Point-of-care Device Anode Materials in Cancer Screening
SEP 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cancer Screening POC Device Anode Materials Background and Objectives
Point-of-care (POC) cancer screening devices represent a revolutionary approach to early cancer detection, offering rapid, accessible, and cost-effective diagnostic capabilities outside traditional laboratory settings. The evolution of these devices has been marked by significant technological advancements over the past two decades, transitioning from basic immunoassay platforms to sophisticated electrochemical biosensors capable of detecting multiple cancer biomarkers simultaneously with high sensitivity and specificity.
The anode materials used in these electrochemical POC devices play a crucial role in determining device performance, sensitivity, and reliability. Historically, conventional materials such as gold, platinum, and carbon-based electrodes dominated early POC device development. However, limitations in sensitivity, biocompatibility, and manufacturing scalability necessitated exploration of alternative materials and composite structures.
Recent technological trends indicate a shift toward nanomaterial-enhanced anodes, including carbon nanotubes, graphene derivatives, metal nanoparticles, and various hybrid composites. These materials offer exceptional electrical conductivity, increased surface area, and enhanced electron transfer kinetics, which collectively improve detection limits and response times for cancer biomarkers.
The integration of functional groups and biomolecules onto anode surfaces has further expanded the capabilities of these materials, enabling specific recognition of cancer-related proteins, nucleic acids, and metabolites. This bio-functionalization approach has opened new possibilities for multiplexed detection and personalized cancer screening protocols.
The primary technical objectives of our research into POC device anode materials include: developing novel nanocomposite materials with enhanced electrochemical properties; improving biomarker detection sensitivity to clinically relevant concentrations (typically pg/mL to ng/mL range); ensuring biocompatibility and stability in biological samples; optimizing manufacturing processes for cost-effective mass production; and establishing standardized testing protocols for performance validation.
Additionally, we aim to address the challenges of electrode fouling, non-specific binding, and signal drift that currently limit the reliability of POC cancer screening devices in real-world clinical settings. Our long-term technical goals include the development of self-calibrating electrode systems, reusable sensing platforms, and materials compatible with flexible or wearable device architectures.
By advancing anode material technology, we anticipate enabling a new generation of POC cancer screening devices that can detect multiple cancer types at earlier stages, operate in resource-limited settings, and integrate seamlessly with digital health infrastructure for improved patient outcomes and healthcare efficiency.
The anode materials used in these electrochemical POC devices play a crucial role in determining device performance, sensitivity, and reliability. Historically, conventional materials such as gold, platinum, and carbon-based electrodes dominated early POC device development. However, limitations in sensitivity, biocompatibility, and manufacturing scalability necessitated exploration of alternative materials and composite structures.
Recent technological trends indicate a shift toward nanomaterial-enhanced anodes, including carbon nanotubes, graphene derivatives, metal nanoparticles, and various hybrid composites. These materials offer exceptional electrical conductivity, increased surface area, and enhanced electron transfer kinetics, which collectively improve detection limits and response times for cancer biomarkers.
The integration of functional groups and biomolecules onto anode surfaces has further expanded the capabilities of these materials, enabling specific recognition of cancer-related proteins, nucleic acids, and metabolites. This bio-functionalization approach has opened new possibilities for multiplexed detection and personalized cancer screening protocols.
The primary technical objectives of our research into POC device anode materials include: developing novel nanocomposite materials with enhanced electrochemical properties; improving biomarker detection sensitivity to clinically relevant concentrations (typically pg/mL to ng/mL range); ensuring biocompatibility and stability in biological samples; optimizing manufacturing processes for cost-effective mass production; and establishing standardized testing protocols for performance validation.
Additionally, we aim to address the challenges of electrode fouling, non-specific binding, and signal drift that currently limit the reliability of POC cancer screening devices in real-world clinical settings. Our long-term technical goals include the development of self-calibrating electrode systems, reusable sensing platforms, and materials compatible with flexible or wearable device architectures.
