Comparative Analysis of Perchloric Acid and Hydrochloric Acid on Alloy Corrosion
AUG 4, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Corrosion Research Background and Objectives
Corrosion research has been a critical field of study for decades, driven by the significant economic and safety implications of material degradation in various industries. The comparative analysis of perchloric acid and hydrochloric acid on alloy corrosion represents a crucial area of investigation within this broader context. This research aims to elucidate the differential effects of these two strong acids on various alloys, providing valuable insights for material selection and corrosion prevention strategies.
The historical development of corrosion science has seen a progression from empirical observations to sophisticated electrochemical and materials science approaches. Early studies focused primarily on visual inspection and weight loss measurements, while modern techniques incorporate advanced spectroscopic and microscopic methods. This evolution has enabled researchers to gain a deeper understanding of corrosion mechanisms at the atomic and molecular levels.
In recent years, the focus has shifted towards more environmentally friendly and cost-effective corrosion prevention methods. This trend aligns with global sustainability goals and the need for more efficient resource utilization. The comparative study of perchloric and hydrochloric acids contributes to this objective by potentially identifying less corrosive alternatives or more resistant materials for specific applications.
The primary objective of this research is to systematically compare the corrosive effects of perchloric acid and hydrochloric acid on various alloys commonly used in industrial settings. This includes analyzing the rate of corrosion, the nature of corrosion products formed, and the morphological changes in the alloy surfaces. By understanding these differences, researchers aim to develop more targeted corrosion prevention strategies and improve material selection processes for acid-intensive applications.
Additionally, this study seeks to explore the underlying mechanisms that contribute to the differential corrosive behavior of these acids. Factors such as pH, oxidizing power, and the specific interactions between acid anions and alloy components will be investigated. This mechanistic understanding is crucial for predicting corrosion behavior in complex environments and for developing new corrosion-resistant alloys.
The research also aims to address the practical implications of using perchloric acid versus hydrochloric acid in industrial processes. This includes considerations of safety, handling procedures, and potential environmental impacts. By providing a comprehensive comparison, the study will inform decision-making processes in industries where these acids are commonly used, such as metal processing, chemical manufacturing, and electronics.
Furthermore, this comparative analysis will contribute to the broader field of corrosion science by potentially revealing new insights into acid-induced corrosion processes. These findings may have implications beyond the specific acids studied, leading to a more nuanced understanding of corrosion phenomena in general.
The historical development of corrosion science has seen a progression from empirical observations to sophisticated electrochemical and materials science approaches. Early studies focused primarily on visual inspection and weight loss measurements, while modern techniques incorporate advanced spectroscopic and microscopic methods. This evolution has enabled researchers to gain a deeper understanding of corrosion mechanisms at the atomic and molecular levels.
In recent years, the focus has shifted towards more environmentally friendly and cost-effective corrosion prevention methods. This trend aligns with global sustainability goals and the need for more efficient resource utilization. The comparative study of perchloric and hydrochloric acids contributes to this objective by potentially identifying less corrosive alternatives or more resistant materials for specific applications.
The primary objective of this research is to systematically compare the corrosive effects of perchloric acid and hydrochloric acid on various alloys commonly used in industrial settings. This includes analyzing the rate of corrosion, the nature of corrosion products formed, and the morphological changes in the alloy surfaces. By understanding these differences, researchers aim to develop more targeted corrosion prevention strategies and improve material selection processes for acid-intensive applications.
Additionally, this study seeks to explore the underlying mechanisms that contribute to the differential corrosive behavior of these acids. Factors such as pH, oxidizing power, and the specific interactions between acid anions and alloy components will be investigated. This mechanistic understanding is crucial for predicting corrosion behavior in complex environments and for developing new corrosion-resistant alloys.
The research also aims to address the practical implications of using perchloric acid versus hydrochloric acid in industrial processes. This includes considerations of safety, handling procedures, and potential environmental impacts. By providing a comprehensive comparison, the study will inform decision-making processes in industries where these acids are commonly used, such as metal processing, chemical manufacturing, and electronics.
Furthermore, this comparative analysis will contribute to the broader field of corrosion science by potentially revealing new insights into acid-induced corrosion processes. These findings may have implications beyond the specific acids studied, leading to a more nuanced understanding of corrosion phenomena in general.
