Point-of-care Devices and Their Role in Population Health Analytics
SEP 19, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Point-of-care Testing Evolution and Objectives
Point-of-care testing (POCT) has evolved significantly over the past several decades, transforming from basic diagnostic tools to sophisticated systems capable of delivering laboratory-quality results at or near the patient's location. The evolution began in the 1960s with simple glucose testing strips, progressing through the 1980s with the introduction of handheld blood analyzers, and accelerating in the 2000s with the integration of digital technologies and connectivity features.
The technological progression of POCT devices has been driven by miniaturization of analytical components, advancements in microfluidics, improvements in biosensor technology, and the integration of data processing capabilities. Modern POCT devices incorporate sophisticated sample preparation, precise analyte detection, and automated result interpretation, all within compact, user-friendly formats accessible to healthcare professionals with minimal specialized training.
A pivotal shift in POCT evolution occurred with the emergence of connectivity features, enabling these devices to transmit results directly to electronic health records and population health databases. This connectivity represents a crucial bridge between individual diagnostic events and broader population health analytics, transforming isolated clinical data points into valuable epidemiological insights.
The primary objectives of contemporary POCT development focus on enhancing accessibility, improving accuracy, reducing cost, and expanding the range of testable analytes. Accessibility improvements target both geographic reach—bringing diagnostic capabilities to remote or underserved areas—and temporal availability, enabling immediate testing regardless of laboratory operating hours. Accuracy objectives center on achieving performance metrics comparable to central laboratory testing while maintaining the convenience of point-of-care delivery.
From a population health perspective, POCT objectives have expanded to include real-time disease surveillance, identification of emerging health trends, and support for preventive healthcare initiatives. These devices aim to facilitate early detection of disease outbreaks, monitor chronic disease prevalence, and evaluate the effectiveness of public health interventions across diverse populations.
The integration of artificial intelligence and machine learning algorithms represents the newest frontier in POCT evolution, with objectives focused on pattern recognition, predictive analytics, and decision support capabilities. These advanced features aim to transform raw diagnostic data into actionable clinical insights, supporting both individual treatment decisions and population-level health management strategies.
Looking forward, POCT development objectives increasingly emphasize interoperability with broader healthcare information systems, enabling seamless data exchange between diagnostic devices, electronic health records, and population health analytics platforms. This interconnectedness represents a critical step toward realizing the full potential of POCT in supporting comprehensive, data-driven approaches to both individual and community health management.
The technological progression of POCT devices has been driven by miniaturization of analytical components, advancements in microfluidics, improvements in biosensor technology, and the integration of data processing capabilities. Modern POCT devices incorporate sophisticated sample preparation, precise analyte detection, and automated result interpretation, all within compact, user-friendly formats accessible to healthcare professionals with minimal specialized training.
A pivotal shift in POCT evolution occurred with the emergence of connectivity features, enabling these devices to transmit results directly to electronic health records and population health databases. This connectivity represents a crucial bridge between individual diagnostic events and broader population health analytics, transforming isolated clinical data points into valuable epidemiological insights.
The primary objectives of contemporary POCT development focus on enhancing accessibility, improving accuracy, reducing cost, and expanding the range of testable analytes. Accessibility improvements target both geographic reach—bringing diagnostic capabilities to remote or underserved areas—and temporal availability, enabling immediate testing regardless of laboratory operating hours. Accuracy objectives center on achieving performance metrics comparable to central laboratory testing while maintaining the convenience of point-of-care delivery.
From a population health perspective, POCT objectives have expanded to include real-time disease surveillance, identification of emerging health trends, and support for preventive healthcare initiatives. These devices aim to facilitate early detection of disease outbreaks, monitor chronic disease prevalence, and evaluate the effectiveness of public health interventions across diverse populations.
The integration of artificial intelligence and machine learning algorithms represents the newest frontier in POCT evolution, with objectives focused on pattern recognition, predictive analytics, and decision support capabilities. These advanced features aim to transform raw diagnostic data into actionable clinical insights, supporting both individual treatment decisions and population-level health management strategies.
Looking forward, POCT development objectives increasingly emphasize interoperability with broader healthcare information systems, enabling seamless data exchange between diagnostic devices, electronic health records, and population health analytics platforms. This interconnectedness represents a critical step toward realizing the full potential of POCT in supporting comprehensive, data-driven approaches to both individual and community health management.