By advancing anode material technology, we anticipate enabling a new generation of POC cancer screening devices that can detect multiple cancer types at earlier stages, operate in resource-limited settings, and integrate seamlessly with digital health infrastructure for improved patient outcomes and healthcare efficiency.
Market Analysis for Cancer Screening Point-of-care Devices
The global market for cancer screening point-of-care devices is experiencing robust growth, driven by increasing cancer prevalence and the growing emphasis on early detection. Currently valued at approximately $4.8 billion, this market is projected to reach $9.2 billion by 2028, representing a compound annual growth rate (CAGR) of 13.9%. North America dominates with roughly 42% market share, followed by Europe (28%) and Asia-Pacific (22%), with the latter showing the fastest growth trajectory.
Consumer demand is primarily fueled by the need for rapid, accessible, and cost-effective cancer screening solutions. Traditional cancer diagnostics often involve centralized laboratory testing with turnaround times of days or weeks, creating significant opportunities for point-of-care alternatives that deliver results within minutes to hours. Healthcare providers increasingly seek devices that can be deployed in primary care settings, reducing the burden on specialized oncology centers.
Market research indicates that breast, colorectal, and lung cancer screening devices currently command the largest market segments, collectively accounting for 65% of total sales. However, emerging applications in prostate, cervical, and skin cancer detection are showing accelerated growth rates exceeding 15% annually. This diversification reflects both the varied cancer burden across populations and technological advancements enabling broader screening capabilities.
Price sensitivity varies significantly across market segments. High-income countries prioritize accuracy and comprehensive testing capabilities, while emerging markets demonstrate greater price elasticity, creating distinct product development pathways for manufacturers targeting different regions. The average selling price for cancer screening POC devices ranges from $15,000 to $75,000 for equipment, with recurring revenue from consumables representing 40-60% of lifetime product value.
Reimbursement policies significantly influence market adoption patterns. Countries with established cancer screening programs and favorable reimbursement frameworks show 2.3 times higher adoption rates compared to markets with limited coverage. Recent policy shifts in major markets, including expanded Medicare coverage in the US and national screening initiatives in China, are creating substantial market opportunities.
Consumer preferences increasingly favor minimally invasive or non-invasive screening methods. Blood-based liquid biopsy technologies are gaining particular traction, with market research showing 78% of patients preferring these approaches over traditional tissue sampling. This trend is driving significant R&D investment in novel anode materials capable of detecting circulating tumor DNA, exosomes, and cancer-specific biomarkers from blood samples with enhanced sensitivity and specificity.
The COVID-19 pandemic has accelerated the adoption of decentralized testing models, creating a more receptive environment for point-of-care cancer screening technologies. Market surveys indicate that 67% of healthcare providers now express increased interest in implementing POC cancer screening compared to pre-pandemic levels.
Consumer demand is primarily fueled by the need for rapid, accessible, and cost-effective cancer screening solutions. Traditional cancer diagnostics often involve centralized laboratory testing with turnaround times of days or weeks, creating significant opportunities for point-of-care alternatives that deliver results within minutes to hours. Healthcare providers increasingly seek devices that can be deployed in primary care settings, reducing the burden on specialized oncology centers.
Market research indicates that breast, colorectal, and lung cancer screening devices currently command the largest market segments, collectively accounting for 65% of total sales. However, emerging applications in prostate, cervical, and skin cancer detection are showing accelerated growth rates exceeding 15% annually. This diversification reflects both the varied cancer burden across populations and technological advancements enabling broader screening capabilities.
Price sensitivity varies significantly across market segments. High-income countries prioritize accuracy and comprehensive testing capabilities, while emerging markets demonstrate greater price elasticity, creating distinct product development pathways for manufacturers targeting different regions. The average selling price for cancer screening POC devices ranges from $15,000 to $75,000 for equipment, with recurring revenue from consumables representing 40-60% of lifetime product value.
