expanded, so did the need for more sophisticated protection methods. The introduction of impressed current cathodic protection (ICCP) in the mid-20th century represented a significant leap forward, allowing for more precise control and broader application.
The objectives of cathodic protection have remained consistent throughout its evolution: to prevent or mitigate corrosion in metal structures exposed to electrolytes. However, the scope and complexity of these objectives have expanded. Modern cathodic protection systems aim not only to extend the lifespan of structures but also to ensure safety, reduce maintenance costs, and comply with increasingly stringent environmental regulations.
Recent technological advancements have focused on improving the efficiency and effectiveness of cathodic protection systems. The integration of remote monitoring and control systems has enabled real-time adjustments and predictive maintenance, significantly enhancing the reliability of protection measures. Additionally, the development of more durable and efficient anode materials has extended the lifespan of cathodic protection systems, reducing the frequency of replacements and interventions.
A critical objective in the current landscape of cathodic protection is understanding and mitigating its impact on hydrogen uptake in protected structures. This aspect has gained prominence due to the potential for hydrogen embrittlement, particularly in high-strength steels used in critical applications. Engineers are now tasked with balancing effective corrosion protection against the risk of hydrogen-induced damage, necessitating a more nuanced approach to cathodic protection design and implementation.
Looking forward, the evolution of cathodic protection is likely to be influenced by advancements in materials science, data analytics, and sustainable energy sources. The integration of artificial intelligence and machine learning algorithms promises to optimize protection strategies, predicting corrosion patterns and adjusting protection parameters dynamically. Furthermore, the development of environmentally friendly anode materials and the use of renewable energy sources for impressed current systems align with global sustainability goals.
Market Demand Analysis for Cathodic Protection Systems
The market demand for cathodic protection systems has been steadily growing due to the increasing awareness of corrosion-related issues across various industries. The global cathodic protection market is expected to reach significant value in the coming years, driven by the rising need to protect critical infrastructure and assets from corrosion damage.
One of the primary factors fueling market demand is the aging infrastructure in developed countries. Many pipelines, bridges, and industrial facilities are reaching the end of their designed lifespan, necessitating enhanced corrosion protection measures. This has led to a surge in retrofitting projects and the implementation of cathodic protection systems to extend the life of existing structures.
The oil and gas industry remains a major consumer of cathodic protection systems, particularly for protecting offshore platforms, pipelines, and storage tanks. As exploration and production activities expand into more challenging environments, the demand for advanced cathodic protection solutions continues to rise. Additionally, the growing emphasis on environmental protection has led to stricter regulations regarding pipeline integrity and leak prevention, further driving the adoption of cathodic protection technologies.
The marine and shipping industry represents another significant market for cathodic protection systems. With the increasing global trade and the expansion of port infrastructure, there is a growing need to protect ships, offshore structures, and harbor facilities from corrosion caused by seawater. This has led to a rise in demand for both impressed current and sacrificial anode cathodic protection systems in marine applications.
In the construction sector, the use of cathodic protection systems for reinforced concrete structures has gained traction. As awareness of the long-term benefits of corrosion prevention in buildings and bridges grows, more project owners and engineers are incorporating cathodic protection into their designs. This trend is particularly evident in coastal areas and regions with harsh environmental conditions.
The water and wastewater treatment industry is another emerging market for cathodic protection systems. With the increasing focus on water conservation and the need to maintain aging water infrastructure, utilities are investing in corrosion protection measures for pipelines, storage tanks, and treatment facilities. This sector is expected to contribute significantly to the overall market growth in the coming years.
Geographically, North America and Europe currently dominate the cathodic protection market due to their extensive industrial infrastructure and stringent regulatory environment. However, rapid industrialization and infrastructure development in Asia-Pacific and the Middle East are creating new opportunities for market expansion. These regions are witnessing increased investments in oil and gas, power generation, and transportation sectors, driving the demand for cathodic protection systems.
One of the primary factors fueling market demand is the aging infrastructure in developed countries. Many pipelines, bridges, and industrial facilities are reaching the end of their designed lifespan, necessitating enhanced corrosion protection measures. This has led to a surge in retrofitting projects and the implementation of cathodic protection systems to extend the life of existing structures.