Market Analysis for POC Health Analytics Solutions
The global Point-of-Care (POC) health analytics market is experiencing robust growth, driven by increasing demand for real-time patient monitoring and data-driven healthcare decision making. Current market valuations place this sector at approximately $18.8 billion in 2023, with projections indicating a compound annual growth rate of 12.7% through 2030. This growth trajectory is significantly outpacing traditional healthcare IT segments, reflecting the strategic importance of POC analytics in modern healthcare delivery systems.
North America currently dominates the market landscape, accounting for roughly 42% of global market share, followed by Europe at 28% and Asia-Pacific at 21%. The remaining regions collectively represent about 9% of the market. This regional distribution correlates strongly with healthcare infrastructure development and technology adoption rates across different geographies.
Market segmentation reveals distinct categories within the POC health analytics space. Hardware components, including portable diagnostic devices and wearable sensors, constitute approximately 35% of the market. Software solutions, encompassing data analytics platforms and integration systems, represent 40%. Services, including implementation support and data management, make up the remaining 25%. This distribution highlights the increasing importance of software capabilities in extracting actionable insights from POC devices.
Key market drivers include the rising prevalence of chronic diseases requiring continuous monitoring, growing patient preference for home-based care, and healthcare systems' push toward preventive and value-based care models. Additionally, technological advancements in miniaturization, connectivity, and artificial intelligence are enabling more sophisticated analytics capabilities in compact POC devices.
Significant market restraints include concerns regarding data security and privacy, interoperability challenges with existing healthcare IT infrastructure, and varying regulatory frameworks across different regions. Reimbursement uncertainties also present obstacles to widespread adoption, particularly in emerging markets where healthcare funding models are still evolving.
The competitive landscape features both established medical device manufacturers expanding into analytics and specialized health IT companies developing POC-specific solutions. Recent market consolidation through mergers and acquisitions indicates strategic positioning by major players to offer comprehensive POC analytics ecosystems rather than standalone products.
Emerging market opportunities include integration with telehealth platforms, expansion of consumer-grade health monitoring devices with clinical-grade analytics, and development of population health management solutions leveraging aggregated POC data. The COVID-19 pandemic has accelerated market growth by highlighting the value of decentralized testing and remote monitoring capabilities, creating a favorable environment for continued innovation and investment in this sector.
North America currently dominates the market landscape, accounting for roughly 42% of global market share, followed by Europe at 28% and Asia-Pacific at 21%. The remaining regions collectively represent about 9% of the market. This regional distribution correlates strongly with healthcare infrastructure development and technology adoption rates across different geographies.
Market segmentation reveals distinct categories within the POC health analytics space. Hardware components, including portable diagnostic devices and wearable sensors, constitute approximately 35% of the market. Software solutions, encompassing data analytics platforms and integration systems, represent 40%. Services, including implementation support and data management, make up the remaining 25%. This distribution highlights the increasing importance of software capabilities in extracting actionable insights from POC devices.
Key market drivers include the rising prevalence of chronic diseases requiring continuous monitoring, growing patient preference for home-based care, and healthcare systems' push toward preventive and value-based care models. Additionally, technological advancements in miniaturization, connectivity, and artificial intelligence are enabling more sophisticated analytics capabilities in compact POC devices.
Significant market restraints include concerns regarding data security and privacy, interoperability challenges with existing healthcare IT infrastructure, and varying regulatory frameworks across different regions. Reimbursement uncertainties also present obstacles to widespread adoption, particularly in emerging markets where healthcare funding models are still evolving.
The competitive landscape features both established medical device manufacturers expanding into analytics and specialized health IT companies developing POC-specific solutions. Recent market consolidation through mergers and acquisitions indicates strategic positioning by major players to offer comprehensive POC analytics ecosystems rather than standalone products.
Emerging market opportunities include integration with telehealth platforms, expansion of consumer-grade health monitoring devices with clinical-grade analytics, and development of population health management solutions leveraging aggregated POC data. The COVID-19 pandemic has accelerated market growth by highlighting the value of decentralized testing and remote monitoring capabilities, creating a favorable environment for continued innovation and investment in this sector.