Market Demand for Corrosion-Resistant Alloys
The market demand for corrosion-resistant alloys has been steadily increasing across various industries due to the growing awareness of the economic and safety implications of corrosion. Industries such as oil and gas, chemical processing, aerospace, and marine engineering are particularly driving this demand as they operate in highly corrosive environments.
In the oil and gas sector, the need for corrosion-resistant alloys is paramount. Offshore platforms, pipelines, and refinery equipment are constantly exposed to harsh marine environments and corrosive substances. The industry's push towards deeper water exploration and more challenging extraction conditions has further amplified the demand for advanced corrosion-resistant materials.
The chemical processing industry also represents a significant market for corrosion-resistant alloys. Chemical plants handle a wide range of corrosive substances, including acids, alkalis, and organic compounds. The need for equipment that can withstand these aggressive environments while maintaining operational efficiency and safety is crucial.
Aerospace is another sector with a growing demand for corrosion-resistant alloys. Aircraft components are exposed to various corrosive factors, including atmospheric moisture, de-icing fluids, and high-temperature exhaust gases. The industry's focus on lightweight materials that can withstand these conditions while maintaining structural integrity drives the demand for innovative corrosion-resistant alloys.
The marine industry, including shipbuilding and offshore structures, continues to be a significant consumer of corrosion-resistant alloys. Seawater's corrosive nature necessitates the use of materials that can withstand long-term exposure without degradation. This demand is further intensified by the increasing focus on sustainable and long-lasting marine infrastructure.
Power generation, particularly in nuclear and renewable energy sectors, also contributes to the market demand. Corrosion-resistant alloys are essential in these applications due to the extreme operating conditions and the critical nature of the equipment involved.
The global trend towards sustainable and durable infrastructure has also boosted the demand for corrosion-resistant alloys in construction and civil engineering projects. Bridges, coastal structures, and high-rise buildings in corrosive environments increasingly utilize these materials to ensure longevity and reduce maintenance costs.
As environmental regulations become more stringent, industries are compelled to adopt materials that minimize the risk of corrosion-related failures and subsequent environmental contamination. This regulatory pressure further drives the market for corrosion-resistant alloys across various sectors.
The ongoing research and development in the field of corrosion-resistant alloys, focusing on improving performance while reducing costs, is expected to open up new market opportunities. Emerging applications in areas such as renewable energy storage, advanced manufacturing, and biomedical implants are likely to further expand the market demand for these specialized materials in the coming years.
In the oil and gas sector, the need for corrosion-resistant alloys is paramount. Offshore platforms, pipelines, and refinery equipment are constantly exposed to harsh marine environments and corrosive substances. The industry's push towards deeper water exploration and more challenging extraction conditions has further amplified the demand for advanced corrosion-resistant materials.
The chemical processing industry also represents a significant market for corrosion-resistant alloys. Chemical plants handle a wide range of corrosive substances, including acids, alkalis, and organic compounds. The need for equipment that can withstand these aggressive environments while maintaining operational efficiency and safety is crucial.
Aerospace is another sector with a growing demand for corrosion-resistant alloys. Aircraft components are exposed to various corrosive factors, including atmospheric moisture, de-icing fluids, and high-temperature exhaust gases. The industry's focus on lightweight materials that can withstand these conditions while maintaining structural integrity drives the demand for innovative corrosion-resistant alloys.
The marine industry, including shipbuilding and offshore structures, continues to be a significant consumer of corrosion-resistant alloys. Seawater's corrosive nature necessitates the use of materials that can withstand long-term exposure without degradation. This demand is further intensified by the increasing focus on sustainable and long-lasting marine infrastructure.
Power generation, particularly in nuclear and renewable energy sectors, also contributes to the market demand. Corrosion-resistant alloys are essential in these applications due to the extreme operating conditions and the critical nature of the equipment involved.
The global trend towards sustainable and durable infrastructure has also boosted the demand for corrosion-resistant alloys in construction and civil engineering projects. Bridges, coastal structures, and high-rise buildings in corrosive environments increasingly utilize these materials to ensure longevity and reduce maintenance costs.
As environmental regulations become more stringent, industries are compelled to adopt materials that minimize the risk of corrosion-related failures and subsequent environmental contamination. This regulatory pressure further drives the market for corrosion-resistant alloys across various sectors.
