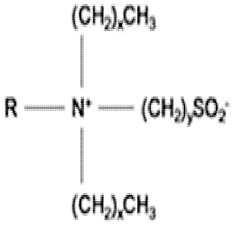
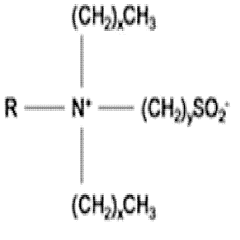
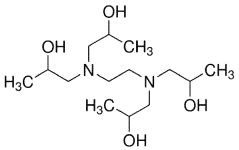
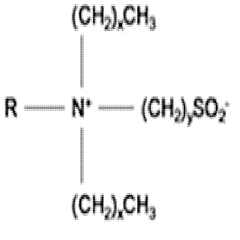
Characterization of Supramolecular Assemblies of Sulphanilic Acid
JUL 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Supramolecular Assembly Background and Objectives
Supramolecular assemblies have emerged as a fascinating field of study in materials science and chemistry over the past few decades. These assemblies are formed through non-covalent interactions between molecular components, resulting in complex structures with unique properties. Sulphanilic acid, an aromatic compound with both amino and sulfonic acid groups, has shown promising potential in the formation of supramolecular assemblies due to its ability to engage in various intermolecular interactions.
The study of supramolecular assemblies of sulphanilic acid aims to explore the fundamental principles governing their formation, structure, and properties. This research is driven by the growing demand for advanced materials with tailored functionalities in diverse fields such as drug delivery, sensing, and catalysis. By understanding the self-assembly mechanisms of sulphanilic acid, researchers can potentially develop novel materials with enhanced performance and controllable characteristics.
The evolution of supramolecular chemistry has paved the way for the investigation of sulphanilic acid assemblies. Early studies focused on simple hydrogen-bonded systems, gradually progressing to more complex architectures involving multiple non-covalent interactions. The advent of advanced characterization techniques, such as high-resolution microscopy and spectroscopy, has significantly contributed to our ability to analyze these assemblies at the molecular level.
The primary objective of characterizing supramolecular assemblies of sulphanilic acid is to elucidate their structural features, formation kinetics, and physicochemical properties. This includes determining the precise arrangement of molecules within the assemblies, identifying the dominant intermolecular forces, and understanding how external factors such as pH, temperature, and solvent composition influence the assembly process.
Furthermore, researchers aim to establish structure-property relationships that can guide the design of functional materials based on sulphanilic acid assemblies. This involves investigating how variations in molecular structure and assembly conditions affect the resulting properties, such as optical behavior, mechanical strength, and responsiveness to external stimuli.
Another crucial goal is to explore the potential applications of sulphanilic acid supramolecular assemblies. Given the compound's unique chemical structure, these assemblies may exhibit interesting properties for use in areas like environmental remediation, biomedical applications, and optoelectronic devices. By thoroughly characterizing these systems, researchers can identify promising avenues for practical implementation and further development.
In conclusion, the characterization of supramolecular assemblies of sulphanilic acid represents a significant area of research with far-reaching implications. By delving into the fundamental aspects of these assemblies and setting clear objectives for their study, scientists aim to unlock new possibilities in materials design and expand our understanding of complex molecular systems.
The study of supramolecular assemblies of sulphanilic acid aims to explore the fundamental principles governing their formation, structure, and properties. This research is driven by the growing demand for advanced materials with tailored functionalities in diverse fields such as drug delivery, sensing, and catalysis. By understanding the self-assembly mechanisms of sulphanilic acid, researchers can potentially develop novel materials with enhanced performance and controllable characteristics.
The evolution of supramolecular chemistry has paved the way for the investigation of sulphanilic acid assemblies. Early studies focused on simple hydrogen-bonded systems, gradually progressing to more complex architectures involving multiple non-covalent interactions. The advent of advanced characterization techniques, such as high-resolution microscopy and spectroscopy, has significantly contributed to our ability to analyze these assemblies at the molecular level.