Technical Challenges in POC Device Implementation
Despite the promising potential of Point-of-Care (POC) devices in population health analytics, their implementation faces numerous technical challenges that must be addressed for widespread adoption. One of the primary obstacles is the miniaturization of complex diagnostic technologies while maintaining accuracy and reliability. Engineers must balance the need for portable, user-friendly devices with the requirement for laboratory-grade precision, often requiring innovative approaches to microfluidics, biosensors, and signal processing.
Data integration presents another significant hurdle, as POC devices must seamlessly connect with existing healthcare information systems. The lack of standardized communication protocols and data formats creates interoperability issues, preventing the smooth flow of information between POC devices and electronic health records. This fragmentation limits the potential for comprehensive population health analytics and hinders the creation of unified patient profiles.
Power management remains a critical challenge, particularly for devices deployed in resource-limited settings. Many advanced POC technologies require substantial energy for operation, creating a tension between functionality and battery life. While renewable energy solutions offer potential alternatives, their integration into medical devices introduces additional complexity in terms of reliability and regulatory compliance.
Calibration and quality control represent ongoing technical concerns that impact the trustworthiness of POC-generated data. Unlike controlled laboratory environments, POC devices operate in diverse settings with varying environmental conditions that can affect measurement accuracy. Developing robust calibration methods that can function reliably across different contexts without requiring technical expertise from end-users remains an engineering challenge.
Cybersecurity vulnerabilities pose increasing risks as POC devices become more connected. The transmission of sensitive health data requires sophisticated encryption and authentication mechanisms, yet these must be implemented within the constraints of limited computing resources and power availability. Balancing security requirements with usability and performance is particularly challenging for devices intended for non-specialist users.
Manufacturing scalability presents another barrier, as many innovative POC technologies rely on specialized materials or fabrication processes that are difficult to scale economically. The transition from laboratory prototypes to mass-produced devices often requires significant redesign to accommodate manufacturing constraints while preserving diagnostic performance.
Regulatory compliance adds another layer of complexity, with different regions maintaining distinct requirements for medical device approval. POC devices intended for global deployment must navigate these varying standards, which can significantly impact design decisions and technical specifications, particularly for devices incorporating novel sensing technologies or analytical methods.
Data integration presents another significant hurdle, as POC devices must seamlessly connect with existing healthcare information systems. The lack of standardized communication protocols and data formats creates interoperability issues, preventing the smooth flow of information between POC devices and electronic health records. This fragmentation limits the potential for comprehensive population health analytics and hinders the creation of unified patient profiles.
Power management remains a critical challenge, particularly for devices deployed in resource-limited settings. Many advanced POC technologies require substantial energy for operation, creating a tension between functionality and battery life. While renewable energy solutions offer potential alternatives, their integration into medical devices introduces additional complexity in terms of reliability and regulatory compliance.
Calibration and quality control represent ongoing technical concerns that impact the trustworthiness of POC-generated data. Unlike controlled laboratory environments, POC devices operate in diverse settings with varying environmental conditions that can affect measurement accuracy. Developing robust calibration methods that can function reliably across different contexts without requiring technical expertise from end-users remains an engineering challenge.
Cybersecurity vulnerabilities pose increasing risks as POC devices become more connected. The transmission of sensitive health data requires sophisticated encryption and authentication mechanisms, yet these must be implemented within the constraints of limited computing resources and power availability. Balancing security requirements with usability and performance is particularly challenging for devices intended for non-specialist users.
Manufacturing scalability presents another barrier, as many innovative POC technologies rely on specialized materials or fabrication processes that are difficult to scale economically. The transition from laboratory prototypes to mass-produced devices often requires significant redesign to accommodate manufacturing constraints while preserving diagnostic performance.
Regulatory compliance adds another layer of complexity, with different regions maintaining distinct requirements for medical device approval. POC devices intended for global deployment must navigate these varying standards, which can significantly impact design decisions and technical specifications, particularly for devices incorporating novel sensing technologies or analytical methods.