The ongoing research and development in the field of corrosion-resistant alloys, focusing on improving performance while reducing costs, is expected to open up new market opportunities. Emerging applications in areas such as renewable energy storage, advanced manufacturing, and biomedical implants are likely to further expand the market demand for these specialized materials in the coming years.
Current Challenges in Acid-Induced Corrosion
Acid-induced corrosion remains a significant challenge in various industries, particularly in the context of alloy materials. The comparative analysis of perchloric acid and hydrochloric acid on alloy corrosion highlights several pressing issues that researchers and engineers are currently grappling with.
One of the primary challenges is the complexity of corrosion mechanisms in different acid environments. Perchloric acid and hydrochloric acid exhibit distinct corrosive behaviors, making it difficult to develop universal protection strategies. The interaction between these acids and various alloy compositions leads to diverse corrosion patterns, necessitating tailored approaches for each specific acid-alloy combination.
The accelerated corrosion rates induced by these strong acids pose another significant challenge. Both perchloric and hydrochloric acids are known for their aggressive nature, capable of rapidly degrading alloy surfaces. This rapid deterioration complicates the implementation of real-time monitoring and control systems, as the window for intervention is often extremely narrow.
Furthermore, the formation of complex corrosion products presents obstacles in accurately assessing the extent of damage. These products can mask underlying corrosion, leading to underestimation of the actual material degradation. In the case of perchloric acid, the formation of perchlorates adds an additional layer of complexity, as these compounds can further contribute to the corrosion process.
The environmental and safety concerns associated with handling these acids also present significant challenges. Perchloric acid, in particular, is known for its explosive properties when in contact with organic materials, necessitating stringent safety protocols. This complicates research efforts and industrial applications, as specialized facilities and equipment are required for safe experimentation and usage.
Another pressing issue is the development of effective corrosion inhibitors that can withstand the harsh acidic environments created by perchloric and hydrochloric acids. Traditional inhibitors often prove inadequate in these extreme conditions, necessitating the exploration of novel materials and formulations capable of providing long-lasting protection.
The impact of temperature and concentration variations on corrosion rates adds another layer of complexity to the challenge. Both perchloric and hydrochloric acids exhibit temperature-dependent corrosivity, with higher temperatures generally accelerating the corrosion process. Developing models that accurately predict corrosion behavior across a range of temperatures and concentrations remains an ongoing challenge.
Lastly, the long-term effects of acid-induced corrosion on alloy mechanical properties are not fully understood. While immediate corrosion damage is often visible, the subtle changes in alloy microstructure and mechanical integrity over time require further investigation. This knowledge gap hampers the accurate prediction of component lifespans and the development of effective preventive maintenance strategies.
One of the primary challenges is the complexity of corrosion mechanisms in different acid environments. Perchloric acid and hydrochloric acid exhibit distinct corrosive behaviors, making it difficult to develop universal protection strategies. The interaction between these acids and various alloy compositions leads to diverse corrosion patterns, necessitating tailored approaches for each specific acid-alloy combination.
The accelerated corrosion rates induced by these strong acids pose another significant challenge. Both perchloric and hydrochloric acids are known for their aggressive nature, capable of rapidly degrading alloy surfaces. This rapid deterioration complicates the implementation of real-time monitoring and control systems, as the window for intervention is often extremely narrow.
Furthermore, the formation of complex corrosion products presents obstacles in accurately assessing the extent of damage. These products can mask underlying corrosion, leading to underestimation of the actual material degradation. In the case of perchloric acid, the formation of perchlorates adds an additional layer of complexity, as these compounds can further contribute to the corrosion process.
The environmental and safety concerns associated with handling these acids also present significant challenges. Perchloric acid, in particular, is known for its explosive properties when in contact with organic materials, necessitating stringent safety protocols. This complicates research efforts and industrial applications, as specialized facilities and equipment are required for safe experimentation and usage.
Another pressing issue is the development of effective corrosion inhibitors that can withstand the harsh acidic environments created by perchloric and hydrochloric acids. Traditional inhibitors often prove inadequate in these extreme conditions, necessitating the exploration of novel materials and formulations capable of providing long-lasting protection.
The impact of temperature and concentration variations on corrosion rates adds another layer of complexity to the challenge. Both perchloric and hydrochloric acids exhibit temperature-dependent corrosivity, with higher temperatures generally accelerating the corrosion process. Developing models that accurately predict corrosion behavior across a range of temperatures and concentrations remains an ongoing challenge.