The oil and gas industry remains a major consumer of cathodic protection systems, particularly for protecting offshore platforms, pipelines, and storage tanks. As exploration and production activities expand into more challenging environments, the demand for advanced cathodic protection solutions continues to rise. Additionally, the growing emphasis on environmental protection has led to stricter regulations regarding pipeline integrity and leak prevention, further driving the adoption of cathodic protection technologies.
The marine and shipping industry represents another significant market for cathodic protection systems. With the increasing global trade and the expansion of port infrastructure, there is a growing need to protect ships, offshore structures, and harbor facilities from corrosion caused by seawater. This has led to a rise in demand for both impressed current and sacrificial anode cathodic protection systems in marine applications.
In the construction sector, the use of cathodic protection systems for reinforced concrete structures has gained traction. As awareness of the long-term benefits of corrosion prevention in buildings and bridges grows, more project owners and engineers are incorporating cathodic protection into their designs. This trend is particularly evident in coastal areas and regions with harsh environmental conditions.
The water and wastewater treatment industry is another emerging market for cathodic protection systems. With the increasing focus on water conservation and the need to maintain aging water infrastructure, utilities are investing in corrosion protection measures for pipelines, storage tanks, and treatment facilities. This sector is expected to contribute significantly to the overall market growth in the coming years.
Geographically, North America and Europe currently dominate the cathodic protection market due to their extensive industrial infrastructure and stringent regulatory environment. However, rapid industrialization and infrastructure development in Asia-Pacific and the Middle East are creating new opportunities for market expansion. These regions are witnessing increased investments in oil and gas, power generation, and transportation sectors, driving the demand for cathodic protection systems.
Current Challenges in Cathodic Protection Technology
Cathodic protection (CP) technology, while effective in mitigating corrosion, faces several significant challenges in its current implementation and impact on hydrogen uptake. One of the primary concerns is the potential for overprotection, which can lead to increased hydrogen generation and subsequent hydrogen embrittlement in protected structures. This phenomenon is particularly problematic in high-strength steels and other susceptible materials used in critical infrastructure.
Another challenge lies in the accurate measurement and control of protection potentials. The dynamic nature of corrosive environments, coupled with variations in soil resistivity and other environmental factors, makes it difficult to maintain optimal protection levels consistently. This can result in either inadequate protection or overprotection, both of which can have detrimental effects on the integrity of the protected structures.
The design and placement of anodes present another significant challenge. Achieving uniform current distribution across large or complex structures is often problematic, leading to areas of under-protection or overprotection. This is especially challenging in offshore environments or in structures with complex geometries, where current distribution can be highly non-uniform.
The interference between different CP systems or with other underground metallic structures is an ongoing issue. Stray currents from nearby CP systems or other sources can disrupt the intended protection and potentially accelerate corrosion in unintended areas. This interference can be particularly challenging in urban environments with multiple buried utilities.
The long-term effectiveness and sustainability of CP systems also pose challenges. The depletion of sacrificial anodes and the degradation of impressed current anodes over time necessitate regular monitoring and replacement, which can be costly and logistically challenging, especially for remote or underwater structures.
Furthermore, the energy requirements for impressed current cathodic protection (ICCP) systems can be substantial, raising concerns about the environmental impact and operational costs of these systems. This is particularly relevant in the context of increasing focus on sustainable and energy-efficient technologies.
The integration of CP systems with modern monitoring and control technologies presents both opportunities and challenges. While remote monitoring and automated control systems offer the potential for more efficient and responsive protection, they also introduce complexities in terms of data management, cybersecurity, and system reliability.
Lastly, the impact of CP on coating systems is an area of ongoing concern. While CP can complement protective coatings, excessive protection potentials can lead to coating disbondment, particularly in the case of certain types of organic coatings. This interaction between CP and coatings requires careful consideration in the design and operation of protection systems.
Another challenge lies in the accurate measurement and control of protection potentials. The dynamic nature of corrosive environments, coupled with variations in soil resistivity and other environmental factors, makes it difficult to maintain optimal protection levels consistently. This can result in either inadequate protection or overprotection, both of which can have detrimental effects on the integrity of the protected structures.
The design and placement of anodes present another significant challenge. Achieving uniform current distribution across large or complex structures is often problematic, leading to areas of under-protection or overprotection. This is especially challenging in offshore environments or in structures with complex geometries, where current distribution can be highly non-uniform.
