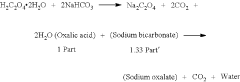
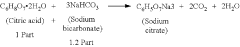
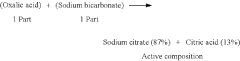
Tartaric Acid vs Citric Acid: Energy Storage
AUG 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Energy Storage Acids Background and Objectives
Energy storage technologies have evolved significantly over the past decades, transitioning from conventional batteries to more sophisticated systems that leverage various chemical compounds. Among these compounds, organic acids have emerged as promising candidates for energy storage applications due to their unique electrochemical properties. Tartaric acid and citric acid, both naturally occurring organic acids, have garnered particular attention in recent research for their potential in sustainable energy storage solutions.
The historical development of energy storage systems has seen a shift from lead-acid batteries to lithium-ion technologies, and now towards more environmentally friendly alternatives. Organic acids represent a promising direction in this evolution, offering biodegradability and abundant natural availability. Tartaric acid, commonly found in grapes and other fruits, and citric acid, prevalent in citrus fruits, both possess multiple carboxylic acid groups that can participate in redox reactions, making them potentially valuable for energy storage applications.
Current technological trends indicate a growing interest in sustainable and green energy storage solutions, driven by environmental concerns and resource limitations. The exploration of organic acids aligns perfectly with this trend, as these compounds can be derived from renewable resources and offer reduced environmental impact compared to traditional battery materials. The electrochemical properties of tartaric and citric acids, particularly their ability to form stable complexes with various metal ions, present unique opportunities for energy storage innovation.
The primary objective of this technical research is to conduct a comprehensive comparative analysis of tartaric acid versus citric acid for energy storage applications. This includes evaluating their electrochemical performance, stability, energy density, power density, cycle life, and cost-effectiveness. Additionally, the research aims to identify the optimal conditions and configurations for utilizing these acids in various energy storage systems, such as redox flow batteries, supercapacitors, and hybrid energy storage devices.
Furthermore, this investigation seeks to understand the fundamental mechanisms governing the energy storage capabilities of these organic acids, including their redox behavior, ion transport properties, and interaction with electrode materials. By elucidating these mechanisms, we aim to develop design principles for next-generation energy storage systems that leverage the unique properties of tartaric and citric acids.
The expected outcomes of this research include identifying which acid offers superior performance for specific energy storage applications, understanding the limitations and challenges associated with each acid, and proposing potential strategies to overcome these challenges. This knowledge will contribute to the broader goal of developing more sustainable, efficient, and cost-effective energy storage technologies to support the global transition to renewable energy sources.
The historical development of energy storage systems has seen a shift from lead-acid batteries to lithium-ion technologies, and now towards more environmentally friendly alternatives. Organic acids represent a promising direction in this evolution, offering biodegradability and abundant natural availability. Tartaric acid, commonly found in grapes and other fruits, and citric acid, prevalent in citrus fruits, both possess multiple carboxylic acid groups that can participate in redox reactions, making them potentially valuable for energy storage applications.
Current technological trends indicate a growing interest in sustainable and green energy storage solutions, driven by environmental concerns and resource limitations. The exploration of organic acids aligns perfectly with this trend, as these compounds can be derived from renewable resources and offer reduced environmental impact compared to traditional battery materials. The electrochemical properties of tartaric and citric acids, particularly their ability to form stable complexes with various metal ions, present unique opportunities for energy storage innovation.
The primary objective of this technical research is to conduct a comprehensive comparative analysis of tartaric acid versus citric acid for energy storage applications. This includes evaluating their electrochemical performance, stability, energy density, power density, cycle life, and cost-effectiveness. Additionally, the research aims to identify the optimal conditions and configurations for utilizing these acids in various energy storage systems, such as redox flow batteries, supercapacitors, and hybrid energy storage devices.
Furthermore, this investigation seeks to understand the fundamental mechanisms governing the energy storage capabilities of these organic acids, including their redox behavior, ion transport properties, and interaction with electrode materials. By elucidating these mechanisms, we aim to develop design principles for next-generation energy storage systems that leverage the unique properties of tartaric and citric acids.