Lastly, the long-term effects of acid-induced corrosion on alloy mechanical properties are not fully understood. While immediate corrosion damage is often visible, the subtle changes in alloy microstructure and mechanical integrity over time require further investigation. This knowledge gap hampers the accurate prediction of component lifespans and the development of effective preventive maintenance strategies.
Existing Methodologies for Acid Corrosion Analysis
01 Corrosion-resistant materials for acid environments
Various materials have been developed to resist corrosion from perchloric and hydrochloric acids. These include specialized alloys, coatings, and composite materials that can withstand the harsh acidic environment. The selection of appropriate materials is crucial for equipment and infrastructure exposed to these acids.- Corrosion-resistant materials for acid environments: Various materials have been developed to resist corrosion from perchloric and hydrochloric acids. These include specialized alloys, coatings, and composite materials that can withstand the highly corrosive nature of these acids. The selection of appropriate materials is crucial for equipment and infrastructure in industries dealing with these acids.
- Protective coatings and surface treatments: Protective coatings and surface treatments are applied to enhance the corrosion resistance of materials exposed to perchloric and hydrochloric acids. These treatments can include chemical conversion coatings, electroplating, and advanced polymer coatings that create a barrier between the acid and the underlying material.
- Corrosion inhibitors and additives: Chemical additives and inhibitors are used to reduce the corrosive effects of perchloric and hydrochloric acids. These substances can be added directly to the acids or incorporated into protective coatings. They work by forming protective films on metal surfaces or by altering the electrochemical properties of the corrosive environment.
- Monitoring and control systems for acid corrosion: Advanced monitoring and control systems have been developed to detect and mitigate corrosion caused by perchloric and hydrochloric acids. These systems use sensors, data analysis, and automated responses to identify corrosion risks and implement preventive measures in real-time, helping to extend the lifespan of equipment and infrastructure.
- Acid-resistant equipment design: Specialized equipment designs have been created to withstand the corrosive effects of perchloric and hydrochloric acids. These designs incorporate features such as improved sealing, optimized fluid flow patterns, and strategic material selection to minimize exposure and extend equipment life in acid-rich environments.
02 Protective coatings and surface treatments
Protective coatings and surface treatments are applied to enhance the corrosion resistance of materials exposed to perchloric and hydrochloric acids. These treatments can include chemical conversion coatings, electroplating, and advanced polymer coatings that create a barrier between the acid and the underlying material.Expand Specific Solutions03 Corrosion inhibitors and additives
Chemical additives and inhibitors are used to reduce the corrosive effects of perchloric and hydrochloric acids. These substances can be added directly to the acids or incorporated into protective formulations. They work by forming protective films on metal surfaces or by altering the electrochemical properties of the corrosive environment.Expand Specific Solutions04 Acid-resistant equipment design
Specialized equipment designs have been developed to minimize corrosion in perchloric and hydrochloric acid environments. These designs incorporate features such as improved sealing, optimized fluid flow, and strategic material selection to enhance durability and reduce maintenance requirements in acidic conditions.Expand Specific Solutions05 Monitoring and control systems for acid corrosion
Advanced monitoring and control systems have been implemented to detect and mitigate corrosion caused by perchloric and hydrochloric acids. These systems use sensors, data analysis, and automated responses to identify corrosion risks, adjust process parameters, and initiate preventive measures to extend the lifespan of equipment and materials exposed to acidic environments.Expand Specific Solutions
Key Players in Corrosion Research and Alloy Manufacturing
The comparative analysis of perchloric and hydrochloric acids on alloy corrosion is in a mature stage of development, with a well-established market and significant industry players. The global corrosion inhibitors market, valued at approximately $7.5 billion in 2020, is expected to grow steadily. Key companies like NIPPON STEEL CORP., Ecolab USA, Inc., and Henkel AG & Co. KGaA are at the forefront of research and development in this field. These industry leaders, along with academic institutions such as Instituto Superior Técnico de Lisboa and Northeastern University, are driving technological advancements in corrosion prevention and mitigation strategies, focusing on improving the efficiency and environmental sustainability of acid-based corrosion inhibitors for various alloys.
NIPPON STEEL CORP.
