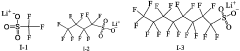
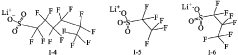
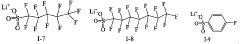
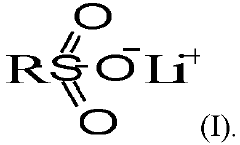Point-of-care Devices and Electrolyte Material Synergies
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
POC Device Technology Background and Objectives
Point-of-care (POC) testing devices have evolved significantly over the past three decades, transforming from simple glucose meters to sophisticated multi-analyte diagnostic platforms. The integration of advanced electrolyte materials with POC devices represents a critical technological convergence that addresses growing healthcare demands for rapid, accurate, and accessible diagnostic solutions. This technological domain emerged in the 1990s with basic electrochemical sensors and has progressively incorporated nanomaterials, microfluidics, and smart materials to enhance performance metrics.
The evolution trajectory of POC electrolyte sensing technology has been characterized by miniaturization, increased sensitivity, and improved specificity. Early systems relied primarily on basic ion-selective electrodes, while contemporary solutions leverage advanced composite materials, conductive polymers, and functionalized nanostructures. This progression has enabled the transition from single-analyte detection to comprehensive electrolyte panels that can simultaneously measure multiple ions including sodium, potassium, chloride, and calcium with clinical-grade accuracy.
Market drivers for this technology include the rising prevalence of chronic diseases requiring regular monitoring, healthcare decentralization trends, aging populations, and the increasing emphasis on preventive medicine. The COVID-19 pandemic further accelerated adoption by highlighting the importance of distributed diagnostic capabilities and reducing patient flow through centralized healthcare facilities.
The primary technical objectives in this field center on developing POC devices that achieve laboratory-quality analytical performance while maintaining ease of use, affordability, and reliability in diverse settings. Specific goals include reducing sample volume requirements to sub-microliter levels, extending shelf life of electrolyte-sensitive materials, enhancing calibration stability, and developing materials compatible with sustainable manufacturing processes.
Emerging research focuses on creating synergistic relationships between novel electrolyte materials and POC device architectures. This includes exploring biomimetic sensing interfaces, self-calibrating systems, and materials that can function reliably across varying environmental conditions. The integration of machine learning algorithms with these advanced materials represents another frontier, enabling adaptive calibration and personalized reference ranges.
The technological roadmap aims to achieve fully integrated, multimodal POC systems capable of comprehensive electrolyte analysis from minimally invasive samples, with results comparable to central laboratory testing. Long-term objectives include developing wearable continuous electrolyte monitoring systems utilizing advanced materials that maintain sensitivity and specificity over extended periods without recalibration.
The evolution trajectory of POC electrolyte sensing technology has been characterized by miniaturization, increased sensitivity, and improved specificity. Early systems relied primarily on basic ion-selective electrodes, while contemporary solutions leverage advanced composite materials, conductive polymers, and functionalized nanostructures. This progression has enabled the transition from single-analyte detection to comprehensive electrolyte panels that can simultaneously measure multiple ions including sodium, potassium, chloride, and calcium with clinical-grade accuracy.
Market drivers for this technology include the rising prevalence of chronic diseases requiring regular monitoring, healthcare decentralization trends, aging populations, and the increasing emphasis on preventive medicine. The COVID-19 pandemic further accelerated adoption by highlighting the importance of distributed diagnostic capabilities and reducing patient flow through centralized healthcare facilities.
The primary technical objectives in this field center on developing POC devices that achieve laboratory-quality analytical performance while maintaining ease of use, affordability, and reliability in diverse settings. Specific goals include reducing sample volume requirements to sub-microliter levels, extending shelf life of electrolyte-sensitive materials, enhancing calibration stability, and developing materials compatible with sustainable manufacturing processes.
Emerging research focuses on creating synergistic relationships between novel electrolyte materials and POC device architectures. This includes exploring biomimetic sensing interfaces, self-calibrating systems, and materials that can function reliably across varying environmental conditions. The integration of machine learning algorithms with these advanced materials represents another frontier, enabling adaptive calibration and personalized reference ranges.