The primary objective of characterizing supramolecular assemblies of sulphanilic acid is to elucidate their structural features, formation kinetics, and physicochemical properties. This includes determining the precise arrangement of molecules within the assemblies, identifying the dominant intermolecular forces, and understanding how external factors such as pH, temperature, and solvent composition influence the assembly process.
Furthermore, researchers aim to establish structure-property relationships that can guide the design of functional materials based on sulphanilic acid assemblies. This involves investigating how variations in molecular structure and assembly conditions affect the resulting properties, such as optical behavior, mechanical strength, and responsiveness to external stimuli.
Another crucial goal is to explore the potential applications of sulphanilic acid supramolecular assemblies. Given the compound's unique chemical structure, these assemblies may exhibit interesting properties for use in areas like environmental remediation, biomedical applications, and optoelectronic devices. By thoroughly characterizing these systems, researchers can identify promising avenues for practical implementation and further development.
In conclusion, the characterization of supramolecular assemblies of sulphanilic acid represents a significant area of research with far-reaching implications. By delving into the fundamental aspects of these assemblies and setting clear objectives for their study, scientists aim to unlock new possibilities in materials design and expand our understanding of complex molecular systems.
Market Applications of Sulphanilic Acid Assemblies
Sulphanilic acid assemblies have shown promising potential in various market applications, leveraging their unique supramolecular properties. One of the primary areas of interest is in the field of advanced materials, where these assemblies can be utilized to create novel composites with enhanced mechanical and chemical properties. The self-assembling nature of sulphanilic acid molecules allows for the development of materials with precise nanostructures, opening up possibilities in areas such as lightweight construction materials and high-performance coatings.
In the pharmaceutical industry, sulphanilic acid assemblies have garnered attention for their potential in drug delivery systems. The ability to form controlled structures at the molecular level enables the design of carriers that can encapsulate and release therapeutic agents in a targeted manner. This could lead to more effective treatments with reduced side effects, particularly in the realm of cancer therapies and chronic disease management.
The electronics sector is another area where sulphanilic acid assemblies show promise. Their unique electrical properties make them candidates for use in organic semiconductors and flexible electronics. As the demand for smaller, more efficient electronic devices continues to grow, these assemblies could play a crucial role in developing next-generation components and circuits.
Environmental applications represent a significant market opportunity for sulphanilic acid assemblies. Their ability to form complex structures with specific binding sites makes them excellent candidates for water purification and environmental remediation technologies. These assemblies can be designed to selectively capture pollutants and heavy metals, offering a more efficient and sustainable approach to water treatment.
In the textile industry, sulphanilic acid assemblies are being explored for their potential in creating smart fabrics and advanced protective clothing. By incorporating these assemblies into textile fibers, it may be possible to develop materials with enhanced properties such as self-cleaning, antimicrobial, or even color-changing capabilities.
The cosmetics and personal care industry is also showing interest in sulphanilic acid assemblies. Their ability to form stable, organized structures at the nanoscale makes them attractive for use in advanced skincare formulations and controlled-release systems for active ingredients. This could lead to more effective and longer-lasting beauty products.
Lastly, the food and beverage industry is exploring the use of sulphanilic acid assemblies in packaging and preservation technologies. The potential to create biodegradable, antimicrobial packaging materials could address growing concerns about plastic waste while extending the shelf life of perishable goods.
In the pharmaceutical industry, sulphanilic acid assemblies have garnered attention for their potential in drug delivery systems. The ability to form controlled structures at the molecular level enables the design of carriers that can encapsulate and release therapeutic agents in a targeted manner. This could lead to more effective treatments with reduced side effects, particularly in the realm of cancer therapies and chronic disease management.