Technical Solution: NIPPON STEEL CORP. has developed advanced corrosion-resistant alloys and surface treatment technologies to mitigate the effects of both perchloric and hydrochloric acids. Their approach involves the use of high-chromium stainless steels with added molybdenum and nitrogen for enhanced corrosion resistance[1]. They have also implemented a duplex coating system, combining a chromium-rich base layer with a nickel-phosphorus top coat, which has shown superior resistance to both acids in comparative tests[2]. Recent studies by the company have focused on the synergistic effects of alloying elements in improving resistance to localized corrosion in acidic environments[3].
Strengths: Extensive experience in alloy development, strong R&D capabilities, and a wide range of corrosion-resistant products. Weaknesses: Higher production costs for specialized alloys may limit market penetration in cost-sensitive sectors.
Henkel AG & Co. KGaA
Technical Solution: Henkel AG & Co. KGaA has developed innovative solutions for protecting alloys against corrosion from perchloric and hydrochloric acids. Their approach centers on advanced surface treatment technologies and smart coating systems. Henkel's Bonderite range includes acid-resistant conversion coatings that form a protective layer on metal surfaces, significantly reducing corrosion rates in both acid environments[1]. They have also introduced nano-ceramic coatings that provide exceptional resistance to chemical attack. Recent developments include self-healing coatings that can repair minor damage and prevent acid penetration[2]. Henkel's research has shown that their latest generation of epoxy-based coatings, enhanced with graphene oxide, offers superior protection against both perchloric and hydrochloric acid corrosion compared to traditional coatings[3].
Strengths: Wide range of surface treatment solutions, strong focus on environmentally friendly technologies, and global distribution network. Weaknesses: Some advanced solutions may require specialized application processes, potentially limiting adoption in certain markets.
Critical Innovations in Corrosion-Resistant Alloy Design
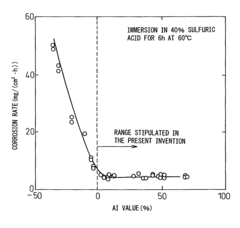
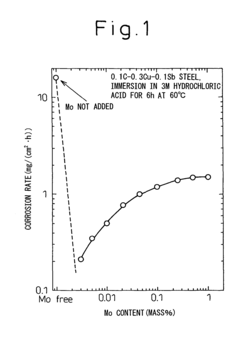
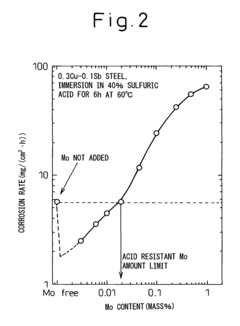
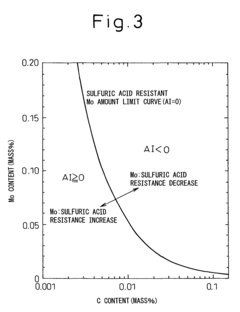
Low alloy steel and weld joint thereof excellent in corrosion resistance to hydrochloric acid and sulfuric acid
PatentInactiveUS7731896B2
Innovation
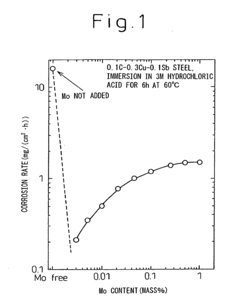
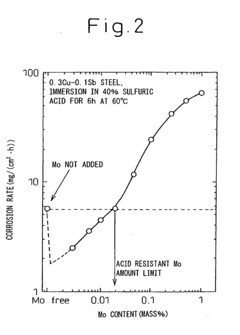
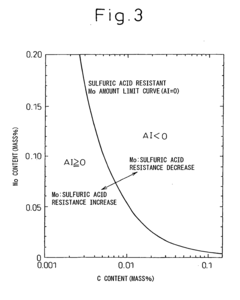
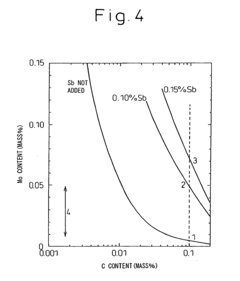
- A low alloy steel composition with specific additions of Mo, Sb, and other elements, optimized to maintain excellent sulfuric acid resistance while improving hydrochloric acid resistance, and a corresponding weld joint composition that ensures acid corrosion resistance indices are positive, ensuring both base materials and welds have comparable corrosion resistance.
