Comparative Study of Biochemical Sensors in Market-ready Point-of-care Devices
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Biochemical Sensor Evolution and Objectives
Biochemical sensors have undergone a remarkable evolution over the past several decades, transforming from bulky laboratory equipment to miniaturized, portable devices capable of rapid and accurate detection. The journey began in the 1950s with the development of the first oxygen electrode by Leland Clark, often considered the father of biosensors. This innovation laid the groundwork for subsequent advancements in electrochemical sensing technologies that remain fundamental to many modern point-of-care (POC) devices.
The 1970s and 1980s witnessed significant breakthroughs with the introduction of enzyme-based glucose sensors, which revolutionized diabetes management by enabling blood glucose monitoring outside clinical settings. This period marked the beginning of the transition from laboratory-confined testing to patient-centric diagnostic approaches, establishing a clear trajectory toward what we now recognize as point-of-care testing.
The 1990s and early 2000s saw rapid miniaturization and integration of sensing technologies, driven by advances in microelectronics, nanotechnology, and materials science. These developments enabled the creation of more sophisticated, multi-analyte sensors with improved sensitivity, specificity, and reliability. Concurrently, the emergence of smartphone technology created new opportunities for connectivity and data analysis, further enhancing the utility of biochemical sensors in clinical applications.
Recent years have witnessed an acceleration in biochemical sensor innovation, particularly in the realm of wearable and implantable devices. Technologies such as continuous glucose monitors have transformed chronic disease management, while advances in molecular recognition elements, including aptamers and molecularly imprinted polymers, have expanded the range of detectable analytes beyond traditional targets.
The primary objective of modern biochemical sensors in point-of-care devices is to provide rapid, accurate, and actionable diagnostic information at the patient's location, eliminating delays associated with centralized laboratory testing. This goal encompasses several key aims: achieving laboratory-comparable analytical performance, ensuring user-friendly operation by non-specialists, maintaining affordability to enable widespread adoption, and incorporating connectivity features for seamless integration with healthcare information systems.
Additional objectives include expanding the range of detectable biomarkers to encompass emerging clinical needs, reducing sample volume requirements to enhance patient comfort, extending shelf life and stability under various environmental conditions, and developing multiplexed sensing capabilities to simultaneously detect multiple analytes from a single sample. These technological aspirations collectively drive the ongoing evolution of biochemical sensors toward increasingly sophisticated, accessible, and clinically valuable point-of-care solutions.
The 1970s and 1980s witnessed significant breakthroughs with the introduction of enzyme-based glucose sensors, which revolutionized diabetes management by enabling blood glucose monitoring outside clinical settings. This period marked the beginning of the transition from laboratory-confined testing to patient-centric diagnostic approaches, establishing a clear trajectory toward what we now recognize as point-of-care testing.
The 1990s and early 2000s saw rapid miniaturization and integration of sensing technologies, driven by advances in microelectronics, nanotechnology, and materials science. These developments enabled the creation of more sophisticated, multi-analyte sensors with improved sensitivity, specificity, and reliability. Concurrently, the emergence of smartphone technology created new opportunities for connectivity and data analysis, further enhancing the utility of biochemical sensors in clinical applications.
Recent years have witnessed an acceleration in biochemical sensor innovation, particularly in the realm of wearable and implantable devices. Technologies such as continuous glucose monitors have transformed chronic disease management, while advances in molecular recognition elements, including aptamers and molecularly imprinted polymers, have expanded the range of detectable analytes beyond traditional targets.
The primary objective of modern biochemical sensors in point-of-care devices is to provide rapid, accurate, and actionable diagnostic information at the patient's location, eliminating delays associated with centralized laboratory testing. This goal encompasses several key aims: achieving laboratory-comparable analytical performance, ensuring user-friendly operation by non-specialists, maintaining affordability to enable widespread adoption, and incorporating connectivity features for seamless integration with healthcare information systems.
Additional objectives include expanding the range of detectable biomarkers to encompass emerging clinical needs, reducing sample volume requirements to enhance patient comfort, extending shelf life and stability under various environmental conditions, and developing multiplexed sensing capabilities to simultaneously detect multiple analytes from a single sample. These technological aspirations collectively drive the ongoing evolution of biochemical sensors toward increasingly sophisticated, accessible, and clinically valuable point-of-care solutions.