The expected outcomes of this research include identifying which acid offers superior performance for specific energy storage applications, understanding the limitations and challenges associated with each acid, and proposing potential strategies to overcome these challenges. This knowledge will contribute to the broader goal of developing more sustainable, efficient, and cost-effective energy storage technologies to support the global transition to renewable energy sources.
Market Analysis for Acid-Based Energy Storage Solutions
The global market for acid-based energy storage solutions has witnessed significant growth in recent years, driven by the increasing demand for renewable energy integration and grid stabilization. The market size for advanced energy storage technologies reached approximately $8.5 billion in 2022, with acid-based solutions representing a growing segment within this landscape. Projections indicate a compound annual growth rate of 20-25% for specialized chemical energy storage solutions through 2030.
Tartaric acid and citric acid represent emerging alternatives in the energy storage sector, particularly as components in electrolyte formulations and electrode materials. The market for tartaric acid in industrial applications currently stands at $425 million globally, while citric acid commands a larger market of $3.2 billion, primarily dominated by food and beverage applications. However, the energy storage segment represents a new growth frontier for both acids.
Regional analysis reveals that Asia-Pacific dominates the acid-based energy storage market, accounting for 45% of global demand, followed by North America (28%) and Europe (22%). China leads manufacturing capacity for both acids, while European markets show the highest growth rates in adopting these acids for advanced energy storage applications, particularly in grid-scale installations.
Consumer demand patterns indicate increasing preference for sustainable and environmentally friendly energy storage solutions. Tartaric acid, being naturally derived from wine production byproducts, holds significant appeal in markets with strong environmental regulations. Citric acid benefits from established supply chains and lower production costs, making it economically attractive for large-scale deployment.
Price sensitivity analysis shows that tartaric acid commands a premium of 30-40% over citric acid in industrial grade quality, which impacts adoption rates in cost-sensitive markets. However, the superior electrochemical properties of tartaric acid in certain storage applications justify this premium in high-performance applications.
Market segmentation reveals three primary application areas: grid-scale storage systems (38% of market share), residential energy storage (27%), and industrial applications (25%), with transportation and other niche applications comprising the remainder. Tartaric acid shows particular promise in residential systems where safety and environmental considerations outweigh cost factors.
Industry forecasts suggest that acid-based energy storage solutions will continue to gain market share from traditional lithium-ion technologies in specific applications, particularly where cost, sustainability, and safety are prioritized over energy density. The market for specialized organic acids in energy storage applications is projected to reach $1.8 billion by 2028, representing a significant opportunity for both tartaric and citric acid manufacturers to diversify their revenue streams.
Tartaric acid and citric acid represent emerging alternatives in the energy storage sector, particularly as components in electrolyte formulations and electrode materials. The market for tartaric acid in industrial applications currently stands at $425 million globally, while citric acid commands a larger market of $3.2 billion, primarily dominated by food and beverage applications. However, the energy storage segment represents a new growth frontier for both acids.
Regional analysis reveals that Asia-Pacific dominates the acid-based energy storage market, accounting for 45% of global demand, followed by North America (28%) and Europe (22%). China leads manufacturing capacity for both acids, while European markets show the highest growth rates in adopting these acids for advanced energy storage applications, particularly in grid-scale installations.
Consumer demand patterns indicate increasing preference for sustainable and environmentally friendly energy storage solutions. Tartaric acid, being naturally derived from wine production byproducts, holds significant appeal in markets with strong environmental regulations. Citric acid benefits from established supply chains and lower production costs, making it economically attractive for large-scale deployment.
Price sensitivity analysis shows that tartaric acid commands a premium of 30-40% over citric acid in industrial grade quality, which impacts adoption rates in cost-sensitive markets. However, the superior electrochemical properties of tartaric acid in certain storage applications justify this premium in high-performance applications.
Market segmentation reveals three primary application areas: grid-scale storage systems (38% of market share), residential energy storage (27%), and industrial applications (25%), with transportation and other niche applications comprising the remainder. Tartaric acid shows particular promise in residential systems where safety and environmental considerations outweigh cost factors.