The electronics sector is another area where sulphanilic acid assemblies show promise. Their unique electrical properties make them candidates for use in organic semiconductors and flexible electronics. As the demand for smaller, more efficient electronic devices continues to grow, these assemblies could play a crucial role in developing next-generation components and circuits.
Environmental applications represent a significant market opportunity for sulphanilic acid assemblies. Their ability to form complex structures with specific binding sites makes them excellent candidates for water purification and environmental remediation technologies. These assemblies can be designed to selectively capture pollutants and heavy metals, offering a more efficient and sustainable approach to water treatment.
In the textile industry, sulphanilic acid assemblies are being explored for their potential in creating smart fabrics and advanced protective clothing. By incorporating these assemblies into textile fibers, it may be possible to develop materials with enhanced properties such as self-cleaning, antimicrobial, or even color-changing capabilities.
The cosmetics and personal care industry is also showing interest in sulphanilic acid assemblies. Their ability to form stable, organized structures at the nanoscale makes them attractive for use in advanced skincare formulations and controlled-release systems for active ingredients. This could lead to more effective and longer-lasting beauty products.
Lastly, the food and beverage industry is exploring the use of sulphanilic acid assemblies in packaging and preservation technologies. The potential to create biodegradable, antimicrobial packaging materials could address growing concerns about plastic waste while extending the shelf life of perishable goods.
Current Challenges in Supramolecular Characterization
The characterization of supramolecular assemblies of sulphanilic acid presents several significant challenges in the field of supramolecular chemistry. One of the primary difficulties lies in the dynamic nature of these assemblies, which can undergo rapid structural changes in response to environmental factors such as temperature, pH, and solvent composition. This inherent instability makes it challenging to capture and analyze the precise structural arrangements of these assemblies using traditional characterization techniques.
Another major hurdle is the complexity of the interactions involved in supramolecular assemblies. Sulphanilic acid can form various types of non-covalent interactions, including hydrogen bonding, π-π stacking, and electrostatic interactions. The interplay of these forces creates a multifaceted system that is difficult to deconvolute and quantify accurately. Researchers often struggle to determine the relative contributions of each interaction type to the overall assembly structure and stability.
The size and polydispersity of supramolecular assemblies of sulphanilic acid also pose significant characterization challenges. These assemblies can range from small oligomers to large, complex structures, and their size distribution can be highly heterogeneous. This variability makes it challenging to obtain consistent and representative data using techniques such as light scattering or microscopy, as the results may not accurately reflect the entire population of assemblies present in the sample.
Furthermore, the characterization of these assemblies in their native environment presents additional complications. Many traditional analytical techniques require sample preparation steps that can disrupt or alter the delicate supramolecular structures. For instance, drying or crystallization processes used in X-ray diffraction studies may not preserve the solution-phase assembly structure, leading to potential misinterpretation of the data.
The time-dependent nature of supramolecular assembly formation and disassembly adds another layer of complexity to the characterization process. Capturing the kinetics of assembly formation, as well as any intermediate states, requires sophisticated time-resolved techniques that are not always readily available or easily implemented. This temporal aspect of supramolecular chemistry often necessitates the development of new, specialized characterization methods.
Lastly, the integration and correlation of data from multiple characterization techniques remain a significant challenge. While individual methods may provide valuable insights into specific aspects of the supramolecular assemblies, combining these diverse data sets to construct a comprehensive understanding of the system's structure, dynamics, and properties is a complex task that requires advanced data analysis and modeling approaches.
Another major hurdle is the complexity of the interactions involved in supramolecular assemblies. Sulphanilic acid can form various types of non-covalent interactions, including hydrogen bonding, π-π stacking, and electrostatic interactions. The interplay of these forces creates a multifaceted system that is difficult to deconvolute and quantify accurately. Researchers often struggle to determine the relative contributions of each interaction type to the overall assembly structure and stability.