Current POC Data Integration Architectures
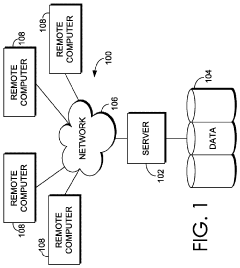
01 Integration of diagnostic capabilities in point-of-care devices
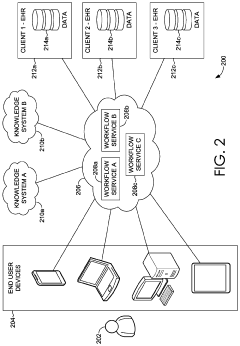
Point-of-care devices can be integrated with diagnostic capabilities to provide immediate health analytics at the patient's location. These devices enable healthcare providers to perform tests and obtain results quickly, facilitating timely decision-making. The integration of diagnostic technologies in portable devices allows for real-time monitoring and analysis of patient data, improving the efficiency of healthcare delivery and patient outcomes.- Integration of diagnostic capabilities in point-of-care devices: Point-of-care devices can be integrated with diagnostic capabilities to provide immediate health analytics at the patient's location. These devices enable healthcare providers to perform tests and obtain results quickly, facilitating timely decision-making. The integration of diagnostic technologies in portable devices allows for real-time monitoring and analysis of patient data, improving the efficiency of healthcare delivery and patient outcomes.
- Data management systems for health analytics: Health analytics platforms incorporate data management systems that collect, store, and analyze patient information from point-of-care devices. These systems enable healthcare providers to access comprehensive patient data, identify trends, and make informed decisions. The integration of data management capabilities in health analytics platforms facilitates the efficient handling of large volumes of medical data, supporting evidence-based healthcare practices and personalized treatment approaches.
- Remote monitoring and telemedicine solutions: Point-of-care devices can be equipped with remote monitoring capabilities, allowing healthcare providers to track patient health metrics from a distance. These telemedicine solutions enable continuous monitoring of patients' conditions, early detection of health issues, and timely interventions. The integration of remote monitoring technologies in health analytics platforms supports the delivery of healthcare services outside traditional clinical settings, improving access to care and patient convenience.
- Predictive analytics and decision support systems: Health analytics platforms incorporate predictive analytics and decision support systems that analyze data from point-of-care devices to forecast health outcomes and guide clinical decisions. These systems use advanced algorithms to identify patterns in patient data, predict disease progression, and recommend appropriate interventions. The integration of predictive analytics in healthcare supports proactive management of patient conditions, optimization of treatment plans, and improved resource allocation.
- Electronic health record integration and interoperability: Point-of-care devices and health analytics platforms can be integrated with electronic health record (EHR) systems to ensure seamless data exchange and comprehensive patient information management. This interoperability enables healthcare providers to access and update patient records in real-time, facilitating coordinated care across different healthcare settings. The integration of point-of-care data with EHR systems supports continuity of care, reduces duplication of tests, and improves the overall quality of healthcare delivery.
02 Data management systems for health analytics
Health analytics platforms incorporate data management systems that collect, store, and analyze patient information from point-of-care devices. These systems enable healthcare providers to track patient health trends, identify patterns, and make data-driven decisions. By centralizing health data from various sources, these platforms facilitate comprehensive analysis and reporting, supporting both individual patient care and population health management.Expand Specific Solutions03 Remote monitoring and telemedicine integration
Point-of-care devices can be designed with remote monitoring capabilities that transmit health data to centralized analytics platforms. This integration enables healthcare providers to monitor patients remotely, reducing the need for in-person visits while maintaining quality care. Telemedicine features allow for virtual consultations based on the collected data, making healthcare more accessible, especially for patients in remote areas or with mobility limitations.Expand Specific Solutions04 Predictive analytics and clinical decision support
Health analytics systems incorporate predictive algorithms that analyze data from point-of-care devices to forecast potential health issues before they become critical. These systems provide clinical decision support by offering evidence-based recommendations to healthcare providers. By leveraging machine learning and artificial intelligence, these platforms can identify risk factors, suggest interventions, and help optimize treatment plans based on individual patient data.Expand Specific Solutions05 Healthcare resource management and cost optimization
Point-of-care health analytics systems help optimize healthcare resource allocation and reduce costs through efficient patient management. These systems enable healthcare facilities to track resource utilization, identify inefficiencies, and implement cost-saving measures. By providing insights into patient flow, treatment outcomes, and operational metrics, these analytics platforms support strategic decision-making and help healthcare organizations improve their financial performance while maintaining quality care.Expand Specific Solutions
Leading Manufacturers and Healthcare Integrators
Point-of-care (POC) devices for population health analytics are in a growth phase, with the market expanding rapidly due to increasing demand for real-time diagnostics and remote patient monitoring. The global POC diagnostics market is projected to reach significant value as healthcare systems prioritize decentralized testing. Leading players like Abbott Point of Care, Roche Diagnostics, and Becton, Dickinson & Co. have established mature technologies, while Philips, T2 Biosystems, and Hemex Health are advancing innovative solutions. Companies like IBM and Intel are integrating data analytics capabilities, transforming these devices from simple diagnostic tools into comprehensive health analytics platforms. The competitive landscape is characterized by increasing consolidation and strategic partnerships to enhance technological capabilities and market reach.