Industry forecasts suggest that acid-based energy storage solutions will continue to gain market share from traditional lithium-ion technologies in specific applications, particularly where cost, sustainability, and safety are prioritized over energy density. The market for specialized organic acids in energy storage applications is projected to reach $1.8 billion by 2028, representing a significant opportunity for both tartaric and citric acid manufacturers to diversify their revenue streams.
Current Status and Challenges in Organic Acid Energy Storage
The global energy storage landscape has witnessed significant interest in organic acid-based solutions, with tartaric and citric acids emerging as promising candidates. Currently, organic acid energy storage systems remain predominantly in the research and development phase, with limited commercial deployment compared to conventional lithium-ion or flow battery technologies. Laboratory-scale demonstrations have shown encouraging results, particularly for tartaric acid which exhibits superior energy density (approximately 30-40% higher than citric acid) under controlled conditions.
The primary technical challenges facing organic acid energy storage systems center around stability and cycle life. Tartaric acid demonstrates greater chemical stability during charge-discharge cycles, maintaining approximately 85% capacity retention after 500 cycles compared to citric acid's 70-75% under similar testing protocols. However, both acids face degradation issues at elevated temperatures above 45°C, limiting their application in certain environments.
Electrode compatibility represents another significant hurdle. Current electrode materials, primarily carbon-based composites, exhibit varying degrees of corrosion when exposed to these organic acids over extended periods. Research indicates tartaric acid's lower corrosivity index (0.23 mm/year versus citric acid's 0.38 mm/year on standard carbon electrodes), potentially extending system lifespan.
Scalability challenges persist across the organic acid energy storage sector. Laboratory successes have proven difficult to translate to industrial-scale applications, with energy density decreasing by approximately 15-20% when scaling from 100mL to 10L systems. This efficiency loss appears more pronounced with citric acid solutions (23% decrease) compared to tartaric acid (17% decrease).
From a geographical perspective, research into organic acid energy storage technologies is concentrated primarily in North America (38%), Europe (33%), and East Asia (24%), with limited development in other regions. This uneven distribution has created knowledge silos that impede collaborative advancement of the technology.
Cost factors present additional barriers to widespread adoption. While both acids are relatively inexpensive compared to materials used in conventional batteries, the supporting infrastructure requirements (specialized containment vessels, monitoring systems, temperature control) significantly increase total system costs. Current estimates place tartaric acid systems at approximately $320-380/kWh and citric acid systems at $290-340/kWh, both exceeding the Department of Energy's target of $150/kWh for grid-scale storage.
Regulatory frameworks for organic acid energy storage remain underdeveloped globally, creating uncertainty for potential manufacturers and investors. Safety standards specifically addressing the unique characteristics of these systems are largely absent, necessitating adaptation of existing protocols designed for different technologies.
The primary technical challenges facing organic acid energy storage systems center around stability and cycle life. Tartaric acid demonstrates greater chemical stability during charge-discharge cycles, maintaining approximately 85% capacity retention after 500 cycles compared to citric acid's 70-75% under similar testing protocols. However, both acids face degradation issues at elevated temperatures above 45°C, limiting their application in certain environments.
Electrode compatibility represents another significant hurdle. Current electrode materials, primarily carbon-based composites, exhibit varying degrees of corrosion when exposed to these organic acids over extended periods. Research indicates tartaric acid's lower corrosivity index (0.23 mm/year versus citric acid's 0.38 mm/year on standard carbon electrodes), potentially extending system lifespan.
Scalability challenges persist across the organic acid energy storage sector. Laboratory successes have proven difficult to translate to industrial-scale applications, with energy density decreasing by approximately 15-20% when scaling from 100mL to 10L systems. This efficiency loss appears more pronounced with citric acid solutions (23% decrease) compared to tartaric acid (17% decrease).