The interference between different CP systems or with other underground metallic structures is an ongoing issue. Stray currents from nearby CP systems or other sources can disrupt the intended protection and potentially accelerate corrosion in unintended areas. This interference can be particularly challenging in urban environments with multiple buried utilities.
The long-term effectiveness and sustainability of CP systems also pose challenges. The depletion of sacrificial anodes and the degradation of impressed current anodes over time necessitate regular monitoring and replacement, which can be costly and logistically challenging, especially for remote or underwater structures.
Furthermore, the energy requirements for impressed current cathodic protection (ICCP) systems can be substantial, raising concerns about the environmental impact and operational costs of these systems. This is particularly relevant in the context of increasing focus on sustainable and energy-efficient technologies.
The integration of CP systems with modern monitoring and control technologies presents both opportunities and challenges. While remote monitoring and automated control systems offer the potential for more efficient and responsive protection, they also introduce complexities in terms of data management, cybersecurity, and system reliability.
Lastly, the impact of CP on coating systems is an area of ongoing concern. While CP can complement protective coatings, excessive protection potentials can lead to coating disbondment, particularly in the case of certain types of organic coatings. This interaction between CP and coatings requires careful consideration in the design and operation of protection systems.
Existing Solutions for Hydrogen Uptake Mitigation
01 Monitoring and control of cathodic protection systems
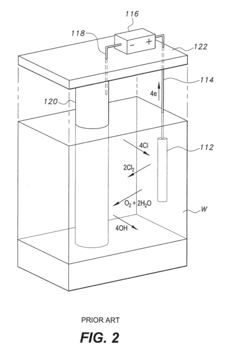
Advanced monitoring and control systems are used to optimize cathodic protection and minimize hydrogen uptake. These systems employ sensors, data analysis, and automated adjustments to maintain optimal protection levels while reducing the risk of hydrogen embrittlement.- Monitoring and control of cathodic protection systems: Advanced monitoring and control systems are used to optimize cathodic protection and minimize hydrogen uptake. These systems employ various sensors and data analysis techniques to adjust protection levels in real-time, ensuring effective corrosion prevention while reducing the risk of hydrogen embrittlement.
- Coating technologies for hydrogen uptake prevention: Specialized coatings are developed to create barriers that prevent hydrogen uptake during cathodic protection. These coatings are designed to be electrically conductive to allow for effective cathodic protection while simultaneously blocking hydrogen penetration into the protected metal structure.
- Optimization of cathodic protection parameters: Research focuses on optimizing cathodic protection parameters such as current density, potential, and duration to minimize hydrogen uptake. This involves studying the relationship between protection levels and hydrogen generation, and developing guidelines for balancing corrosion protection with hydrogen uptake prevention.
- Hydrogen detection and measurement techniques: Advanced methods for detecting and measuring hydrogen uptake in cathodically protected structures are developed. These techniques include electrochemical sensors, acoustic emission monitoring, and spectroscopic analysis, allowing for early detection of hydrogen-related issues and timely intervention.
- Alternative cathodic protection methods: Novel cathodic protection methods are explored to reduce hydrogen uptake while maintaining corrosion protection. These include pulsed current techniques, hybrid protection systems combining cathodic protection with other methods, and the use of specialized sacrificial anodes designed to minimize hydrogen evolution.
02 Electrochemical techniques for hydrogen uptake prevention
Various electrochemical techniques are employed to prevent or reduce hydrogen uptake during cathodic protection. These methods include the use of specialized coatings, controlled potential application, and the introduction of inhibitors to mitigate hydrogen absorption into the protected metal.Expand Specific Solutions03 Hydrogen detection and measurement in cathodic protection systems
Specialized sensors and measurement techniques are developed to detect and quantify hydrogen uptake in metals under cathodic protection. These methods allow for real-time monitoring of hydrogen levels, enabling prompt adjustments to protection parameters to prevent embrittlement.Expand Specific Solutions04 Material selection and surface treatments for hydrogen uptake resistance
The choice of materials and surface treatments plays a crucial role in reducing hydrogen uptake during cathodic protection. Researchers develop and test various alloys, coatings, and surface modification techniques to enhance resistance to hydrogen absorption while maintaining effective corrosion protection.Expand Specific Solutions05 Optimization of cathodic protection parameters to minimize hydrogen uptake
Research focuses on optimizing cathodic protection parameters such as current density, potential, and duration to achieve effective corrosion prevention while minimizing hydrogen uptake. This involves studying the relationship between protection levels and hydrogen generation, and developing guidelines for optimal operation.Expand Specific Solutions
Key Players in Cathodic Protection Industry
The cathodic protection market is in a growth phase, driven by increasing awareness of corrosion prevention in various industries. The global market size is projected to reach several billion dollars by 2025, with a compound annual growth rate of around 5-6%. Technologically, cathodic protection is well-established but continues to evolve, particularly in areas like remote monitoring and control systems. Key players like Marathon Petroleum, Saudi Aramco, and PetroChina are investing in advanced cathodic protection solutions to safeguard their extensive infrastructure. Smaller specialized firms such as Farwest Corrosion Control and CESCOR are also contributing to technological advancements, focusing on innovative materials and application techniques to enhance the efficiency and reliability of cathodic protection systems.