Abbott Point of Care, Inc.
Technical Solution: Abbott Point of Care has developed the i-STAT system, a handheld blood analyzer that provides real-time, lab-quality results within minutes at the patient's bedside. The system integrates advanced biosensor technology with sophisticated data management capabilities to deliver immediate diagnostic information. Their i-STAT Alinity platform further enhances this capability by connecting to hospital information systems via wireless networking, enabling seamless integration of point-of-care testing results with electronic health records. This connectivity allows for real-time population health analytics by aggregating data across patient populations. The system includes comprehensive quality management features and remote monitoring capabilities that enable healthcare organizations to track testing patterns, identify disease trends, and implement targeted interventions based on aggregated data[1][3]. Abbott has also developed NAVICA, a mobile app that pairs with their ID NOW COVID-19 rapid test, demonstrating how point-of-care testing can be linked to digital platforms for broader population health monitoring and management.
Strengths: Provides immediate actionable results that reduce time to treatment decisions; offers wireless connectivity for real-time data integration with healthcare systems; supports comprehensive quality management and compliance tracking. Weaknesses: Relatively higher cost per test compared to centralized laboratory testing; requires regular calibration and quality control; limited test menu compared to full laboratory capabilities.
Koninklijke Philips NV
Technical Solution: Philips has developed an integrated approach to point-of-care diagnostics through their Patient Monitoring and Analytics solutions. Their system combines bedside monitoring devices with advanced analytics platforms that collect and analyze patient data in real-time. The Philips IntelliVue Guardian Solution with Early Warning Scoring (EWS) automatically acquires vital signs and calculates risk scores to identify deteriorating patients before critical events occur. This technology integrates with their HealthSuite Digital Platform, which aggregates data across devices and care settings to provide population-level insights. Philips' point-of-care ultrasound devices, such as Lumify, connect to standard smartphones and tablets, allowing clinicians to perform diagnostic imaging at the bedside and immediately share results through secure cloud connections. Their eICU Program enables remote monitoring of ICU patients across multiple hospitals, creating virtual care networks that can analyze patterns across large patient populations[2][5]. Philips has also developed specific analytics tools that identify high-risk patients within populations and track outcomes of interventions, supporting value-based care initiatives.
Strengths: Comprehensive ecosystem approach integrating multiple diagnostic modalities with analytics platforms; strong interoperability with existing hospital systems; advanced predictive algorithms for early intervention. Weaknesses: Complex implementation requiring significant IT infrastructure; higher initial investment costs; potential challenges in data standardization across diverse care settings and devices.
Key Patents in POC Analytics Technology
Analytics at the point of care
PatentActiveUS11355222B2
Innovation
- Integrating multi-source data analytics into clinical decision workflows by aggregating data from knowledge systems and electronic health records (EHRs) to provide data-driven recommendations to clinicians at the point of care, allowing for informed decision-making and enabling feedback to improve future recommendations.
Patent
Innovation
- Integration of real-time data collection and analysis capabilities in point-of-care devices, enabling immediate clinical decision support at the patient care site.
- Development of machine learning algorithms that can identify population health trends from aggregated point-of-care testing data, facilitating early detection of disease outbreaks and public health concerns.
- Implementation of secure, HIPAA-compliant data transmission protocols that protect patient privacy while enabling valuable population health insights from distributed point-of-care testing locations.