Point-of-care Testing Market Analysis
The global Point-of-Care Testing (POCT) market has experienced substantial growth in recent years, valued at approximately $29.5 billion in 2020 and projected to reach $50.6 billion by 2025, representing a compound annual growth rate (CAGR) of 11.4%. This remarkable expansion is driven by several key factors, including the increasing prevalence of chronic and infectious diseases, growing demand for rapid diagnostic results, technological advancements in biosensor technologies, and the shift toward decentralized healthcare delivery models.
The COVID-19 pandemic has significantly accelerated market growth, creating unprecedented demand for rapid diagnostic solutions that can be deployed outside traditional laboratory settings. This has catalyzed innovation in the biochemical sensor space, particularly for respiratory pathogens, with major manufacturers rapidly developing and commercializing new POCT platforms.
Geographically, North America currently dominates the POCT market with approximately 40% market share, followed by Europe (30%) and Asia-Pacific (20%). However, the Asia-Pacific region is expected to witness the highest growth rate over the next five years due to improving healthcare infrastructure, increasing healthcare expenditure, and growing awareness about early disease diagnosis in countries like China and India.
By application segment, glucose monitoring continues to lead the market, accounting for roughly 25% of the total POCT market value. This is followed by infectious disease testing (20%), cardiac markers (15%), and coagulation monitoring (10%). The infectious disease segment has seen particularly rapid growth since 2020, driven by COVID-19 testing requirements.
The end-user landscape is dominated by hospitals and clinics (45%), followed by home care settings (30%), and diagnostic laboratories (15%). The home care segment is projected to grow at the fastest rate as patient-centric healthcare models gain traction and technological improvements make self-testing more accessible and reliable.
Key market trends include miniaturization of testing devices, integration with digital health platforms and electronic health records, development of multiplexed testing capabilities, and increasing focus on non-invasive sampling methods. The market is also witnessing a shift toward subscription-based business models and testing-as-a-service approaches, particularly for chronic disease management applications.
Regulatory considerations remain a significant factor influencing market dynamics, with varying approval pathways across different regions creating challenges for global product launches. Recent regulatory changes, such as the EU's In Vitro Diagnostic Regulation (IVDR), are reshaping market entry strategies and product development timelines for biochemical sensor-based POCT devices.
The COVID-19 pandemic has significantly accelerated market growth, creating unprecedented demand for rapid diagnostic solutions that can be deployed outside traditional laboratory settings. This has catalyzed innovation in the biochemical sensor space, particularly for respiratory pathogens, with major manufacturers rapidly developing and commercializing new POCT platforms.
Geographically, North America currently dominates the POCT market with approximately 40% market share, followed by Europe (30%) and Asia-Pacific (20%). However, the Asia-Pacific region is expected to witness the highest growth rate over the next five years due to improving healthcare infrastructure, increasing healthcare expenditure, and growing awareness about early disease diagnosis in countries like China and India.
By application segment, glucose monitoring continues to lead the market, accounting for roughly 25% of the total POCT market value. This is followed by infectious disease testing (20%), cardiac markers (15%), and coagulation monitoring (10%). The infectious disease segment has seen particularly rapid growth since 2020, driven by COVID-19 testing requirements.
The end-user landscape is dominated by hospitals and clinics (45%), followed by home care settings (30%), and diagnostic laboratories (15%). The home care segment is projected to grow at the fastest rate as patient-centric healthcare models gain traction and technological improvements make self-testing more accessible and reliable.
Key market trends include miniaturization of testing devices, integration with digital health platforms and electronic health records, development of multiplexed testing capabilities, and increasing focus on non-invasive sampling methods. The market is also witnessing a shift toward subscription-based business models and testing-as-a-service approaches, particularly for chronic disease management applications.
Regulatory considerations remain a significant factor influencing market dynamics, with varying approval pathways across different regions creating challenges for global product launches. Recent regulatory changes, such as the EU's In Vitro Diagnostic Regulation (IVDR), are reshaping market entry strategies and product development timelines for biochemical sensor-based POCT devices.