The size and polydispersity of supramolecular assemblies of sulphanilic acid also pose significant characterization challenges. These assemblies can range from small oligomers to large, complex structures, and their size distribution can be highly heterogeneous. This variability makes it challenging to obtain consistent and representative data using techniques such as light scattering or microscopy, as the results may not accurately reflect the entire population of assemblies present in the sample.
Furthermore, the characterization of these assemblies in their native environment presents additional complications. Many traditional analytical techniques require sample preparation steps that can disrupt or alter the delicate supramolecular structures. For instance, drying or crystallization processes used in X-ray diffraction studies may not preserve the solution-phase assembly structure, leading to potential misinterpretation of the data.
The time-dependent nature of supramolecular assembly formation and disassembly adds another layer of complexity to the characterization process. Capturing the kinetics of assembly formation, as well as any intermediate states, requires sophisticated time-resolved techniques that are not always readily available or easily implemented. This temporal aspect of supramolecular chemistry often necessitates the development of new, specialized characterization methods.
Lastly, the integration and correlation of data from multiple characterization techniques remain a significant challenge. While individual methods may provide valuable insights into specific aspects of the supramolecular assemblies, combining these diverse data sets to construct a comprehensive understanding of the system's structure, dynamics, and properties is a complex task that requires advanced data analysis and modeling approaches.
Existing Characterization Methods for Sulphanilic Acid
01 Synthesis and characterization of sulphanilic acid-based supramolecular assemblies
Research focuses on the synthesis and characterization of supramolecular assemblies using sulphanilic acid as a building block. These assemblies are formed through various non-covalent interactions, such as hydrogen bonding and π-π stacking. The resulting structures are analyzed using spectroscopic and microscopic techniques to understand their properties and potential applications.- Synthesis and characterization of sulphanilic acid-based supramolecular assemblies: This point focuses on the methods for synthesizing and characterizing supramolecular assemblies using sulphanilic acid as a building block. These assemblies can be formed through various non-covalent interactions, such as hydrogen bonding, π-π stacking, and electrostatic interactions. The resulting structures are analyzed using techniques like spectroscopy, microscopy, and X-ray diffraction to understand their properties and behavior.
- Applications of sulphanilic acid supramolecular assemblies in drug delivery: Supramolecular assemblies of sulphanilic acid can be utilized as drug delivery systems. These assemblies can encapsulate or bind to various therapeutic agents, providing controlled release, improved solubility, and enhanced bioavailability. The unique properties of these assemblies, such as stimuli-responsiveness and targetability, make them promising candidates for advanced drug delivery applications.
- Sulphanilic acid-based supramolecular assemblies for environmental applications: This point explores the use of sulphanilic acid supramolecular assemblies in environmental applications, such as water treatment and pollutant removal. These assemblies can form complexes with heavy metals or organic pollutants, facilitating their removal from contaminated water sources. Additionally, they can be used as sensors for detecting environmental contaminants.
- Functional materials derived from sulphanilic acid supramolecular assemblies: Sulphanilic acid-based supramolecular assemblies can be used to create functional materials with unique properties. These materials can exhibit conductivity, optical properties, or responsive behavior, making them suitable for applications in electronics, sensors, and smart materials. The self-assembly process allows for the creation of well-defined nanostructures with tailored functionalities.
- Modification and functionalization of sulphanilic acid for enhanced supramolecular assembly: This point focuses on strategies to modify and functionalize sulphanilic acid to improve its supramolecular assembly properties. Chemical modifications can be made to introduce new functional groups, alter the electronic properties, or enhance the binding affinity of sulphanilic acid. These modifications can lead to the formation of more stable, diverse, or responsive supramolecular structures.