Low alloy steel excellent in resistance to corrosion by hydrochloric acid and corrosion by sulfuric acid and weld joint comprising the same
PatentInactiveEP1460145B1
Innovation
- A low alloy steel with specific compositions, including controlled amounts of Mo, Sb, and Cu, is developed to enhance both sulfuric acid and hydrochloric acid corrosion resistance, along with a weld joint composition that maintains a positive acid corrosion resistance index, ensuring excellent corrosion resistance in environments with varying acid concentrations.
Environmental Impact of Acid Corrosion Processes
The environmental impact of acid corrosion processes, particularly those involving perchloric acid and hydrochloric acid on alloy corrosion, is a significant concern in various industrial applications. These processes can lead to substantial environmental consequences if not properly managed and controlled.
Acid corrosion processes involving perchloric acid and hydrochloric acid can result in the release of harmful substances into the environment. These acids, when used in industrial settings, may contaminate soil and water sources if not adequately contained or treated. The corrosion products, often containing dissolved metal ions, can have detrimental effects on ecosystems and aquatic life.
The use of perchloric acid in corrosion processes poses unique environmental challenges due to its strong oxidizing properties. It can react vigorously with organic materials, potentially leading to fires or explosions if mishandled. This reactivity also increases the risk of atmospheric pollution through the release of chlorine-containing compounds.
Hydrochloric acid, while less reactive than perchloric acid, still presents significant environmental concerns. Its corrosive nature can lead to the degradation of infrastructure and equipment, potentially resulting in leaks and spills. These incidents can cause localized environmental damage and pose risks to human health and safety.
The disposal of waste products from acid corrosion processes is another critical environmental consideration. Improper disposal methods can lead to soil and groundwater contamination, affecting both terrestrial and aquatic ecosystems. The accumulation of heavy metals from corroded alloys in the environment can have long-lasting impacts on biodiversity and food chains.
To mitigate these environmental risks, industries employing acid corrosion processes must implement robust containment and treatment systems. This includes the use of corrosion-resistant materials for storage and handling, as well as advanced wastewater treatment technologies to neutralize and remove harmful substances before discharge.
Regulatory frameworks play a crucial role in minimizing the environmental impact of acid corrosion processes. Stringent guidelines for the handling, storage, and disposal of acids and their byproducts are essential to protect ecosystems and public health. Regular monitoring and reporting of emissions and waste management practices are necessary to ensure compliance with environmental standards.
Research into more environmentally friendly alternatives to traditional acid corrosion processes is ongoing. This includes the development of less hazardous acids, improved corrosion inhibitors, and novel surface treatment techniques that reduce the overall environmental footprint of alloy corrosion prevention and management.
Acid corrosion processes involving perchloric acid and hydrochloric acid can result in the release of harmful substances into the environment. These acids, when used in industrial settings, may contaminate soil and water sources if not adequately contained or treated. The corrosion products, often containing dissolved metal ions, can have detrimental effects on ecosystems and aquatic life.
The use of perchloric acid in corrosion processes poses unique environmental challenges due to its strong oxidizing properties. It can react vigorously with organic materials, potentially leading to fires or explosions if mishandled. This reactivity also increases the risk of atmospheric pollution through the release of chlorine-containing compounds.
Hydrochloric acid, while less reactive than perchloric acid, still presents significant environmental concerns. Its corrosive nature can lead to the degradation of infrastructure and equipment, potentially resulting in leaks and spills. These incidents can cause localized environmental damage and pose risks to human health and safety.
The disposal of waste products from acid corrosion processes is another critical environmental consideration. Improper disposal methods can lead to soil and groundwater contamination, affecting both terrestrial and aquatic ecosystems. The accumulation of heavy metals from corroded alloys in the environment can have long-lasting impacts on biodiversity and food chains.
To mitigate these environmental risks, industries employing acid corrosion processes must implement robust containment and treatment systems. This includes the use of corrosion-resistant materials for storage and handling, as well as advanced wastewater treatment technologies to neutralize and remove harmful substances before discharge.
Regulatory frameworks play a crucial role in minimizing the environmental impact of acid corrosion processes. Stringent guidelines for the handling, storage, and disposal of acids and their byproducts are essential to protect ecosystems and public health. Regular monitoring and reporting of emissions and waste management practices are necessary to ensure compliance with environmental standards.
Research into more environmentally friendly alternatives to traditional acid corrosion processes is ongoing. This includes the development of less hazardous acids, improved corrosion inhibitors, and novel surface treatment techniques that reduce the overall environmental footprint of alloy corrosion prevention and management.