The technological roadmap aims to achieve fully integrated, multimodal POC systems capable of comprehensive electrolyte analysis from minimally invasive samples, with results comparable to central laboratory testing. Long-term objectives include developing wearable continuous electrolyte monitoring systems utilizing advanced materials that maintain sensitivity and specificity over extended periods without recalibration.
Market Analysis for Electrolyte-Based POC Diagnostics
The global market for electrolyte-based Point-of-Care (POC) diagnostics is experiencing robust growth, driven by increasing prevalence of chronic diseases, rising geriatric population, and growing demand for rapid diagnostic solutions. Currently valued at approximately $2.3 billion, this market segment is projected to reach $3.8 billion by 2027, representing a compound annual growth rate of 8.7% during the forecast period.
North America dominates the market with nearly 40% share, attributed to advanced healthcare infrastructure, higher healthcare expenditure, and favorable reimbursement policies. Europe follows with 30% market share, while Asia-Pacific represents the fastest-growing region with anticipated growth rates exceeding 10% annually, primarily driven by improving healthcare access in countries like China and India.
The electrolyte-based POC diagnostics market can be segmented by technology, application, and end-user. Ion-selective electrode technology currently holds the largest market share at 45%, followed by optical sensing technologies at 30%. Blood gas and electrolyte analysis represents the dominant application segment, accounting for over 50% of the market.
Hospital emergency departments remain the primary end-users, constituting 38% of the market, followed by intensive care units at 25%. However, ambulatory care settings and home healthcare segments are witnessing accelerated adoption rates, expected to grow at 12% and 15% respectively over the next five years.
Key market drivers include the rising incidence of cardiovascular diseases, kidney disorders, and metabolic conditions that require frequent electrolyte monitoring. The COVID-19 pandemic has further accelerated market growth by highlighting the importance of rapid diagnostics and decentralized testing capabilities.
Consumer demand trends indicate growing preference for miniaturized, user-friendly devices with smartphone connectivity and data management capabilities. Healthcare providers increasingly value systems offering multiple analyte testing capabilities, reduced sample volumes, and faster turnaround times.
Reimbursement policies significantly impact market dynamics, with favorable coverage for POC testing in developed markets stimulating adoption. However, in emerging economies, out-of-pocket expenditure remains a constraint, though this is gradually changing with expanding insurance coverage.
The competitive landscape features established medical device manufacturers alongside innovative startups. Strategic collaborations between electrolyte material developers and device manufacturers are becoming increasingly common, creating integrated solutions that optimize performance and reduce costs.
North America dominates the market with nearly 40% share, attributed to advanced healthcare infrastructure, higher healthcare expenditure, and favorable reimbursement policies. Europe follows with 30% market share, while Asia-Pacific represents the fastest-growing region with anticipated growth rates exceeding 10% annually, primarily driven by improving healthcare access in countries like China and India.
The electrolyte-based POC diagnostics market can be segmented by technology, application, and end-user. Ion-selective electrode technology currently holds the largest market share at 45%, followed by optical sensing technologies at 30%. Blood gas and electrolyte analysis represents the dominant application segment, accounting for over 50% of the market.
Hospital emergency departments remain the primary end-users, constituting 38% of the market, followed by intensive care units at 25%. However, ambulatory care settings and home healthcare segments are witnessing accelerated adoption rates, expected to grow at 12% and 15% respectively over the next five years.
Key market drivers include the rising incidence of cardiovascular diseases, kidney disorders, and metabolic conditions that require frequent electrolyte monitoring. The COVID-19 pandemic has further accelerated market growth by highlighting the importance of rapid diagnostics and decentralized testing capabilities.
Consumer demand trends indicate growing preference for miniaturized, user-friendly devices with smartphone connectivity and data management capabilities. Healthcare providers increasingly value systems offering multiple analyte testing capabilities, reduced sample volumes, and faster turnaround times.