Saudi Arabian Oil Co.
Technical Solution: Saudi Aramco has developed advanced cathodic protection systems for its extensive oil and gas infrastructure. Their approach combines impressed current cathodic protection (ICCP) with sacrificial anode cathodic protection (SACP) to provide comprehensive corrosion control. The company utilizes remote monitoring and control systems to optimize cathodic protection performance across vast pipeline networks[1]. To address hydrogen uptake concerns, Saudi Aramco employs specialized coatings and careful potential control to minimize hydrogen evolution at protected surfaces. They have also implemented hydrogen monitoring programs to assess the impact of cathodic protection on steel embrittlement in critical components[2].
Strengths: Extensive experience in protecting large-scale oil and gas infrastructure; advanced monitoring and control systems. Weaknesses: Potential for overprotection in some areas, leading to increased hydrogen uptake risk.
PetroChina Co., Ltd.
Technical Solution: PetroChina has implemented a comprehensive cathodic protection strategy for its pipeline network, focusing on minimizing hydrogen uptake. The company utilizes a combination of high-efficiency sacrificial anodes and advanced impressed current systems. PetroChina's approach includes the use of specialized backfill materials to enhance anode performance and reduce current requirements. To address hydrogen uptake, they have developed a proprietary potential control algorithm that maintains protection levels while minimizing hydrogen evolution[3]. Additionally, PetroChina employs hydrogen permeation monitoring techniques to assess the effectiveness of their cathodic protection systems in reducing hydrogen uptake[4].
Strengths: Innovative potential control algorithms; extensive pipeline protection experience. Weaknesses: Potential challenges in applying the technology to diverse environmental conditions across China's varied geography.
Core Innovations in Cathodic Protection Methods
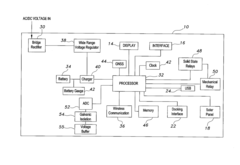
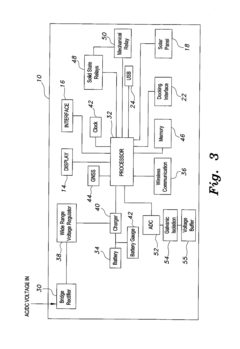
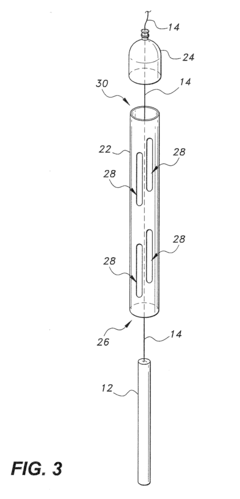
Portable cathodic protection current interrupter
PatentActiveUS20160268078A1
Innovation
- A programmable portable cathodic protection current interrupter with a processor, relay, display, user interface, onboard clock, and GNSS, featuring selective switching between main, testing, and external interruption channels, and powered by solar panels or rechargeable batteries, allowing for flexible and precise control of current interruption cycles.
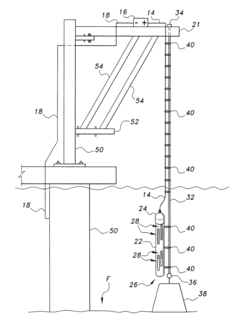
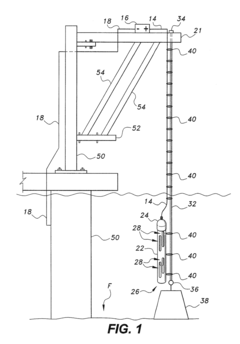
Anode assembly for cathodic protection of offshore steel piles
PatentActiveUS20180230604A1
Innovation
- An anode assembly with a protective housing and support cable system, where the housing has an open upper and lower end, secured by a cap and anchored with a weight on the ocean floor, and supported by a cable connected to an external support above the water, ensuring stable positioning and electrical connectivity.