Regulatory Framework for POC Health Analytics
The regulatory landscape for Point-of-Care (POC) health analytics devices presents a complex framework that varies significantly across global jurisdictions. In the United States, the Food and Drug Administration (FDA) classifies POC devices based on risk levels, with most diagnostic devices falling under Class II (moderate risk) requiring 510(k) clearance or Class III (high risk) requiring premarket approval (PMA). The FDA has recently introduced the Digital Health Software Precertification Program, specifically addressing software components in POC analytics systems.
The European Union implements the In Vitro Diagnostic Regulation (IVDR 2017/746), which replaced the previous directive in May 2022, introducing more stringent requirements for clinical evidence, post-market surveillance, and risk classification. This regulation particularly impacts POC devices that incorporate AI algorithms for population health analytics, requiring manufacturers to demonstrate ongoing validation of their analytical systems.
In emerging markets, regulatory frameworks are evolving rapidly but remain inconsistent. China's National Medical Products Administration (NMPA) has established specific pathways for innovative medical devices, while India's Central Drugs Standard Control Organization (CDSCO) is developing frameworks that balance innovation with safety concerns in resource-limited settings.
Data privacy regulations significantly impact POC analytics systems that process population health data. The General Data Protection Regulation (GDPR) in Europe, the Health Insurance Portability and Accountability Act (HIPAA) in the US, and similar regulations worldwide impose strict requirements on data collection, storage, processing, and sharing. These regulations necessitate robust data governance frameworks within POC analytics systems, particularly those that aggregate population-level insights.
Interoperability standards represent another critical regulatory consideration. The Fast Healthcare Interoperability Resources (FHIR) standard, promoted by regulatory bodies globally, facilitates seamless data exchange between POC devices and broader healthcare information systems. Compliance with these standards is increasingly becoming a regulatory requirement rather than an optional feature.
Regulatory bodies are also addressing the unique challenges posed by AI and machine learning in POC analytics. The FDA's proposed regulatory framework for AI/ML-based Software as a Medical Device (SaMD) introduces the concept of "predetermined change control plans" that allow for algorithm updates while maintaining regulatory compliance. This approach acknowledges the iterative nature of AI systems used in population health analytics.
The global harmonization of regulations through initiatives like the International Medical Device Regulators Forum (IMDRF) is gradually creating more consistent pathways for POC analytics devices, though significant regional variations persist that manufacturers must navigate carefully when deploying solutions across multiple markets.
The European Union implements the In Vitro Diagnostic Regulation (IVDR 2017/746), which replaced the previous directive in May 2022, introducing more stringent requirements for clinical evidence, post-market surveillance, and risk classification. This regulation particularly impacts POC devices that incorporate AI algorithms for population health analytics, requiring manufacturers to demonstrate ongoing validation of their analytical systems.
In emerging markets, regulatory frameworks are evolving rapidly but remain inconsistent. China's National Medical Products Administration (NMPA) has established specific pathways for innovative medical devices, while India's Central Drugs Standard Control Organization (CDSCO) is developing frameworks that balance innovation with safety concerns in resource-limited settings.
Data privacy regulations significantly impact POC analytics systems that process population health data. The General Data Protection Regulation (GDPR) in Europe, the Health Insurance Portability and Accountability Act (HIPAA) in the US, and similar regulations worldwide impose strict requirements on data collection, storage, processing, and sharing. These regulations necessitate robust data governance frameworks within POC analytics systems, particularly those that aggregate population-level insights.
Interoperability standards represent another critical regulatory consideration. The Fast Healthcare Interoperability Resources (FHIR) standard, promoted by regulatory bodies globally, facilitates seamless data exchange between POC devices and broader healthcare information systems. Compliance with these standards is increasingly becoming a regulatory requirement rather than an optional feature.
Regulatory bodies are also addressing the unique challenges posed by AI and machine learning in POC analytics. The FDA's proposed regulatory framework for AI/ML-based Software as a Medical Device (SaMD) introduces the concept of "predetermined change control plans" that allow for algorithm updates while maintaining regulatory compliance. This approach acknowledges the iterative nature of AI systems used in population health analytics.
The global harmonization of regulations through initiatives like the International Medical Device Regulators Forum (IMDRF) is gradually creating more consistent pathways for POC analytics devices, though significant regional variations persist that manufacturers must navigate carefully when deploying solutions across multiple markets.