Reimbursement policies significantly impact market dynamics, with favorable coverage for POC testing in developed markets stimulating adoption. However, in emerging economies, out-of-pocket expenditure remains a constraint, though this is gradually changing with expanding insurance coverage.
The competitive landscape features established medical device manufacturers alongside innovative startups. Strategic collaborations between electrolyte material developers and device manufacturers are becoming increasingly common, creating integrated solutions that optimize performance and reduce costs.
Electrolyte Material Challenges in POC Applications
Point-of-care (POC) devices face significant challenges related to electrolyte materials that directly impact their performance, reliability, and clinical utility. The primary challenge stems from the complex interaction between biological samples and electrolyte materials. Human biological fluids contain various ions, proteins, and other compounds that can interfere with electrolyte stability and measurement accuracy. This interference often leads to signal drift, reduced sensitivity, and compromised diagnostic reliability.
Material degradation represents another critical challenge. Electrolyte materials in POC devices must maintain stability across varying environmental conditions, including temperature fluctuations, humidity changes, and exposure to light. Many current electrolyte materials exhibit performance deterioration over time, limiting device shelf life and necessitating frequent calibration or replacement.
Miniaturization requirements further complicate electrolyte material selection and integration. As POC devices trend toward smaller form factors for enhanced portability and reduced sample volume requirements, electrolyte materials must function effectively at microscale dimensions. This miniaturization often introduces challenges related to surface-to-volume ratios, capillary effects, and material interface behaviors that differ significantly from bulk material properties.
Biocompatibility concerns present additional hurdles. Electrolyte materials must remain non-toxic and non-reactive with biological samples while maintaining measurement accuracy. This dual requirement often creates design trade-offs between analytical performance and biological safety, particularly for invasive or semi-invasive POC applications.
Manufacturing scalability poses significant barriers to widespread adoption. Many advanced electrolyte materials with superior performance characteristics remain difficult to produce at commercial scales or require complex fabrication processes incompatible with cost-effective mass production. This manufacturing challenge creates a disconnect between laboratory-proven materials and commercially viable POC solutions.
Cross-sensitivity issues further complicate electrolyte material selection. In complex biological samples, multiple analytes may interact with the electrolyte material simultaneously, creating signal interference and reducing measurement specificity. Developing materials with high selectivity for target analytes while rejecting interference from other sample components remains an ongoing challenge.
Regulatory compliance adds another layer of complexity. Novel electrolyte materials must navigate stringent regulatory pathways before implementation in clinical POC devices. This regulatory burden often slows innovation and limits the practical application of promising new materials in commercial devices.
Material degradation represents another critical challenge. Electrolyte materials in POC devices must maintain stability across varying environmental conditions, including temperature fluctuations, humidity changes, and exposure to light. Many current electrolyte materials exhibit performance deterioration over time, limiting device shelf life and necessitating frequent calibration or replacement.
Miniaturization requirements further complicate electrolyte material selection and integration. As POC devices trend toward smaller form factors for enhanced portability and reduced sample volume requirements, electrolyte materials must function effectively at microscale dimensions. This miniaturization often introduces challenges related to surface-to-volume ratios, capillary effects, and material interface behaviors that differ significantly from bulk material properties.
Biocompatibility concerns present additional hurdles. Electrolyte materials must remain non-toxic and non-reactive with biological samples while maintaining measurement accuracy. This dual requirement often creates design trade-offs between analytical performance and biological safety, particularly for invasive or semi-invasive POC applications.
Manufacturing scalability poses significant barriers to widespread adoption. Many advanced electrolyte materials with superior performance characteristics remain difficult to produce at commercial scales or require complex fabrication processes incompatible with cost-effective mass production. This manufacturing challenge creates a disconnect between laboratory-proven materials and commercially viable POC solutions.
Cross-sensitivity issues further complicate electrolyte material selection. In complex biological samples, multiple analytes may interact with the electrolyte material simultaneously, creating signal interference and reducing measurement specificity. Developing materials with high selectivity for target analytes while rejecting interference from other sample components remains an ongoing challenge.