02 Applications of sulphanilic acid supramolecular assemblies in drug delivery
Sulphanilic acid-based supramolecular assemblies are explored for their potential in drug delivery systems. These assemblies can encapsulate various therapeutic agents and provide controlled release mechanisms. The biocompatibility and biodegradability of these systems are investigated to ensure their safety and efficacy in pharmaceutical applications.Expand Specific Solutions03 Sulphanilic acid supramolecular assemblies in environmental remediation
The use of sulphanilic acid-based supramolecular assemblies in environmental remediation processes is investigated. These assemblies show promise in the removal of pollutants from water and soil through adsorption and catalytic degradation. The recyclability and efficiency of these materials in various environmental applications are studied.Expand Specific Solutions04 Functionalization of sulphanilic acid supramolecular assemblies
Research explores the functionalization of sulphanilic acid-based supramolecular assemblies to enhance their properties and expand their applications. Various chemical modifications and incorporation of additional functional groups are investigated to tailor the assemblies for specific uses in fields such as sensing, catalysis, and materials science.Expand Specific Solutions05 Computational studies of sulphanilic acid supramolecular assemblies
Computational methods are employed to study the formation, structure, and properties of sulphanilic acid-based supramolecular assemblies. Molecular dynamics simulations and density functional theory calculations are used to predict and understand the behavior of these assemblies under various conditions, guiding experimental design and interpretation of results.Expand Specific Solutions
Key Players in Supramolecular Chemistry Research
The characterization of supramolecular assemblies of sulphanilic acid is an emerging field in materials science and nanotechnology. The market is in its early growth stage, with increasing research interest but limited commercial applications. The global market size for supramolecular assemblies is projected to grow steadily as potential applications in drug delivery, sensing, and materials engineering develop. Technologically, the field is still maturing, with academic institutions like Northwestern University, Johns Hopkins University, and King Abdullah University of Science & Technology leading research efforts. Companies such as Saudi Arabian Oil Co. and Aramco Services Co. are also investing in this area, indicating growing industrial interest in potential applications.
Northwestern University
Technical Solution: Northwestern University has developed advanced characterization techniques for supramolecular assemblies of sulphanilic acid. They utilize a combination of spectroscopic methods, including UV-Vis, fluorescence, and circular dichroism, to probe the self-assembly process and structural features of these systems[1]. Their approach also incorporates advanced microscopy techniques such as atomic force microscopy (AFM) and transmission electron microscopy (TEM) to visualize the morphology and hierarchical organization of the assemblies at different scales[2]. Additionally, they employ small-angle X-ray scattering (SAXS) to investigate the internal structure and packing of the supramolecular aggregates[3]. This multi-technique approach allows for a comprehensive understanding of the assembly mechanisms and properties of sulphanilic acid-based supramolecular structures.
Strengths: Comprehensive characterization approach, combining spectroscopic, microscopic, and scattering techniques. Weaknesses: May require access to specialized equipment and expertise, potentially limiting widespread application.
The Johns Hopkins University
Technical Solution: The Johns Hopkins University has developed a novel approach to characterize supramolecular assemblies of sulphanilic acid using advanced nuclear magnetic resonance (NMR) spectroscopy techniques. Their method employs a combination of 1D and 2D NMR experiments, including DOSY (Diffusion Ordered Spectroscopy) and NOESY (Nuclear Overhauser Effect Spectroscopy), to probe the molecular interactions and spatial arrangements within the assemblies[1]. They have also integrated computational modeling with experimental data to predict and validate the structure of these supramolecular systems[2]. Furthermore, the university has pioneered the use of solid-state NMR techniques to study the packing and dynamics of sulphanilic acid assemblies in the solid state, providing insights into their behavior in different physical forms[3]. This comprehensive NMR-based approach allows for detailed structural characterization at the molecular level, offering unique insights into the assembly process and stability of these supramolecular systems.
Strengths: High-resolution structural information at the molecular level, ability to study dynamics and interactions in solution and solid state. Weaknesses: Requires expensive NMR equipment and specialized expertise, may be limited in characterizing very large assemblies.