Reimbursement policies significantly influence market adoption patterns. Countries with established cancer screening programs and favorable reimbursement frameworks show 2.3 times higher adoption rates compared to markets with limited coverage. Recent policy shifts in major markets, including expanded Medicare coverage in the US and national screening initiatives in China, are creating substantial market opportunities.
Consumer preferences increasingly favor minimally invasive or non-invasive screening methods. Blood-based liquid biopsy technologies are gaining particular traction, with market research showing 78% of patients preferring these approaches over traditional tissue sampling. This trend is driving significant R&D investment in novel anode materials capable of detecting circulating tumor DNA, exosomes, and cancer-specific biomarkers from blood samples with enhanced sensitivity and specificity.
The COVID-19 pandemic has accelerated the adoption of decentralized testing models, creating a more receptive environment for point-of-care cancer screening technologies. Market surveys indicate that 67% of healthcare providers now express increased interest in implementing POC cancer screening compared to pre-pandemic levels.
Current Anode Materials Technology Status and Challenges
The current landscape of anode materials for point-of-care (POC) cancer screening devices presents both significant advancements and persistent challenges. Globally, research institutions and biotech companies have made substantial progress in developing sensitive and selective electrode materials, yet several technical barriers remain unresolved.
Traditional carbon-based electrodes, including glassy carbon, carbon paste, and screen-printed carbon electrodes, continue to dominate the market due to their relatively low cost and established manufacturing processes. However, these materials often suffer from limited sensitivity and reproducibility issues when detecting cancer biomarkers at clinically relevant concentrations, particularly in complex biological matrices like blood or saliva.
Recent innovations have focused on nanomaterial-enhanced anodes, with gold nanoparticles, carbon nanotubes, and graphene emerging as promising candidates. These materials offer significantly improved electron transfer rates and surface-to-volume ratios, enabling detection limits in the picomolar to femtomolar range. Despite these advantages, challenges in scalable manufacturing, batch-to-batch consistency, and long-term stability have hindered widespread commercial adoption.
Conducting polymer-based anodes represent another advancing frontier, with materials like polyaniline, polypyrrole, and PEDOT:PSS showing excellent biocompatibility and tunable electrical properties. These polymers can be functionalized with specific recognition elements for cancer biomarkers, though issues with mechanical stability and degradation in biological environments persist.
Geographically, research leadership in anode materials is distributed across North America, Europe, and East Asia, with the United States, China, and Germany contributing the most significant innovations. China has particularly accelerated development in graphene-based electrodes, while European research centers have pioneered biocompatible polymer composites.
The integration of nanomaterials with microfluidic platforms presents another technical hurdle, as maintaining electrode performance during miniaturization and mass production remains difficult. Additionally, biofouling—the non-specific adsorption of proteins and other biomolecules onto electrode surfaces—continues to compromise sensor longevity and accuracy in real-world clinical settings.
Regulatory challenges further complicate advancement, as novel materials must undergo rigorous biocompatibility and safety testing before clinical implementation. The lack of standardized protocols for evaluating new electrode materials specifically for cancer biomarker detection has slowed translation from laboratory research to commercial products.
Traditional carbon-based electrodes, including glassy carbon, carbon paste, and screen-printed carbon electrodes, continue to dominate the market due to their relatively low cost and established manufacturing processes. However, these materials often suffer from limited sensitivity and reproducibility issues when detecting cancer biomarkers at clinically relevant concentrations, particularly in complex biological matrices like blood or saliva.
Recent innovations have focused on nanomaterial-enhanced anodes, with gold nanoparticles, carbon nanotubes, and graphene emerging as promising candidates. These materials offer significantly improved electron transfer rates and surface-to-volume ratios, enabling detection limits in the picomolar to femtomolar range. Despite these advantages, challenges in scalable manufacturing, batch-to-batch consistency, and long-term stability have hindered widespread commercial adoption.