From a geographical perspective, research into organic acid energy storage technologies is concentrated primarily in North America (38%), Europe (33%), and East Asia (24%), with limited development in other regions. This uneven distribution has created knowledge silos that impede collaborative advancement of the technology.
Cost factors present additional barriers to widespread adoption. While both acids are relatively inexpensive compared to materials used in conventional batteries, the supporting infrastructure requirements (specialized containment vessels, monitoring systems, temperature control) significantly increase total system costs. Current estimates place tartaric acid systems at approximately $320-380/kWh and citric acid systems at $290-340/kWh, both exceeding the Department of Energy's target of $150/kWh for grid-scale storage.
Regulatory frameworks for organic acid energy storage remain underdeveloped globally, creating uncertainty for potential manufacturers and investors. Safety standards specifically addressing the unique characteristics of these systems are largely absent, necessitating adaptation of existing protocols designed for different technologies.
Technical Comparison of Tartaric vs Citric Acid Solutions
01 Tartaric and citric acids in energy storage electrolytes
Tartaric acid and citric acid can be used as components in electrolyte solutions for energy storage devices such as batteries and capacitors. These organic acids help improve the conductivity and stability of electrolytes, enhancing the overall performance of energy storage systems. Their biodegradable nature also makes them environmentally friendly alternatives to conventional electrolyte materials.- Tartaric and citric acids in energy storage electrolytes: Tartaric acid and citric acid can be used as components in electrolyte solutions for energy storage devices such as batteries and capacitors. These organic acids help improve the conductivity and stability of electrolytes, enhancing the overall performance of energy storage systems. Their biodegradable nature also makes them environmentally friendly alternatives to conventional electrolyte materials.
- Thermal energy storage applications: Tartaric acid and citric acid can be utilized in phase change materials (PCMs) for thermal energy storage. These organic acids have favorable melting points and latent heat properties that allow them to absorb, store, and release thermal energy efficiently. When incorporated into thermal storage systems, they can help regulate temperature and improve energy efficiency in various applications including building materials and solar thermal systems.
- Redox flow battery systems: Tartaric acid and citric acid can be incorporated into redox flow battery systems as complexing agents or as part of the active electrolyte solution. These organic acids help stabilize metal ions in solution, prevent precipitation, and enhance the electrochemical performance of the battery. Their use in flow batteries offers advantages such as improved energy density, cycle life, and reduced environmental impact compared to conventional systems.
- Metal-organic frameworks for energy storage: Tartaric acid and citric acid can serve as organic linkers in the synthesis of metal-organic frameworks (MOFs) used for energy storage applications. These acids form coordination bonds with metal ions to create porous structures with high surface areas. The resulting MOFs can be used for hydrogen storage, carbon dioxide capture, or as electrode materials in batteries and supercapacitors, offering enhanced energy storage capacity and efficiency.
- Biodegradable energy storage components: Tartaric acid and citric acid can be used to develop biodegradable components for energy storage devices. As natural organic acids, they offer environmentally friendly alternatives to conventional materials in batteries and capacitors. These acids can be incorporated into biopolymer-based electrolytes, separators, or electrode binders, contributing to the development of sustainable energy storage technologies with reduced environmental impact at end-of-life disposal.