Advanced Analytical Techniques for Supramolecular Structures
Composition for single-cell proteomics analysis, sample treatment method using same, and method for unifying labeling of ultra-small amounts of peptides
PatentWO2024136320A1
Innovation
- A composition containing sulfobetaine-based amphoteric surfactant and N,N,N',N-tetrakis(2-hydroxypropyl)ethylenediamine is used for sample pretreatment, enabling unified processes of cell lysis, peptide extraction, and peptide labeling, which are performed in a centrifuge tube with micro-penetrating holes, allowing for quantitative protein analysis without sample loss.
Environmental Impact of Sulphanilic Acid Research
The environmental impact of sulphanilic acid research is a critical aspect to consider in the characterization of supramolecular assemblies. As the use of sulphanilic acid in various industrial processes and research applications continues to grow, it is essential to evaluate its potential effects on ecosystems and human health.
Sulphanilic acid, being a synthetic compound, can persist in the environment for extended periods. Its release into water bodies through industrial effluents or improper disposal of laboratory waste can lead to contamination of aquatic ecosystems. Studies have shown that sulphanilic acid can affect the growth and reproduction of aquatic organisms, potentially disrupting food chains and biodiversity in affected areas.
Furthermore, the production and use of sulphanilic acid in research settings may contribute to air pollution. Volatile organic compounds (VOCs) released during synthesis or handling processes can react with other atmospheric pollutants, potentially forming ground-level ozone and other harmful secondary pollutants. This underscores the importance of proper ventilation and emission control measures in laboratories and production facilities.
Soil contamination is another concern associated with sulphanilic acid research. Accidental spills or improper disposal of solid waste containing this compound can lead to soil pollution. This may affect soil microorganisms, plant growth, and potentially enter the food chain through uptake by crops or grazing animals.
The environmental fate of sulphanilic acid is influenced by various factors, including pH, temperature, and the presence of other chemicals. Understanding these interactions is crucial for predicting its behavior in different environmental compartments and developing effective remediation strategies.
Research into the biodegradation of sulphanilic acid has shown promising results, with certain microorganisms capable of breaking down the compound under specific conditions. This opens up possibilities for bioremediation techniques to mitigate environmental contamination. However, further studies are needed to optimize these processes and assess their feasibility on a larger scale.
As research on supramolecular assemblies of sulphanilic acid progresses, it is imperative to consider the potential environmental implications of scaled-up production and application. Life cycle assessments and environmental impact studies should be integrated into the research process to identify and address potential risks proactively.
Developing green chemistry approaches for the synthesis and use of sulphanilic acid in supramolecular assemblies could significantly reduce its environmental footprint. This may include exploring alternative solvents, optimizing reaction conditions to minimize waste, and designing more efficient purification methods.
Sulphanilic acid, being a synthetic compound, can persist in the environment for extended periods. Its release into water bodies through industrial effluents or improper disposal of laboratory waste can lead to contamination of aquatic ecosystems. Studies have shown that sulphanilic acid can affect the growth and reproduction of aquatic organisms, potentially disrupting food chains and biodiversity in affected areas.
Furthermore, the production and use of sulphanilic acid in research settings may contribute to air pollution. Volatile organic compounds (VOCs) released during synthesis or handling processes can react with other atmospheric pollutants, potentially forming ground-level ozone and other harmful secondary pollutants. This underscores the importance of proper ventilation and emission control measures in laboratories and production facilities.
Soil contamination is another concern associated with sulphanilic acid research. Accidental spills or improper disposal of solid waste containing this compound can lead to soil pollution. This may affect soil microorganisms, plant growth, and potentially enter the food chain through uptake by crops or grazing animals.
The environmental fate of sulphanilic acid is influenced by various factors, including pH, temperature, and the presence of other chemicals. Understanding these interactions is crucial for predicting its behavior in different environmental compartments and developing effective remediation strategies.
Research into the biodegradation of sulphanilic acid has shown promising results, with certain microorganisms capable of breaking down the compound under specific conditions. This opens up possibilities for bioremediation techniques to mitigate environmental contamination. However, further studies are needed to optimize these processes and assess their feasibility on a larger scale.