Current Biochemical Sensing Technologies and Barriers
The biochemical sensing landscape has evolved significantly over the past decade, with point-of-care (POC) devices becoming increasingly sophisticated and accessible. Current market-ready biochemical sensors primarily utilize electrochemical, optical, piezoelectric, and thermal detection methods, each with distinct advantages in specific applications. Electrochemical sensors dominate the POC market due to their cost-effectiveness, miniaturization potential, and compatibility with various biomarkers, exemplified by glucose monitoring systems that represent approximately 70% of the global biosensor market.
Optical sensing technologies, including surface plasmon resonance (SPR) and fluorescence-based methods, offer high sensitivity but face challenges in miniaturization and cost reduction for widespread POC deployment. Recent advancements in smartphone-integrated colorimetric detection have shown promise in democratizing optical sensing technologies, though standardization remains problematic across different device cameras and lighting conditions.
Piezoelectric sensors, utilizing quartz crystal microbalance (QCM) and surface acoustic wave (SAW) principles, excel in label-free detection but struggle with specificity in complex biological samples. Meanwhile, thermal biosensors, though highly specific, remain limited by their relatively slow response times and higher power requirements, restricting their application in portable POC devices.
Despite technological advancements, significant barriers persist in biochemical sensor development for POC applications. Sample preparation remains a critical bottleneck, as many sensors require purified samples to function optimally, necessitating additional processing steps that complicate device design and user experience. The integration of sample preparation into seamless microfluidic systems represents an ongoing challenge for truly user-friendly POC devices.
Sensor stability and shelf-life present another major hurdle, particularly for enzyme-based sensors that degrade over time. Current stabilization techniques using trehalose and other polyols have extended shelf-life but often at the cost of reduced sensitivity or increased response time. Environmental factors such as temperature and humidity continue to affect sensor performance, limiting deployment in resource-constrained settings.
Cross-reactivity and interference from non-target molecules in complex biological matrices (blood, saliva, urine) significantly impact sensor specificity and reliability. While selective membranes and recognition elements have improved specificity, they often increase manufacturing complexity and cost, creating a challenging trade-off between performance and accessibility.
Regulatory compliance presents a substantial barrier to market entry, with different regions maintaining varying requirements for analytical performance, clinical validation, and manufacturing standards. The FDA's recent regulatory framework for Laboratory Developed Tests (LDTs) has introduced additional complexity for POC device manufacturers seeking market approval in the United States.
Optical sensing technologies, including surface plasmon resonance (SPR) and fluorescence-based methods, offer high sensitivity but face challenges in miniaturization and cost reduction for widespread POC deployment. Recent advancements in smartphone-integrated colorimetric detection have shown promise in democratizing optical sensing technologies, though standardization remains problematic across different device cameras and lighting conditions.
Piezoelectric sensors, utilizing quartz crystal microbalance (QCM) and surface acoustic wave (SAW) principles, excel in label-free detection but struggle with specificity in complex biological samples. Meanwhile, thermal biosensors, though highly specific, remain limited by their relatively slow response times and higher power requirements, restricting their application in portable POC devices.
Despite technological advancements, significant barriers persist in biochemical sensor development for POC applications. Sample preparation remains a critical bottleneck, as many sensors require purified samples to function optimally, necessitating additional processing steps that complicate device design and user experience. The integration of sample preparation into seamless microfluidic systems represents an ongoing challenge for truly user-friendly POC devices.
Sensor stability and shelf-life present another major hurdle, particularly for enzyme-based sensors that degrade over time. Current stabilization techniques using trehalose and other polyols have extended shelf-life but often at the cost of reduced sensitivity or increased response time. Environmental factors such as temperature and humidity continue to affect sensor performance, limiting deployment in resource-constrained settings.
Cross-reactivity and interference from non-target molecules in complex biological matrices (blood, saliva, urine) significantly impact sensor specificity and reliability. While selective membranes and recognition elements have improved specificity, they often increase manufacturing complexity and cost, creating a challenging trade-off between performance and accessibility.