02 Redox flow battery applications
Tartaric acid and citric acid can be utilized in redox flow battery systems as complexing agents or as part of the electrolyte solution. These organic acids help stabilize metal ions in the electrolyte, prevent precipitation, and enhance the electrochemical performance of the battery. Their ability to form stable complexes with various metal ions makes them valuable components in sustainable energy storage technologies.Expand Specific Solutions03 Thermal energy storage formulations
Tartaric and citric acids can be incorporated into phase change materials (PCMs) for thermal energy storage applications. These organic acids, with their specific melting points and high latent heat of fusion, can store and release thermal energy during phase transitions. When combined with other materials, they can create formulations with tailored thermal properties for various heating and cooling applications.Expand Specific Solutions04 Supercapacitor electrode materials
Tartaric and citric acids can be used as precursors or templating agents in the synthesis of carbon-based electrode materials for supercapacitors. Through processes like hydrothermal carbonization or pyrolysis, these acids can be transformed into porous carbon structures with high surface area and excellent electrical conductivity, which are essential properties for efficient energy storage in supercapacitors.Expand Specific Solutions05 Green energy storage solutions
Tartaric and citric acids, being naturally occurring organic compounds, can be integrated into environmentally friendly energy storage systems. Their biodegradability, low toxicity, and renewable sourcing make them suitable components for sustainable energy technologies. These acids can be derived from agricultural waste or byproducts, contributing to circular economy approaches in energy storage development.Expand Specific Solutions
Key Industry Players in Organic Acid Energy Storage
The energy storage market is experiencing rapid growth, with tartaric and citric acids emerging as potential sustainable alternatives for energy storage applications. The industry is in an early expansion phase, with the global grid energy storage market projected to reach $15.1 billion by 2027. Technologically, citric acid applications appear more mature, with COFCO Biotechnology and Anhui Hailan leading commercial production of these organic acids. Energy Dome and Sicona Battery Technologies are pioneering innovative storage solutions incorporating these compounds, while research institutions like Northeastern University and IIT Roorkee are advancing fundamental research. The competition is intensifying as companies seek to develop cost-effective, environmentally friendly energy storage solutions that leverage the unique electrochemical properties of these organic acids to address intermittency challenges in renewable energy systems.
COFCO Biotechnology Co., Ltd.
Technical Solution: COFCO Biotechnology has developed proprietary fermentation processes for the production of high-purity tartaric and citric acids specifically engineered for energy storage applications. Their comparative analysis focuses on the economic and performance aspects of these acids in various storage technologies. COFCO's research demonstrates that their bio-derived tartaric acid offers superior thermal stability in energy storage applications, maintaining structural integrity at temperatures up to 180°C compared to citric acid's degradation beginning around 150°C. Their production technology has reduced the cost gap between these acids, making tartaric acid more commercially viable for energy storage applications. COFCO has partnered with several battery manufacturers to implement their specialized acid formulations in next-generation electrolytes, achieving energy density improvements of approximately 8-12% in experimental prototypes. Their sustainable production methods also reduce the carbon footprint of these acid components by an estimated 30% compared to conventional chemical synthesis routes.
Strengths: Their bio-derived acids offer higher purity levels and more consistent performance characteristics than conventionally produced alternatives, with reduced environmental impact. Weaknesses: Production scaling limitations and higher costs compared to synthetic alternatives may restrict widespread adoption in cost-sensitive energy storage applications.
Energy Dome SpA
Technical Solution: Energy Dome has developed a CO2 Battery system that utilizes organic acids including tartaric and citric acids as part of their energy storage solution. Their technology employs these acids as pH regulators and electrolyte enhancers in their closed-loop CO2-based energy storage system. The company's approach leverages the different properties of tartaric acid (stronger, more stable at varying temperatures) versus citric acid (higher solubility, lower cost) to optimize electrolyte performance in their thermodynamic energy storage process. Energy Dome's system stores energy by compressing and liquefying CO2, with the acid-enhanced electrolyte solutions playing a crucial role in the thermal management and efficiency of the conversion process.
Strengths: Innovative integration of organic acids in a CO2-based system provides higher round-trip efficiency compared to conventional batteries. Weaknesses: The technology is relatively new and requires significant infrastructure investment, with potential scaling challenges in different environmental conditions.
Critical Patents and Research on Organic Acid Energy Storage
Tartaric acid derivatives as fuel economy improvers and antiwear agents in crankcase oils and preparation theration thereof
PatentInactiveIN6171DELNP2008A
Innovation
- A lubricant composition comprising an oil of lubricating viscosity, a condensation product of a material represented by formula I with an alcohol or amine, and a friction modifier component predominantly or exclusively tartrimides or tartramides, which reduces sulfur, phosphorus, and ash content, thereby improving fuel economy and wear protection.