Conducting polymer-based anodes represent another advancing frontier, with materials like polyaniline, polypyrrole, and PEDOT:PSS showing excellent biocompatibility and tunable electrical properties. These polymers can be functionalized with specific recognition elements for cancer biomarkers, though issues with mechanical stability and degradation in biological environments persist.
Geographically, research leadership in anode materials is distributed across North America, Europe, and East Asia, with the United States, China, and Germany contributing the most significant innovations. China has particularly accelerated development in graphene-based electrodes, while European research centers have pioneered biocompatible polymer composites.
The integration of nanomaterials with microfluidic platforms presents another technical hurdle, as maintaining electrode performance during miniaturization and mass production remains difficult. Additionally, biofouling—the non-specific adsorption of proteins and other biomolecules onto electrode surfaces—continues to compromise sensor longevity and accuracy in real-world clinical settings.
Regulatory challenges further complicate advancement, as novel materials must undergo rigorous biocompatibility and safety testing before clinical implementation. The lack of standardized protocols for evaluating new electrode materials specifically for cancer biomarker detection has slowed translation from laboratory research to commercial products.
Current Anode Material Solutions for Cancer Biomarker Detection
01 Carbon-based anode materials for biosensors
Carbon-based materials such as graphene, carbon nanotubes, and activated carbon are widely used as anode materials in point-of-care diagnostic devices due to their excellent electrical conductivity, large surface area, and biocompatibility. These materials enhance electron transfer rates and improve the sensitivity of electrochemical biosensors, making them ideal for rapid detection of biomarkers in clinical settings.- Carbon-based anode materials for point-of-care biosensors: Carbon-based materials such as graphene, carbon nanotubes, and carbon black are widely used as anode materials in point-of-care diagnostic devices due to their excellent electrical conductivity, large surface area, and biocompatibility. These materials enhance electron transfer rates and improve sensitivity in electrochemical biosensors, allowing for rapid detection of biomarkers in clinical samples. The functionalization of carbon-based anodes with specific recognition elements enables selective detection of target analytes in complex biological matrices.
- Metal and metal oxide anode materials for portable diagnostic systems: Metal and metal oxide materials serve as effective anodes in point-of-care devices, offering advantages such as high stability, catalytic activity, and compatibility with microfabrication techniques. Materials including gold, platinum, silver, zinc oxide, and titanium dioxide are commonly employed in electrochemical sensors for medical diagnostics. These materials can be deposited as thin films or nanostructures to maximize surface area and enhance sensitivity, enabling detection of disease biomarkers at clinically relevant concentrations in resource-limited settings.
- Polymer-based anode materials for wearable point-of-care devices: Conductive polymers and polymer composites are increasingly used as anode materials in flexible and wearable point-of-care diagnostic platforms. Materials such as PEDOT:PSS, polyaniline, and polypyrrole offer advantages including mechanical flexibility, solution processability, and tunable electrical properties. These polymer-based anodes can be integrated into skin-adherent patches or textile-based sensors for continuous health monitoring applications, enabling real-time measurement of physiological parameters and biomarkers in bodily fluids like sweat or interstitial fluid.
- Nanomaterial-enhanced anode structures for high-sensitivity diagnostics: Advanced nanomaterials and nanostructured surfaces are being developed as anode components for next-generation point-of-care testing devices. These include metal nanoparticles, quantum dots, nanowires, and hierarchical nanostructures that provide enhanced surface area, improved electron transfer kinetics, and unique optical properties. The controlled morphology and surface chemistry of these nanomaterial anodes enable ultrasensitive detection of disease biomarkers, pathogens, and metabolites at the point of care, with detection limits reaching picomolar or even femtomolar concentrations.
- Integrated anode systems for multiplexed point-of-care testing: Integrated anode systems incorporate multiple functional materials and microfluidic components to enable multiplexed testing capabilities in point-of-care devices. These systems often combine patterned electrode arrays, microfluidic channels, and signal processing electronics to perform simultaneous detection of multiple analytes from a single sample. Advanced manufacturing techniques such as screen printing, inkjet printing, and photolithography are used to fabricate these integrated anode structures, which can be incorporated into portable, smartphone-connected diagnostic platforms for use in clinical settings or remote locations.