Regulatory compliance presents a substantial barrier to market entry, with different regions maintaining varying requirements for analytical performance, clinical validation, and manufacturing standards. The FDA's recent regulatory framework for Laboratory Developed Tests (LDTs) has introduced additional complexity for POC device manufacturers seeking market approval in the United States.
Contemporary Biochemical Sensing Solutions
01 Electrochemical biosensors for analyte detection
Electrochemical biosensors utilize electrodes to detect biological analytes through redox reactions. These sensors measure electrical signals generated when target molecules interact with recognition elements on the electrode surface. The technology enables rapid, sensitive detection of various biomarkers, metabolites, and pathogens in medical diagnostics, environmental monitoring, and food safety applications. Advanced designs incorporate nanomaterials and specialized coatings to enhance sensitivity and selectivity.- Optical detection methods for biochemical sensors: Optical detection methods are widely used in biochemical sensors for their high sensitivity and non-invasive nature. These sensors utilize various optical phenomena such as fluorescence, absorbance, and surface plasmon resonance to detect biomolecules. The optical signals can be processed to provide quantitative measurements of target analytes. These methods often incorporate specialized light sources, detectors, and signal processing algorithms to enhance sensitivity and specificity.
- Electrochemical biosensors for analyte detection: Electrochemical biosensors convert biological recognition events into measurable electrical signals. These sensors typically consist of a biological recognition element coupled with an electrochemical transducer. They can detect various analytes including glucose, proteins, and nucleic acids with high sensitivity. The electrochemical methods employed include amperometry, potentiometry, and impedance spectroscopy, which offer advantages such as rapid response times, miniaturization potential, and compatibility with integrated circuits.
- Microfluidic systems for biochemical sensing: Microfluidic systems integrate multiple laboratory functions on a single chip for biochemical sensing applications. These systems manipulate small volumes of fluids through microchannels and chambers, enabling precise control over sample preparation, reaction conditions, and detection. The miniaturized format reduces reagent consumption, accelerates analysis times, and allows for multiplexed detection. Microfluidic biochemical sensors are particularly valuable for point-of-care diagnostics and high-throughput screening applications.
- Nanomaterial-based biochemical sensors: Nanomaterials such as quantum dots, carbon nanotubes, and metal nanoparticles are increasingly incorporated into biochemical sensors to enhance performance. These materials offer unique optical, electrical, and catalytic properties that can significantly improve sensitivity, selectivity, and response time. Nanomaterial-based sensors can detect biomolecules at extremely low concentrations, often reaching single-molecule detection limits. The high surface-to-volume ratio of nanomaterials also provides increased binding sites for target analytes.
- Wearable and implantable biochemical sensors: Wearable and implantable biochemical sensors enable continuous monitoring of physiological parameters in real-time. These sensors are designed to be minimally invasive while providing reliable measurements of various biomarkers in bodily fluids such as sweat, tears, or interstitial fluid. Advanced materials and fabrication techniques are employed to ensure biocompatibility, flexibility, and durability. These sensors often incorporate wireless communication capabilities for data transmission to external devices, facilitating remote monitoring and personalized healthcare.