Standardization of Corrosion Testing Procedures
Standardization of corrosion testing procedures is crucial for ensuring reliable and reproducible results in comparative analyses of perchloric acid and hydrochloric acid on alloy corrosion. This standardization process involves establishing uniform methods, parameters, and conditions for conducting corrosion tests across different laboratories and research institutions.
One of the primary aspects of standardization is the preparation of test specimens. This includes defining the alloy composition, surface finish, and dimensions of the samples to be tested. Standardized procedures for cleaning and degreasing the specimens prior to testing are also essential to eliminate any surface contaminants that may influence the corrosion process.
The composition and concentration of the test solutions, namely perchloric acid and hydrochloric acid, must be precisely defined and controlled. This includes specifying the purity of the acids, their concentration ranges, and any additives or impurities that may be present. Additionally, the volume of the test solution and the ratio of solution volume to specimen surface area should be standardized to ensure consistent exposure conditions.
Temperature control is another critical factor in corrosion testing. Standardized procedures should specify the test temperature and acceptable temperature ranges, as well as methods for maintaining and monitoring temperature throughout the duration of the experiment. This may involve the use of thermostatic baths or environmental chambers to provide stable temperature conditions.
Exposure time is a key parameter that must be standardized to allow for meaningful comparisons between different tests. Procedures should define the duration of exposure for short-term and long-term tests, as well as any intermediate time points for data collection or specimen examination.
Standardization of measurement techniques is essential for quantifying corrosion rates and characterizing corrosion mechanisms. This includes specifying methods for weight loss measurements, electrochemical techniques such as potentiodynamic polarization and electrochemical impedance spectroscopy, and surface analysis techniques like scanning electron microscopy and X-ray photoelectron spectroscopy.
Data analysis and reporting procedures should also be standardized to ensure consistency in the interpretation and presentation of results. This involves defining methods for calculating corrosion rates, statistical analysis of data, and formats for reporting experimental conditions and results.
Interlaboratory testing and round-robin studies play a crucial role in validating and refining standardized procedures. These collaborative efforts help identify sources of variability and improve the reproducibility of test methods across different laboratories and operators.
By implementing standardized corrosion testing procedures, researchers can more accurately compare the effects of perchloric acid and hydrochloric acid on alloy corrosion, leading to more reliable and meaningful results in materials science and corrosion engineering.
One of the primary aspects of standardization is the preparation of test specimens. This includes defining the alloy composition, surface finish, and dimensions of the samples to be tested. Standardized procedures for cleaning and degreasing the specimens prior to testing are also essential to eliminate any surface contaminants that may influence the corrosion process.
The composition and concentration of the test solutions, namely perchloric acid and hydrochloric acid, must be precisely defined and controlled. This includes specifying the purity of the acids, their concentration ranges, and any additives or impurities that may be present. Additionally, the volume of the test solution and the ratio of solution volume to specimen surface area should be standardized to ensure consistent exposure conditions.
Temperature control is another critical factor in corrosion testing. Standardized procedures should specify the test temperature and acceptable temperature ranges, as well as methods for maintaining and monitoring temperature throughout the duration of the experiment. This may involve the use of thermostatic baths or environmental chambers to provide stable temperature conditions.
Exposure time is a key parameter that must be standardized to allow for meaningful comparisons between different tests. Procedures should define the duration of exposure for short-term and long-term tests, as well as any intermediate time points for data collection or specimen examination.
Standardization of measurement techniques is essential for quantifying corrosion rates and characterizing corrosion mechanisms. This includes specifying methods for weight loss measurements, electrochemical techniques such as potentiodynamic polarization and electrochemical impedance spectroscopy, and surface analysis techniques like scanning electron microscopy and X-ray photoelectron spectroscopy.
Data analysis and reporting procedures should also be standardized to ensure consistency in the interpretation and presentation of results. This involves defining methods for calculating corrosion rates, statistical analysis of data, and formats for reporting experimental conditions and results.
Interlaboratory testing and round-robin studies play a crucial role in validating and refining standardized procedures. These collaborative efforts help identify sources of variability and improve the reproducibility of test methods across different laboratories and operators.
By implementing standardized corrosion testing procedures, researchers can more accurately compare the effects of perchloric acid and hydrochloric acid on alloy corrosion, leading to more reliable and meaningful results in materials science and corrosion engineering.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!