As research on supramolecular assemblies of sulphanilic acid progresses, it is imperative to consider the potential environmental implications of scaled-up production and application. Life cycle assessments and environmental impact studies should be integrated into the research process to identify and address potential risks proactively.
Developing green chemistry approaches for the synthesis and use of sulphanilic acid in supramolecular assemblies could significantly reduce its environmental footprint. This may include exploring alternative solvents, optimizing reaction conditions to minimize waste, and designing more efficient purification methods.
Computational Modeling in Supramolecular Design
Computational modeling has become an indispensable tool in the design and characterization of supramolecular assemblies, including those involving sulphanilic acid. This approach offers valuable insights into the structure, dynamics, and properties of these complex systems, complementing experimental techniques and guiding rational design strategies.
Molecular dynamics (MD) simulations play a crucial role in understanding the self-assembly process of sulphanilic acid molecules. These simulations can reveal the formation of various supramolecular structures, such as micelles, vesicles, or more complex hierarchical assemblies. By employing appropriate force fields and simulation parameters, researchers can investigate the influence of factors like concentration, pH, and temperature on the assembly process.
Quantum mechanical calculations, particularly density functional theory (DFT), provide detailed information about the electronic structure and interactions within sulphanilic acid assemblies. These methods are essential for elucidating the nature of non-covalent interactions, including hydrogen bonding, π-π stacking, and electrostatic forces, which govern the formation and stability of supramolecular structures.
Coarse-grained modeling techniques offer a valuable approach for studying larger-scale assemblies and longer time scales than those accessible through atomistic simulations. By simplifying the representation of sulphanilic acid molecules and their interactions, these models can capture the essential features of self-assembly while reducing computational cost.
Machine learning algorithms are increasingly being applied to predict and optimize the properties of supramolecular assemblies. These techniques can be used to analyze large datasets of experimental and computational results, identifying patterns and relationships that may not be apparent through traditional analysis methods. This approach can accelerate the discovery of novel supramolecular structures with desired properties.
Multiscale modeling approaches, combining atomistic, coarse-grained, and continuum methods, provide a comprehensive understanding of sulphanilic acid assemblies across different length and time scales. These integrated models can bridge the gap between molecular-level interactions and macroscopic properties, offering valuable insights for the rational design of functional materials.
Molecular dynamics (MD) simulations play a crucial role in understanding the self-assembly process of sulphanilic acid molecules. These simulations can reveal the formation of various supramolecular structures, such as micelles, vesicles, or more complex hierarchical assemblies. By employing appropriate force fields and simulation parameters, researchers can investigate the influence of factors like concentration, pH, and temperature on the assembly process.
Quantum mechanical calculations, particularly density functional theory (DFT), provide detailed information about the electronic structure and interactions within sulphanilic acid assemblies. These methods are essential for elucidating the nature of non-covalent interactions, including hydrogen bonding, π-π stacking, and electrostatic forces, which govern the formation and stability of supramolecular structures.
Coarse-grained modeling techniques offer a valuable approach for studying larger-scale assemblies and longer time scales than those accessible through atomistic simulations. By simplifying the representation of sulphanilic acid molecules and their interactions, these models can capture the essential features of self-assembly while reducing computational cost.
Machine learning algorithms are increasingly being applied to predict and optimize the properties of supramolecular assemblies. These techniques can be used to analyze large datasets of experimental and computational results, identifying patterns and relationships that may not be apparent through traditional analysis methods. This approach can accelerate the discovery of novel supramolecular structures with desired properties.
Multiscale modeling approaches, combining atomistic, coarse-grained, and continuum methods, provide a comprehensive understanding of sulphanilic acid assemblies across different length and time scales. These integrated models can bridge the gap between molecular-level interactions and macroscopic properties, offering valuable insights for the rational design of functional materials.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!