02 Optical-based biochemical sensing systems
Optical biochemical sensors detect analytes through changes in optical properties such as absorbance, fluorescence, or refractive index. These systems utilize light-matter interactions to identify and quantify target molecules without requiring electrical connections. The technology encompasses various approaches including surface plasmon resonance, fluorescence spectroscopy, and colorimetric detection. Applications range from point-of-care diagnostics to environmental monitoring, offering advantages in multiplexed detection and non-invasive sensing.Expand Specific Solutions03 Microfluidic and lab-on-chip biosensor platforms
Microfluidic biosensor platforms integrate sample preparation, analyte detection, and signal processing into miniaturized devices. These lab-on-chip systems utilize microscale channels and chambers to manipulate small fluid volumes, enabling efficient biochemical reactions and analyses. The technology offers advantages including reduced reagent consumption, faster analysis times, and potential for automation. Applications include point-of-care diagnostics, personalized medicine, and environmental monitoring where portable, integrated sensing solutions are beneficial.Expand Specific Solutions04 Nanomaterial-enhanced biochemical sensors
Nanomaterial-enhanced biochemical sensors incorporate nanoscale structures such as nanoparticles, nanowires, or quantum dots to improve detection capabilities. These materials provide increased surface area, unique optical and electrical properties, and enhanced interaction with target analytes. The integration of nanomaterials enables higher sensitivity, lower detection limits, and improved selectivity in sensing applications. These advanced sensors find applications in early disease detection, environmental monitoring, and food safety where trace-level detection is critical.Expand Specific Solutions05 Wearable and implantable biochemical sensors
Wearable and implantable biochemical sensors enable continuous or on-demand monitoring of physiological parameters and biomarkers. These devices are designed for direct contact with the body, either externally (skin-mounted) or internally (subcutaneous or intravascular). The technology incorporates biocompatible materials, wireless communication capabilities, and power management systems for extended operation. Applications include glucose monitoring for diabetes management, electrolyte sensing for hydration status, and detection of various biomarkers for personalized healthcare and early disease detection.Expand Specific Solutions
Leading Manufacturers and Competitive Landscape
The biochemical sensor market for point-of-care devices is currently in a growth phase, characterized by increasing adoption across healthcare settings. The global market size is estimated at $25-30 billion with projected annual growth of 8-10% through 2027, driven by demand for rapid diagnostics and personalized medicine. Technology maturity varies significantly across sensor types, with established players like Abbott Diabetes Care, Roche Diagnostics, and Becton Dickinson dominating with mature glucose and blood chemistry platforms. Meanwhile, companies like Aviana Molecular Technologies, EnLiSense, and Magnomics are developing next-generation technologies leveraging microfluidics and smartphone connectivity. Academic institutions including University of California and Shanghai Jiao Tong University are advancing novel sensing approaches through industry partnerships with Philips and Hitachi, creating a competitive landscape balanced between established technologies and emerging innovations.
Abbott Diabetes Care, Inc.
Technical Solution: Abbott has pioneered continuous glucose monitoring (CGM) systems with their FreeStyle Libre platform, which uses electrochemical sensing technology to measure glucose levels in interstitial fluid. Their biochemical sensors employ wearable, minimally invasive filaments that last up to 14 days without requiring finger prick calibration. The technology utilizes enzyme-based glucose oxidase reactions coupled with advanced signal processing algorithms to provide real-time glucose readings. Abbott's sensors feature factory calibration and Bluetooth connectivity for seamless data transmission to smartphones. Their latest generation sensors have reduced warm-up times to under 1 hour and improved accuracy with mean absolute relative difference (MARD) values below 10%, making them comparable to traditional blood glucose meters while offering continuous monitoring capabilities.
Strengths: Exceptional user experience with no finger prick calibration required; extended 14-day wear period exceeds competitors; affordable compared to other CGM systems; compact form factor. Weaknesses: Limited to glucose monitoring only; accuracy can be affected by certain medications and physiological conditions; slight measurement lag compared to blood glucose levels.
Becton, Dickinson & Co.
Technical Solution: BD has developed an extensive portfolio of point-of-care biochemical sensing technologies centered around their BD Veritor™ and BD MAX™ systems. Their approach combines lateral flow immunoassay technology with digital image processing for rapid detection of infectious disease biomarkers. BD's biochemical sensors utilize proprietary nanoparticle-conjugated antibodies that provide enhanced sensitivity compared to traditional lateral flow tests. Their systems incorporate automated sample processing with precise microfluidic control to ensure consistent results across different operators and settings. For blood glucose monitoring, BD has pioneered ultra-fine lancet technology that significantly reduces pain during sample collection. Their recent innovations include multiplexed cartridges capable of detecting multiple respiratory pathogens from a single sample within 15 minutes, with built-in controls to verify sample adequacy and reagent functionality. BD's connectivity solutions enable wireless transmission of results to electronic medical records and public health surveillance systems.
Strengths: Exceptional manufacturing scale and quality control; extensive distribution network ensuring global availability; comprehensive regulatory approvals across multiple markets; robust design suitable for challenging environments. Weaknesses: Some systems require dedicated readers increasing total cost of ownership; limited menu of tests compared to laboratory-based systems; relatively higher per-test costs for certain assays.