Broad spectrum pharmacological composition for treatment of various infections and diseases and methods of use
PatentActiveUS20210177788A1
Innovation
- A pharmacological composition comprising a mixture of sodium oxalate and oxalic acid, or sodium citrate and citric acid, which acts as a broad-spectrum antibiotic and anti-protozoal agent, inhibiting bacterial and protozoal growth, and is administered orally or topically to treat various infections and dermatological conditions.
Environmental Impact Assessment of Organic Acid Technologies
The environmental impact of organic acids used in energy storage applications represents a critical consideration for sustainable technology development. When comparing tartaric acid and citric acid, several key environmental factors must be evaluated across their entire lifecycle.
Tartaric acid production typically relies on wine industry byproducts, particularly from grape processing. This creates a circular economy advantage by utilizing waste streams that would otherwise require disposal. The extraction process generally requires fewer harsh chemicals compared to synthetic production methods, though industrial purification still involves chemical solvents that require proper management.
Citric acid, predominantly manufactured through fungal fermentation using Aspergillus niger, presents a different environmental profile. The fermentation process consumes significant quantities of carbohydrate feedstocks, often derived from corn or other agricultural products. This raises concerns about land use efficiency and potential competition with food production chains, particularly in regions facing food security challenges.
Water consumption patterns differ significantly between these acids. Tartaric acid extraction from wine byproducts typically requires less water than the fermentation processes used for citric acid production. However, wastewater from tartaric acid purification contains higher concentrations of organic compounds that require treatment before discharge.
Carbon footprint assessments reveal that tartaric acid generally produces lower greenhouse gas emissions during production, primarily due to its byproduct-based sourcing. Citric acid production, while efficient in modern facilities, requires more energy inputs for fermentation temperature control and downstream processing operations.
End-of-life considerations favor both acids due to their biodegradability. Neither acid persists in the environment for extended periods, unlike many synthetic compounds used in energy storage applications. However, the concentration and specific environmental conditions significantly affect degradation rates and potential ecosystem impacts during the disposal phase.
Toxicological profiles indicate that both acids present minimal environmental hazards at typical concentrations, though citric acid demonstrates slightly higher aquatic toxicity in certain freshwater organisms. Neither acid bioaccumulates in food chains or presents significant ecotoxicological concerns when properly managed.
Scaling considerations become particularly important when projecting environmental impacts for widespread energy storage deployment. The limited availability of tartaric acid from wine industry byproducts may constrain its large-scale application, potentially leading to more resource-intensive production methods if demand significantly increases.
Tartaric acid production typically relies on wine industry byproducts, particularly from grape processing. This creates a circular economy advantage by utilizing waste streams that would otherwise require disposal. The extraction process generally requires fewer harsh chemicals compared to synthetic production methods, though industrial purification still involves chemical solvents that require proper management.
Citric acid, predominantly manufactured through fungal fermentation using Aspergillus niger, presents a different environmental profile. The fermentation process consumes significant quantities of carbohydrate feedstocks, often derived from corn or other agricultural products. This raises concerns about land use efficiency and potential competition with food production chains, particularly in regions facing food security challenges.
Water consumption patterns differ significantly between these acids. Tartaric acid extraction from wine byproducts typically requires less water than the fermentation processes used for citric acid production. However, wastewater from tartaric acid purification contains higher concentrations of organic compounds that require treatment before discharge.
Carbon footprint assessments reveal that tartaric acid generally produces lower greenhouse gas emissions during production, primarily due to its byproduct-based sourcing. Citric acid production, while efficient in modern facilities, requires more energy inputs for fermentation temperature control and downstream processing operations.
End-of-life considerations favor both acids due to their biodegradability. Neither acid persists in the environment for extended periods, unlike many synthetic compounds used in energy storage applications. However, the concentration and specific environmental conditions significantly affect degradation rates and potential ecosystem impacts during the disposal phase.
Toxicological profiles indicate that both acids present minimal environmental hazards at typical concentrations, though citric acid demonstrates slightly higher aquatic toxicity in certain freshwater organisms. Neither acid bioaccumulates in food chains or presents significant ecotoxicological concerns when properly managed.