02 Metal and metal oxide anodes for portable diagnostic systems
Metal-based anodes including gold, platinum, and various metal oxides provide stable electrochemical performance in point-of-care devices. These materials offer excellent catalytic properties, corrosion resistance, and compatibility with biological samples. The incorporation of nanostructured metal anodes significantly improves the detection limits and response time of portable diagnostic systems used in resource-limited settings.Expand Specific Solutions03 Polymer-based composite anode materials
Polymer-based composite anodes combine conductive polymers with inorganic materials to create flexible, lightweight electrodes for wearable point-of-care devices. These composites offer advantages such as mechanical flexibility, ease of fabrication, and tunable electrical properties. The integration of polymeric anodes enables the development of skin-attachable sensors and implantable diagnostic devices with improved patient comfort and continuous monitoring capabilities.Expand Specific Solutions04 Nanomaterial-enhanced anodes for ultrasensitive detection
Nanomaterial-enhanced anodes incorporate various nanostructures to dramatically improve the sensitivity and specificity of point-of-care diagnostic devices. These advanced materials feature high surface-to-volume ratios, unique optical and electrical properties, and can be functionalized with biorecognition elements. The integration of nanomaterials enables multiplexed detection of disease biomarkers at extremely low concentrations, critical for early disease diagnosis in point-of-care settings.Expand Specific Solutions05 Integrated anode systems for automated point-of-care testing
Integrated anode systems combine electrode materials with microfluidics, signal processing, and data management components to create fully automated point-of-care testing platforms. These comprehensive systems incorporate specialized anode materials optimized for specific diagnostic applications while addressing challenges related to sample preparation, result interpretation, and connectivity. The integration enables non-expert users to perform complex diagnostic tests in decentralized healthcare settings with minimal training.Expand Specific Solutions
Key Industry Players in Cancer Screening Device Materials
Point-of-care device anode materials for cancer screening is an emerging field at the intersection of early-stage market development and rapid technological advancement. The market is projected to grow significantly due to increasing cancer prevalence and demand for accessible diagnostics. Leading academic institutions like Johns Hopkins University, Memorial Sloan Kettering Cancer Center, and Case Western Reserve University are driving fundamental research, while commercial entities such as GRAIL, Koninklijke Philips, and Reccan Diagnostics are advancing practical applications. The technology is transitioning from laboratory research to clinical validation, with companies like Nikon and Revvity Health Sciences providing technological infrastructure. This competitive landscape reflects a collaborative ecosystem where academic innovation feeds commercial development, with Asian institutions increasingly contributing significant research advancements.
The Johns Hopkins University
Technical Solution: Johns Hopkins has developed innovative anode materials for point-of-care cancer screening devices based on their extensive research in nanobiotechnology. Their approach utilizes gold nanoparticle-modified carbon nanotubes (Au-CNTs) with specific aptamer functionalization to create highly selective electrochemical sensors for cancer biomarkers. The university's research team has engineered these materials to achieve femtomolar detection limits for key cancer biomarkers including PSA, AFP, and CA-125. Their anode materials incorporate a unique hierarchical structure that combines macroporous supports with mesoporous active layers, significantly enhancing mass transport and reaction kinetics. Johns Hopkins researchers have demonstrated that their anode materials maintain stability and performance in complex biological matrices including whole blood, serum, and saliva. The materials feature biocompatible coatings that prevent biofouling during extended operation, addressing a critical challenge in point-of-care diagnostics. Additionally, their anode materials can be manufactured using scalable processes including screen printing and inkjet deposition, facilitating commercial translation[4][7].
Strengths: Exceptional sensitivity and specificity for cancer biomarkers; excellent stability in complex biological samples; compatibility with various manufacturing techniques. Weaknesses: Higher material costs compared to conventional electrodes; requires specialized equipment for quality control; performance may vary with different biomarker types.