Key Patents and Innovations in Biochemical Sensors
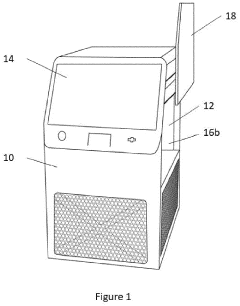
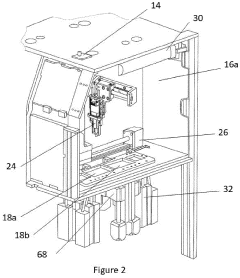
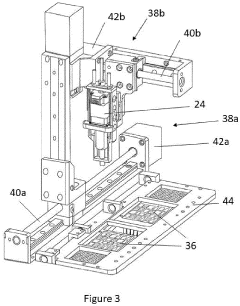
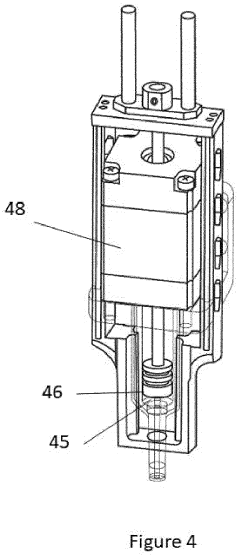
Point-of-care device for carrying out a variety of different biochemical reactions
PatentPendingEP4382915A1
Innovation
- A PoC device with a hermetically sealed housing, a robotic dispenser for liquid transfer and mixing, a thermal control unit for efficient temperature management, and an optical system for colorimetric analysis, combined with a magnetic unit for nucleic acid extraction and a UV lamp for sterilization, allowing for simultaneous and automated performance of multiple biochemical reactions.
Point-of-care multiplexing biosensor array and scalable method of manufacture
PatentPendingUS20250049359A1
Innovation
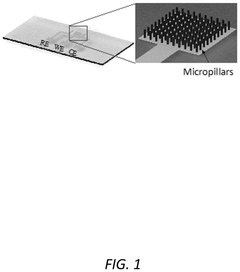
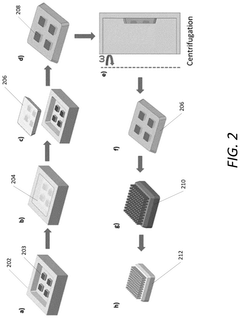
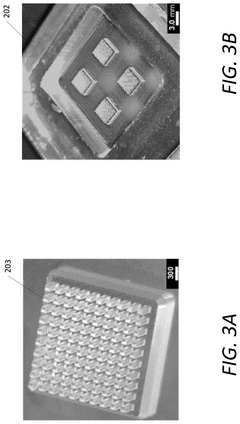
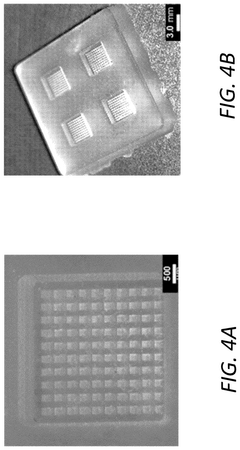
- A multiplexing and scalable electrochemical POC biosensing device is developed, utilizing micromolded electrodes in the form of arrays of micropillars, mounted on a printed circuit board (PCB), and incorporating a molded microfluidic portion for sample fluid flow, enabling rapid and quantitative detection of biomolecules.
Regulatory Framework for POC Diagnostic Devices
The regulatory landscape for Point-of-Care (POC) diagnostic devices presents a complex framework that varies significantly across global markets. In the United States, the Food and Drug Administration (FDA) classifies POC biochemical sensors under medical devices, with most falling into Class II requiring 510(k) clearance or Class III requiring premarket approval (PMA). The regulatory pathway depends on the intended use, risk profile, and novelty of the technology employed in the sensor system.