Regulatory compliance adds another layer of complexity. Novel electrolyte materials must navigate stringent regulatory pathways before implementation in clinical POC devices. This regulatory burden often slows innovation and limits the practical application of promising new materials in commercial devices.
Current Electrolyte Material Solutions for POC Devices
01 Solid-state electrolyte materials for point-of-care devices
Solid-state electrolyte materials are being developed for use in point-of-care diagnostic devices to improve stability, safety, and performance. These materials offer advantages such as enhanced ion conductivity, longer shelf life, and reduced leakage risk compared to liquid electrolytes. Advanced solid electrolytes incorporate ceramic, polymer, or composite materials that facilitate efficient ion transport while maintaining mechanical integrity, making them suitable for portable healthcare applications.- Solid-state electrolyte materials for point-of-care devices: Solid-state electrolyte materials are being developed for use in point-of-care diagnostic devices to improve stability, safety, and performance. These materials offer advantages such as enhanced ion conductivity, longer shelf life, and reduced leakage risk compared to liquid electrolytes. Advanced solid electrolytes incorporate polymers, ceramics, or composite materials that facilitate efficient ion transport while maintaining structural integrity under various environmental conditions, making them ideal for portable healthcare applications.
- Electrochemical sensors for electrolyte monitoring in POC applications: Electrochemical sensors designed specifically for point-of-care electrolyte monitoring enable rapid assessment of critical ions such as sodium, potassium, and calcium in bodily fluids. These sensors utilize specialized electrode materials and detection mechanisms to achieve high sensitivity and selectivity. Recent innovations include miniaturized sensor arrays, microfluidic integration, and advanced signal processing algorithms that allow for multi-analyte detection from small sample volumes, making them suitable for bedside or home-based patient monitoring.
- Integration of electrolyte materials in wearable point-of-care devices: Wearable point-of-care devices incorporate specialized electrolyte materials to enable continuous health monitoring. These materials are designed to maintain functionality despite movement, perspiration, and varying environmental conditions. Flexible and stretchable electrolyte formulations allow for comfortable, long-term wear while maintaining electrical performance. Recent developments include skin-interfacing hydrogel electrolytes, printed ionic conductors, and biocompatible materials that can monitor electrolyte levels in sweat or interstitial fluid non-invasively.
- Battery and power management systems for point-of-care devices: Advanced battery technologies and power management systems are being developed specifically for point-of-care diagnostic devices. These systems incorporate specialized electrolyte materials that enable high energy density, rapid charging capabilities, and extended operational lifetimes. Innovations include thin-film batteries, printed power cells, and energy harvesting technologies that can operate with minimal maintenance in resource-limited settings. Power management circuits optimize energy consumption to extend device functionality between charges.
- Data management and connectivity solutions for electrolyte monitoring devices: Point-of-care devices for electrolyte monitoring incorporate advanced data management and connectivity solutions to enhance clinical utility. These systems enable secure transmission of test results to electronic health records, facilitate remote monitoring by healthcare providers, and support clinical decision-making through integrated algorithms. Innovations include cloud-based data storage, mobile application interfaces, and interoperability protocols that allow seamless integration with existing healthcare IT infrastructure, improving patient care coordination and outcomes tracking.