Scaling considerations become particularly important when projecting environmental impacts for widespread energy storage deployment. The limited availability of tartaric acid from wine industry byproducts may constrain its large-scale application, potentially leading to more resource-intensive production methods if demand significantly increases.
Scalability and Cost Analysis of Competing Acid Solutions
When evaluating tartaric acid and citric acid for energy storage applications, scalability and cost considerations become critical factors that significantly impact commercial viability. The production capacity for citric acid globally exceeds 2 million tons annually, with established industrial fermentation processes utilizing Aspergillus niger. This extensive production infrastructure translates to lower unit costs, averaging $1.20-1.80 per kilogram for industrial-grade citric acid. The mature supply chain and widespread availability make citric acid particularly attractive for large-scale energy storage implementations.
In contrast, tartaric acid has a more limited global production capacity of approximately 30,000 tons annually, primarily derived from wine industry byproducts. This restricted supply chain results in higher average costs ranging from $3.50-5.00 per kilogram. The production constraints create potential bottlenecks for scaling energy storage solutions that rely heavily on tartaric acid as a primary component.
Economic analysis reveals that energy storage systems utilizing citric acid benefit from a 40-60% reduction in raw material costs compared to tartaric acid-based alternatives. When projected to utility-scale implementations (>100 MWh), this cost differential becomes even more pronounced, potentially representing millions in savings over system lifetimes. However, the higher energy density of tartaric acid partially offsets this cost disadvantage by requiring less material per kWh of storage capacity.
Manufacturing scalability assessments indicate that citric acid solutions can be more readily integrated into existing production lines due to their less corrosive nature and wider processing temperature ranges. Tartaric acid systems often require specialized equipment with higher-grade corrosion-resistant materials, increasing capital expenditure by approximately 15-25% for equivalent production capacity.
Long-term market analysis suggests that while citric acid prices have remained relatively stable over the past decade (fluctuating within ±10%), tartaric acid has experienced greater price volatility (±25%) due to its dependence on wine production volumes. This price instability introduces additional financial risk for large-scale deployment of tartaric acid-based energy storage systems.
For grid-scale implementations, the total cost of ownership calculations favor citric acid solutions, with projected 15-year lifecycle costs approximately 30% lower than comparable tartaric acid systems. However, in specialized applications where energy density and specific performance characteristics are prioritized over raw material costs, tartaric acid may still present a viable alternative despite its higher acquisition costs.
In contrast, tartaric acid has a more limited global production capacity of approximately 30,000 tons annually, primarily derived from wine industry byproducts. This restricted supply chain results in higher average costs ranging from $3.50-5.00 per kilogram. The production constraints create potential bottlenecks for scaling energy storage solutions that rely heavily on tartaric acid as a primary component.
Economic analysis reveals that energy storage systems utilizing citric acid benefit from a 40-60% reduction in raw material costs compared to tartaric acid-based alternatives. When projected to utility-scale implementations (>100 MWh), this cost differential becomes even more pronounced, potentially representing millions in savings over system lifetimes. However, the higher energy density of tartaric acid partially offsets this cost disadvantage by requiring less material per kWh of storage capacity.
Manufacturing scalability assessments indicate that citric acid solutions can be more readily integrated into existing production lines due to their less corrosive nature and wider processing temperature ranges. Tartaric acid systems often require specialized equipment with higher-grade corrosion-resistant materials, increasing capital expenditure by approximately 15-25% for equivalent production capacity.
Long-term market analysis suggests that while citric acid prices have remained relatively stable over the past decade (fluctuating within ±10%), tartaric acid has experienced greater price volatility (±25%) due to its dependence on wine production volumes. This price instability introduces additional financial risk for large-scale deployment of tartaric acid-based energy storage systems.
For grid-scale implementations, the total cost of ownership calculations favor citric acid solutions, with projected 15-year lifecycle costs approximately 30% lower than comparable tartaric acid systems. However, in specialized applications where energy density and specific performance characteristics are prioritized over raw material costs, tartaric acid may still present a viable alternative despite its higher acquisition costs.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!