The European Union has implemented the In Vitro Diagnostic Regulation (IVDR 2017/746), which replaced the previous In Vitro Diagnostic Directive (IVDD) with a transition period ending in 2022. This regulation introduces more stringent requirements for clinical evidence, post-market surveillance, and risk classification. POC devices are categorized into four risk classes (A, B, C, D), with biochemical sensors typically falling into classes B or C depending on the analytes measured and clinical implications.
In Asia, regulatory frameworks show considerable heterogeneity. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) requires thorough clinical validation and quality management systems. China's National Medical Products Administration (NMPA) has recently strengthened its regulatory oversight, implementing a classification-based system similar to the FDA but with unique local requirements for clinical trials and manufacturing standards.
Regulatory compliance for biochemical sensors in POC devices necessitates adherence to several international standards, including ISO 13485 for quality management systems, ISO 14971 for risk management, and IEC 61010 for safety requirements. Additionally, the Clinical Laboratory Improvement Amendments (CLIA) in the US categorizes tests based on complexity, with many POC devices designed to meet CLIA-waived status to enable broader deployment outside traditional laboratory settings.
Data privacy and security regulations have emerged as critical considerations in the regulatory framework, particularly for connected POC devices that transmit patient data. Compliance with regulations such as HIPAA in the US, GDPR in Europe, and equivalent frameworks in other regions is mandatory for market access. These regulations impose strict requirements on data encryption, patient consent, and breach notification protocols.
Reimbursement pathways represent another regulatory hurdle for POC diagnostic devices. In the US, obtaining Current Procedural Terminology (CPT) codes and favorable coverage determinations from the Centers for Medicare & Medicaid Services (CMS) is essential for commercial viability. Similar reimbursement challenges exist in other markets, with health technology assessment bodies evaluating clinical utility and cost-effectiveness before approving coverage.
The COVID-19 pandemic has prompted regulatory authorities worldwide to implement emergency use authorization (EUA) pathways, accelerating the approval process for POC diagnostic devices. While these temporary measures have facilitated rapid market entry, manufacturers must prepare for the transition to standard regulatory pathways as emergency provisions are phased out.
The European Union has implemented the In Vitro Diagnostic Regulation (IVDR 2017/746), which replaced the previous In Vitro Diagnostic Directive (IVDD) with a transition period ending in 2022. This regulation introduces more stringent requirements for clinical evidence, post-market surveillance, and risk classification. POC devices are categorized into four risk classes (A, B, C, D), with biochemical sensors typically falling into classes B or C depending on the analytes measured and clinical implications.
In Asia, regulatory frameworks show considerable heterogeneity. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) requires thorough clinical validation and quality management systems. China's National Medical Products Administration (NMPA) has recently strengthened its regulatory oversight, implementing a classification-based system similar to the FDA but with unique local requirements for clinical trials and manufacturing standards.
Regulatory compliance for biochemical sensors in POC devices necessitates adherence to several international standards, including ISO 13485 for quality management systems, ISO 14971 for risk management, and IEC 61010 for safety requirements. Additionally, the Clinical Laboratory Improvement Amendments (CLIA) in the US categorizes tests based on complexity, with many POC devices designed to meet CLIA-waived status to enable broader deployment outside traditional laboratory settings.
Data privacy and security regulations have emerged as critical considerations in the regulatory framework, particularly for connected POC devices that transmit patient data. Compliance with regulations such as HIPAA in the US, GDPR in Europe, and equivalent frameworks in other regions is mandatory for market access. These regulations impose strict requirements on data encryption, patient consent, and breach notification protocols.
Reimbursement pathways represent another regulatory hurdle for POC diagnostic devices. In the US, obtaining Current Procedural Terminology (CPT) codes and favorable coverage determinations from the Centers for Medicare & Medicaid Services (CMS) is essential for commercial viability. Similar reimbursement challenges exist in other markets, with health technology assessment bodies evaluating clinical utility and cost-effectiveness before approving coverage.
The COVID-19 pandemic has prompted regulatory authorities worldwide to implement emergency use authorization (EUA) pathways, accelerating the approval process for POC diagnostic devices. While these temporary measures have facilitated rapid market entry, manufacturers must prepare for the transition to standard regulatory pathways as emergency provisions are phased out.