Data Security and Privacy Considerations
The integration of point-of-care (POC) devices into population health analytics introduces significant data security and privacy challenges that must be addressed comprehensively. As these devices collect sensitive health information from individuals across diverse populations, protecting this data becomes paramount for maintaining trust and compliance with regulatory frameworks.
Healthcare data collected through POC devices falls under stringent regulations such as HIPAA in the United States, GDPR in Europe, and similar frameworks globally. These regulations mandate specific requirements for data encryption, storage, transmission, and access controls. Organizations deploying POC devices must implement robust compliance programs that address these varying international standards while maintaining operational efficiency.
End-to-end encryption represents a critical security measure for POC devices, ensuring that data remains protected from the point of collection through transmission and storage. Advanced encryption protocols must be implemented at both hardware and software levels, with particular attention to securing data during wireless transmission—often a vulnerable point in the data lifecycle. Multi-factor authentication and role-based access controls further strengthen the security architecture by limiting data access to authorized personnel only.
The distributed nature of POC device networks creates unique security challenges. Unlike centralized hospital systems, these devices operate across diverse environments with varying security infrastructures. This necessitates the development of adaptive security protocols that can function effectively across different network conditions while maintaining consistent protection standards. Edge computing solutions that process sensitive data locally before transmission can significantly reduce vulnerability exposure.
De-identification and anonymization techniques play crucial roles in balancing individual privacy with population health analytics requirements. Advanced methods such as differential privacy, k-anonymity, and synthetic data generation allow meaningful population-level insights while protecting individual identities. However, these techniques must be carefully calibrated to preserve analytical utility without compromising privacy guarantees.
Patient consent management presents another complex challenge in the POC ecosystem. Dynamic consent models that allow individuals to control how their data is used across different research and analytical contexts are increasingly important. These systems must be transparent, user-friendly, and capable of managing consent preferences across multiple devices and data repositories.
As artificial intelligence becomes more integrated with POC analytics, additional privacy considerations emerge. Machine learning models can potentially memorize training data, creating risks of information leakage. Privacy-preserving AI techniques such as federated learning—where models are trained across distributed devices without centralizing sensitive data—offer promising solutions for maintaining both analytical power and privacy protection in population health applications.
Healthcare data collected through POC devices falls under stringent regulations such as HIPAA in the United States, GDPR in Europe, and similar frameworks globally. These regulations mandate specific requirements for data encryption, storage, transmission, and access controls. Organizations deploying POC devices must implement robust compliance programs that address these varying international standards while maintaining operational efficiency.
End-to-end encryption represents a critical security measure for POC devices, ensuring that data remains protected from the point of collection through transmission and storage. Advanced encryption protocols must be implemented at both hardware and software levels, with particular attention to securing data during wireless transmission—often a vulnerable point in the data lifecycle. Multi-factor authentication and role-based access controls further strengthen the security architecture by limiting data access to authorized personnel only.
The distributed nature of POC device networks creates unique security challenges. Unlike centralized hospital systems, these devices operate across diverse environments with varying security infrastructures. This necessitates the development of adaptive security protocols that can function effectively across different network conditions while maintaining consistent protection standards. Edge computing solutions that process sensitive data locally before transmission can significantly reduce vulnerability exposure.
De-identification and anonymization techniques play crucial roles in balancing individual privacy with population health analytics requirements. Advanced methods such as differential privacy, k-anonymity, and synthetic data generation allow meaningful population-level insights while protecting individual identities. However, these techniques must be carefully calibrated to preserve analytical utility without compromising privacy guarantees.
Patient consent management presents another complex challenge in the POC ecosystem. Dynamic consent models that allow individuals to control how their data is used across different research and analytical contexts are increasingly important. These systems must be transparent, user-friendly, and capable of managing consent preferences across multiple devices and data repositories.
As artificial intelligence becomes more integrated with POC analytics, additional privacy considerations emerge. Machine learning models can potentially memorize training data, creating risks of information leakage. Privacy-preserving AI techniques such as federated learning—where models are trained across distributed devices without centralizing sensitive data—offer promising solutions for maintaining both analytical power and privacy protection in population health applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!