Environmental Impact of Cathodic Protection Systems
Cathodic protection systems, while effective in preventing corrosion, can have significant environmental impacts that engineers must consider. The primary concern is the potential for increased hydrogen evolution at the cathode surface, which can lead to hydrogen embrittlement in certain materials and contribute to atmospheric hydrogen levels.
One of the most notable environmental impacts is the alteration of local water chemistry. The electrochemical reactions involved in cathodic protection can increase the pH of the surrounding water, potentially affecting aquatic ecosystems. This pH shift may disrupt the delicate balance of marine life, particularly in sensitive coastal areas or enclosed water bodies.
Furthermore, the use of sacrificial anodes in cathodic protection systems can introduce metal ions into the aquatic environment. Depending on the anode material used, this may result in the release of zinc, aluminum, or magnesium ions. While these metals are generally considered less harmful than the corrosion products they prevent, their accumulation over time can still impact water quality and marine organisms.
The energy consumption of impressed current cathodic protection (ICCP) systems is another environmental consideration. These systems require a continuous supply of electrical power, which, depending on the energy source, may contribute to carbon emissions and overall environmental footprint. Engineers should consider renewable energy options or energy-efficient designs to mitigate this impact.
Cathodic protection can also influence the microbial communities in the vicinity of protected structures. The altered electrochemical conditions may favor certain types of bacteria while inhibiting others, potentially leading to shifts in local biodiversity. This is particularly relevant in marine environments where microbial communities play crucial roles in nutrient cycling and ecosystem health.
The physical presence of cathodic protection equipment, such as anodes and cabling, can have direct impacts on marine habitats. Installation and maintenance activities may disturb seabed ecosystems, while the equipment itself can create artificial structures that alter local habitats. Engineers must carefully consider placement and design to minimize these physical disturbances.
Lastly, the disposal of spent anodes and other cathodic protection components at the end of their lifecycle presents environmental challenges. Proper recycling and waste management practices are essential to prevent these materials from contributing to environmental pollution. Engineers should factor in the entire lifecycle of cathodic protection systems, from installation to decommissioning, when assessing their environmental impact.
One of the most notable environmental impacts is the alteration of local water chemistry. The electrochemical reactions involved in cathodic protection can increase the pH of the surrounding water, potentially affecting aquatic ecosystems. This pH shift may disrupt the delicate balance of marine life, particularly in sensitive coastal areas or enclosed water bodies.
Furthermore, the use of sacrificial anodes in cathodic protection systems can introduce metal ions into the aquatic environment. Depending on the anode material used, this may result in the release of zinc, aluminum, or magnesium ions. While these metals are generally considered less harmful than the corrosion products they prevent, their accumulation over time can still impact water quality and marine organisms.
The energy consumption of impressed current cathodic protection (ICCP) systems is another environmental consideration. These systems require a continuous supply of electrical power, which, depending on the energy source, may contribute to carbon emissions and overall environmental footprint. Engineers should consider renewable energy options or energy-efficient designs to mitigate this impact.
Cathodic protection can also influence the microbial communities in the vicinity of protected structures. The altered electrochemical conditions may favor certain types of bacteria while inhibiting others, potentially leading to shifts in local biodiversity. This is particularly relevant in marine environments where microbial communities play crucial roles in nutrient cycling and ecosystem health.
The physical presence of cathodic protection equipment, such as anodes and cabling, can have direct impacts on marine habitats. Installation and maintenance activities may disturb seabed ecosystems, while the equipment itself can create artificial structures that alter local habitats. Engineers must carefully consider placement and design to minimize these physical disturbances.
Lastly, the disposal of spent anodes and other cathodic protection components at the end of their lifecycle presents environmental challenges. Proper recycling and waste management practices are essential to prevent these materials from contributing to environmental pollution. Engineers should factor in the entire lifecycle of cathodic protection systems, from installation to decommissioning, when assessing their environmental impact.