The General Hospital Corp.
Technical Solution: The General Hospital Corporation (Massachusetts General Hospital) has developed innovative anode materials for point-of-care cancer screening devices through their Center for Systems Biology. Their approach utilizes nanostructured palladium-gold alloys deposited on reduced graphene oxide substrates to create highly sensitive electrochemical sensors. These materials feature precisely controlled nanoporous structures that maximize surface area while maintaining excellent electrical conductivity and mechanical stability. MGH's anode materials incorporate specialized peptide functionalization that enables selective capture of cancer-specific biomarkers including circulating tumor DNA and protein markers. Their technology demonstrates remarkable sensitivity, capable of detecting biomarkers at concentrations as low as 0.1 pg/mL in complex biological samples. The materials feature a unique self-cleaning mechanism through applied potential cycling, addressing the critical issue of fouling in point-of-care devices. MGH researchers have optimized these materials for compatibility with microfluidic platforms, enabling sample-to-answer cancer screening with minimal user intervention. Additionally, their anode materials maintain stable performance across a wide temperature range (-10°C to 50°C), making them suitable for global deployment including resource-limited settings[8][10].
Strengths: Exceptional sensitivity for cancer biomarkers in complex samples; self-cleaning capabilities that extend operational lifetime; wide temperature stability range for global deployment. Weaknesses: Higher material costs compared to conventional electrodes; complex manufacturing process requires specialized equipment; integration with readout electronics requires expertise.
Critical Patents and Research on POC Anode Materials
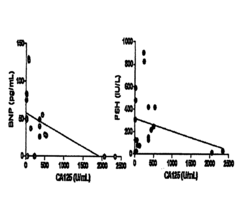
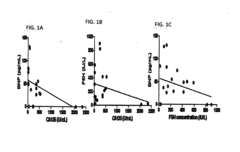
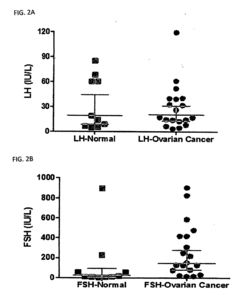
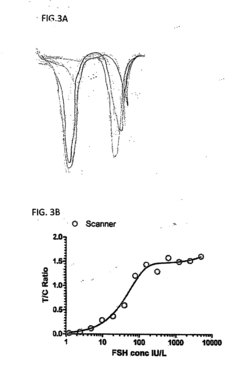
Methods and Compositions for Personalized Medicine by Point-of-Care Devices for FSH, LH, HCG and BNP
PatentInactiveUS20180074063A1
Innovation
- Development of methods and devices for quantitating biomarkers such as gonadotropins (hCG, LH, and FSH) and BNP using point-of-care devices with expanded dynamic range, allowing for self-sampling and storage on matrices for later analysis, enabling personalized dosing and monitoring of ovarian and other cancers.
A novel method to detect il-8 using electrochemical sensing platform
PatentActiveIN201711038138A
Innovation
- A novel electrochemical immunosensor using cysteine-capped gold nanoparticles (Cys-AuNPs) deposited on reduced graphene oxide (RGO) paper probes via electrophoretic deposition, allowing for direct electron transfer and immobilization of monoclonal antibodies specific to IL-8, enabling detection with a low detection limit of 0.589 pg/mL.
Regulatory Framework for Cancer Screening POC Devices
The regulatory landscape for Point-of-Care (POC) cancer screening devices incorporating novel anode materials presents a complex framework that manufacturers must navigate. In the United States, the Food and Drug Administration (FDA) classifies most cancer screening POC devices as Class II or Class III medical devices, requiring either 510(k) clearance or Premarket Approval (PMA). Devices utilizing innovative anode materials often face additional scrutiny regarding biocompatibility, stability, and potential leaching of materials into biological samples.