Clinical Validation and Performance Metrics
Clinical validation represents a critical phase in the development and market readiness assessment of biochemical sensors for point-of-care (POC) devices. The validation process typically involves rigorous testing in clinical settings to evaluate sensor performance against established laboratory methods. Current industry standards require sensitivity levels of 95% or higher and specificity rates exceeding 90% for most FDA-approved POC diagnostic devices.
Performance metrics for biochemical sensors in POC applications encompass several key parameters. Analytical sensitivity, defined as the lowest detectable concentration of an analyte, varies significantly across market-ready devices, with glucose sensors achieving detection limits as low as 0.5 mg/dL while protein biomarker sensors typically operate in the ng/mL range. Precision metrics, expressed as coefficients of variation (CV), generally fall below 5% for market-leading devices, with elite systems achieving CVs under 2% for repeated measurements.
Response time represents another crucial performance indicator, with current market-ready sensors demonstrating significant improvements. Modern electrochemical glucose sensors deliver results in 5-10 seconds, while more complex immunoassay-based POC devices require 10-20 minutes for complete analysis. This temporal efficiency directly impacts clinical workflow integration and patient management decisions.
Stability characteristics, including shelf-life and operational stability, have seen substantial enhancement through advanced materials and preservation technologies. Leading POC biochemical sensors now maintain calibration integrity for 12-24 months under recommended storage conditions, with operational stability during use ranging from 7-30 days depending on sensor type and environmental exposure.
Clinical correlation studies reveal varying degrees of agreement between POC biochemical sensors and reference laboratory methods. Correlation coefficients (r) typically range from 0.85 to 0.98, with higher-end devices approaching the performance of central laboratory analyzers. Bland-Altman analyses demonstrate mean biases under 5% for glucose and electrolyte sensors, while more complex biomarker sensors may exhibit biases of 8-15% compared to reference methods.
Regulatory bodies have established performance requirements that vary by analyte and intended use. The FDA's CLIA waiver requirements stipulate that POC devices must demonstrate 95% agreement with reference methods within defined clinical decision ranges. Similarly, the European Union's IVDR framework mandates comprehensive clinical evidence and performance evaluation data for biochemical sensors in POC applications, with particular emphasis on clinical utility and risk-benefit assessment.
Performance metrics for biochemical sensors in POC applications encompass several key parameters. Analytical sensitivity, defined as the lowest detectable concentration of an analyte, varies significantly across market-ready devices, with glucose sensors achieving detection limits as low as 0.5 mg/dL while protein biomarker sensors typically operate in the ng/mL range. Precision metrics, expressed as coefficients of variation (CV), generally fall below 5% for market-leading devices, with elite systems achieving CVs under 2% for repeated measurements.
Response time represents another crucial performance indicator, with current market-ready sensors demonstrating significant improvements. Modern electrochemical glucose sensors deliver results in 5-10 seconds, while more complex immunoassay-based POC devices require 10-20 minutes for complete analysis. This temporal efficiency directly impacts clinical workflow integration and patient management decisions.
Stability characteristics, including shelf-life and operational stability, have seen substantial enhancement through advanced materials and preservation technologies. Leading POC biochemical sensors now maintain calibration integrity for 12-24 months under recommended storage conditions, with operational stability during use ranging from 7-30 days depending on sensor type and environmental exposure.
Clinical correlation studies reveal varying degrees of agreement between POC biochemical sensors and reference laboratory methods. Correlation coefficients (r) typically range from 0.85 to 0.98, with higher-end devices approaching the performance of central laboratory analyzers. Bland-Altman analyses demonstrate mean biases under 5% for glucose and electrolyte sensors, while more complex biomarker sensors may exhibit biases of 8-15% compared to reference methods.
Regulatory bodies have established performance requirements that vary by analyte and intended use. The FDA's CLIA waiver requirements stipulate that POC devices must demonstrate 95% agreement with reference methods within defined clinical decision ranges. Similarly, the European Union's IVDR framework mandates comprehensive clinical evidence and performance evaluation data for biochemical sensors in POC applications, with particular emphasis on clinical utility and risk-benefit assessment.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!