Regulatory Framework for Cathodic Protection Implementation
The regulatory framework for cathodic protection implementation is a critical aspect that engineers must understand to ensure compliance and effectiveness in corrosion prevention strategies. Various international and national standards govern the application of cathodic protection systems, with the primary aim of safeguarding infrastructure integrity while minimizing environmental impacts.
One of the key regulatory bodies in this field is the National Association of Corrosion Engineers (NACE), which has developed comprehensive standards for cathodic protection. NACE SP0169 and SP0285 are particularly relevant, providing guidelines for the control of external corrosion on underground or submerged metallic piping systems and underground storage tank systems, respectively.
In the United States, the Department of Transportation's Pipeline and Hazardous Materials Safety Administration (PHMSA) enforces regulations for cathodic protection of pipelines through 49 CFR Part 192 for natural gas pipelines and 49 CFR Part 195 for hazardous liquid pipelines. These regulations mandate the implementation and maintenance of cathodic protection systems to mitigate corrosion risks.
The European Union has established its own set of standards through the European Committee for Standardization (CEN). EN 12954 and EN 13636 are notable examples, covering cathodic protection of buried or immersed metallic structures and underground storage tanks, respectively. These standards align with international best practices while addressing specific European requirements.
For marine applications, the International Maritime Organization (IMO) provides guidelines for cathodic protection in ships and offshore structures. The IMO's SOLAS (Safety of Life at Sea) convention includes provisions related to corrosion prevention, indirectly influencing cathodic protection practices in maritime environments.
Engineers must also consider local regulations, which may impose additional requirements or modifications to international standards. For instance, in areas with high soil resistivity or extreme environmental conditions, local authorities may mandate more stringent cathodic protection criteria.
The regulatory landscape also addresses the potential environmental impacts of cathodic protection systems. Concerns about the release of metal ions into the environment and the generation of hydrogen gas have led to the development of guidelines for monitoring and controlling these side effects. The U.S. Environmental Protection Agency (EPA) provides guidance on managing the environmental aspects of cathodic protection, particularly in relation to underground storage tanks and groundwater protection.
As the understanding of hydrogen uptake and its potential effects on material properties evolves, regulatory bodies are beginning to incorporate these considerations into their frameworks. This includes guidelines for monitoring hydrogen evolution rates and assessing the risk of hydrogen embrittlement in protected structures.
One of the key regulatory bodies in this field is the National Association of Corrosion Engineers (NACE), which has developed comprehensive standards for cathodic protection. NACE SP0169 and SP0285 are particularly relevant, providing guidelines for the control of external corrosion on underground or submerged metallic piping systems and underground storage tank systems, respectively.
In the United States, the Department of Transportation's Pipeline and Hazardous Materials Safety Administration (PHMSA) enforces regulations for cathodic protection of pipelines through 49 CFR Part 192 for natural gas pipelines and 49 CFR Part 195 for hazardous liquid pipelines. These regulations mandate the implementation and maintenance of cathodic protection systems to mitigate corrosion risks.
The European Union has established its own set of standards through the European Committee for Standardization (CEN). EN 12954 and EN 13636 are notable examples, covering cathodic protection of buried or immersed metallic structures and underground storage tanks, respectively. These standards align with international best practices while addressing specific European requirements.
For marine applications, the International Maritime Organization (IMO) provides guidelines for cathodic protection in ships and offshore structures. The IMO's SOLAS (Safety of Life at Sea) convention includes provisions related to corrosion prevention, indirectly influencing cathodic protection practices in maritime environments.
Engineers must also consider local regulations, which may impose additional requirements or modifications to international standards. For instance, in areas with high soil resistivity or extreme environmental conditions, local authorities may mandate more stringent cathodic protection criteria.
The regulatory landscape also addresses the potential environmental impacts of cathodic protection systems. Concerns about the release of metal ions into the environment and the generation of hydrogen gas have led to the development of guidelines for monitoring and controlling these side effects. The U.S. Environmental Protection Agency (EPA) provides guidance on managing the environmental aspects of cathodic protection, particularly in relation to underground storage tanks and groundwater protection.
As the understanding of hydrogen uptake and its potential effects on material properties evolves, regulatory bodies are beginning to incorporate these considerations into their frameworks. This includes guidelines for monitoring hydrogen evolution rates and assessing the risk of hydrogen embrittlement in protected structures.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!