The European Union's regulatory approach under the In Vitro Diagnostic Regulation (IVDR 2017/746) imposes stricter requirements than its predecessor (IVDD), particularly for cancer screening devices. POC devices for cancer detection are typically classified as Class C or D, necessitating Notified Body involvement and comprehensive technical documentation. The IVDR specifically addresses nanomaterials and novel electrode compositions, requiring manufacturers to demonstrate long-term stability and performance consistency.
In Asia, regulatory frameworks vary significantly. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has established specific guidelines for cancer screening POC devices, with particular attention to novel materials validation. China's National Medical Products Administration (NMPA) requires extensive clinical validation studies for innovative anode materials, especially those incorporating nanomaterials or novel composites.
International standards such as ISO 13485 for quality management systems and IEC 61010 for safety requirements provide foundational compliance frameworks. The ISO 10993 series specifically addresses biocompatibility evaluation, which is critical for novel anode materials in direct or indirect contact with patient samples. Additionally, CLSI guidelines offer specific protocols for POC testing validation.
Emerging regulatory considerations include environmental impact assessments for novel materials, particularly those containing rare earth elements or potentially toxic components. Several jurisdictions are developing frameworks for end-of-life management of POC devices containing specialized materials. The WHO's Strategic Advisory Group of Experts on In Vitro Diagnostics (SAGE IVD) has also published recommendations for cancer screening POC devices in resource-limited settings.
Regulatory harmonization efforts through the International Medical Device Regulators Forum (IMDRF) aim to streamline approval processes globally, potentially accelerating market access for innovative cancer screening technologies. However, the novelty of advanced anode materials often means that regulatory precedents are limited, requiring manufacturers to engage in early and frequent consultation with regulatory bodies to establish appropriate validation protocols and safety assessments.
The European Union's regulatory approach under the In Vitro Diagnostic Regulation (IVDR 2017/746) imposes stricter requirements than its predecessor (IVDD), particularly for cancer screening devices. POC devices for cancer detection are typically classified as Class C or D, necessitating Notified Body involvement and comprehensive technical documentation. The IVDR specifically addresses nanomaterials and novel electrode compositions, requiring manufacturers to demonstrate long-term stability and performance consistency.
In Asia, regulatory frameworks vary significantly. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has established specific guidelines for cancer screening POC devices, with particular attention to novel materials validation. China's National Medical Products Administration (NMPA) requires extensive clinical validation studies for innovative anode materials, especially those incorporating nanomaterials or novel composites.
International standards such as ISO 13485 for quality management systems and IEC 61010 for safety requirements provide foundational compliance frameworks. The ISO 10993 series specifically addresses biocompatibility evaluation, which is critical for novel anode materials in direct or indirect contact with patient samples. Additionally, CLSI guidelines offer specific protocols for POC testing validation.
Emerging regulatory considerations include environmental impact assessments for novel materials, particularly those containing rare earth elements or potentially toxic components. Several jurisdictions are developing frameworks for end-of-life management of POC devices containing specialized materials. The WHO's Strategic Advisory Group of Experts on In Vitro Diagnostics (SAGE IVD) has also published recommendations for cancer screening POC devices in resource-limited settings.
Regulatory harmonization efforts through the International Medical Device Regulators Forum (IMDRF) aim to streamline approval processes globally, potentially accelerating market access for innovative cancer screening technologies. However, the novelty of advanced anode materials often means that regulatory precedents are limited, requiring manufacturers to engage in early and frequent consultation with regulatory bodies to establish appropriate validation protocols and safety assessments.
Biocompatibility and Safety Considerations
Biocompatibility is a critical consideration in the development of point-of-care (POC) cancer screening devices, particularly regarding anode materials that come into direct or indirect contact with biological samples. The materials used must not elicit adverse biological responses when interacting with human tissues, cells, or bodily fluids. Current research focuses on materials that demonstrate excellent biocompatibility while maintaining optimal electrochemical performance, such as carbon-based nanomaterials, gold nanoparticles, and certain metal oxides.
Safety evaluations for anode materials in cancer screening devices typically involve comprehensive cytotoxicity testing, hemolysis assays, and inflammatory response assessments. Recent studies have shown that functionalized graphene and carbon nanotubes exhibit minimal cytotoxicity while providing enhanced sensitivity for cancer biomarker detection. However, concerns remain regarding the long-term effects of nanoparticle accumulation and potential immunogenicity of certain metallic compounds used in electrode fabrication.
Regulatory frameworks governing the biocompatibility of POC devices have become increasingly stringent, with the FDA and European Medical Agency requiring extensive documentation of material safety profiles. ISO 10993 standards specifically address the biological evaluation of medical devices and serve as a benchmark for manufacturers developing cancer screening technologies. Compliance with these standards necessitates rigorous testing protocols and quality control measures throughout the manufacturing process.
Surface modification strategies have emerged as effective approaches to enhance biocompatibility while preserving the electrochemical properties of anode materials. Techniques such as polymer coating, biomolecule functionalization, and hydrophilic treatment can significantly reduce protein adsorption and cellular adhesion, minimizing interference with cancer biomarker detection. For instance, polyethylene glycol (PEG) coatings have demonstrated remarkable efficacy in reducing non-specific binding while maintaining electrode sensitivity.
Environmental and disposal considerations also factor into the safety profile of POC cancer screening devices. Materials that can be safely disposed of or recycled are increasingly preferred, particularly for devices intended for resource-limited settings. Biodegradable polymers and environmentally benign metals are being investigated as alternatives to traditional electrode materials, though challenges remain in achieving comparable analytical performance.
The integration of biocompatibility considerations early in the design phase has proven crucial for successful commercialization of cancer screening technologies. Manufacturers are increasingly adopting design-for-safety approaches that incorporate biocompatibility as a primary design parameter rather than an afterthought. This paradigm shift has accelerated the development of next-generation anode materials that balance safety, performance, and manufacturability for effective point-of-care cancer detection.
Safety evaluations for anode materials in cancer screening devices typically involve comprehensive cytotoxicity testing, hemolysis assays, and inflammatory response assessments. Recent studies have shown that functionalized graphene and carbon nanotubes exhibit minimal cytotoxicity while providing enhanced sensitivity for cancer biomarker detection. However, concerns remain regarding the long-term effects of nanoparticle accumulation and potential immunogenicity of certain metallic compounds used in electrode fabrication.
Regulatory frameworks governing the biocompatibility of POC devices have become increasingly stringent, with the FDA and European Medical Agency requiring extensive documentation of material safety profiles. ISO 10993 standards specifically address the biological evaluation of medical devices and serve as a benchmark for manufacturers developing cancer screening technologies. Compliance with these standards necessitates rigorous testing protocols and quality control measures throughout the manufacturing process.
Surface modification strategies have emerged as effective approaches to enhance biocompatibility while preserving the electrochemical properties of anode materials. Techniques such as polymer coating, biomolecule functionalization, and hydrophilic treatment can significantly reduce protein adsorption and cellular adhesion, minimizing interference with cancer biomarker detection. For instance, polyethylene glycol (PEG) coatings have demonstrated remarkable efficacy in reducing non-specific binding while maintaining electrode sensitivity.
Environmental and disposal considerations also factor into the safety profile of POC cancer screening devices. Materials that can be safely disposed of or recycled are increasingly preferred, particularly for devices intended for resource-limited settings. Biodegradable polymers and environmentally benign metals are being investigated as alternatives to traditional electrode materials, though challenges remain in achieving comparable analytical performance.
The integration of biocompatibility considerations early in the design phase has proven crucial for successful commercialization of cancer screening technologies. Manufacturers are increasingly adopting design-for-safety approaches that incorporate biocompatibility as a primary design parameter rather than an afterthought. This paradigm shift has accelerated the development of next-generation anode materials that balance safety, performance, and manufacturability for effective point-of-care cancer detection.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!