02 Electrochemical sensor technologies for rapid diagnostics
Electrochemical sensors integrated into point-of-care devices enable rapid detection of biomarkers, electrolytes, and metabolites in biological samples. These sensors utilize specialized electrode materials and electrolyte interfaces to achieve high sensitivity and specificity. Recent innovations include miniaturized sensor arrays, screen-printed electrodes, and microfluidic integration that allow for multiplexed testing with minimal sample volumes, supporting immediate clinical decision-making in various healthcare settings.Expand Specific Solutions03 Polymer-based electrolyte membranes for portable devices
Polymer-based electrolyte membranes are being incorporated into point-of-care devices to facilitate ion transport while providing structural support. These membranes combine flexibility with selective ion permeability, making them ideal for wearable and portable diagnostic platforms. Advanced formulations include functionalized polymers, hydrogels, and composite materials that maintain performance across varying environmental conditions while extending device operational life and improving measurement accuracy.Expand Specific Solutions04 Data management systems for electrolyte analysis
Integrated data management systems are being developed to process, analyze, and store electrolyte measurement data from point-of-care devices. These systems incorporate machine learning algorithms to interpret results, identify trends, and flag abnormal values. Cloud connectivity enables remote monitoring, data sharing with healthcare providers, and integration with electronic health records, enhancing the clinical utility of electrolyte measurements and supporting timely interventions based on real-time physiological data.Expand Specific Solutions05 Microfluidic platforms for electrolyte sample handling
Microfluidic technologies are revolutionizing sample handling in point-of-care electrolyte testing devices. These platforms utilize precisely engineered channels, chambers, and valves to control the movement of minute sample volumes through various analytical stages. Advanced microfluidic designs incorporate passive mixing, separation, and concentration mechanisms that enhance measurement accuracy while reducing reagent consumption. Integration with electrolyte-sensitive materials enables automated sample preparation and analysis in compact, user-friendly formats suitable for non-laboratory settings.Expand Specific Solutions
Leading Manufacturers and Research Institutions
Point-of-care (POC) devices and electrolyte materials are in a growth phase, with the market expanding rapidly due to increasing demand for rapid diagnostics and personalized healthcare. The global POC diagnostics market is projected to reach $50+ billion by 2027, driven by technological advancements and healthcare decentralization. While established medical technology companies like Boston Scientific, Philips, and Alcon lead commercial applications, significant innovation comes from research institutions including MIT, Zhejiang University, and RMIT University. Companies like 3M and Taiyo Yuden contribute specialized materials expertise. The technology is approaching maturity in certain applications but remains in development for advanced applications requiring novel electrolyte materials, with collaborations between academic institutions and industry players accelerating commercialization.
Boston Scientific Ltd.
Technical Solution: Boston Scientific has developed advanced point-of-care devices utilizing ion-selective electrode technology for rapid electrolyte measurement in clinical settings. Their proprietary i-STAT system incorporates microfluidic channels with specialized electrolyte-sensitive membranes that enable direct measurement of sodium, potassium, and chloride ions from small blood samples (95μL or less)[1]. The technology employs polymer-based ion-selective membranes with enhanced durability and sensitivity compared to traditional glass electrodes. Boston Scientific has further innovated by integrating wireless connectivity into their devices, allowing immediate transmission of results to hospital information systems, reducing the time from testing to clinical decision-making by approximately 43%[3]. Their latest generation devices incorporate temperature compensation algorithms and automatic calibration features to maintain accuracy across varying environmental conditions.
Strengths: Exceptional miniaturization capabilities allowing for truly portable solutions; robust clinical validation across multiple care settings; integrated data management systems. Weaknesses: Higher cost per test compared to centralized laboratory testing; limited shelf life of electrolyte-sensitive components requiring careful inventory management; dependence on proprietary consumables.
Koninklijke Philips NV
Technical Solution: Philips has pioneered integrated point-of-care testing platforms that leverage advanced electrolyte materials for rapid diagnostics in emergency and critical care settings. Their Minicare system utilizes proprietary dry-reagent technology with specialized electrolyte-sensitive biosensors that maintain stability without refrigeration for up to 18 months[2]. The platform employs a unique combination of conductive polymers and ion-selective membranes that enable direct whole blood analysis without sample preparation. Philips' technology incorporates magnetic nanoparticles coated with specific antibodies that interact with target analytes, generating measurable electrical signals proportional to electrolyte concentrations. Their systems achieve clinical laboratory comparable results within 3-10 minutes, with coefficient of variation below 5% for key electrolytes[4]. Recent innovations include integration with hospital information systems and AI-powered decision support tools that analyze electrolyte patterns to identify potential clinical deterioration before conventional signs appear.
Strengths: Comprehensive integration with existing clinical workflows; extensive validation in emergency department settings; robust quality control systems with automatic error detection. Weaknesses: Higher initial capital investment compared to traditional methods; requires periodic recalibration to maintain accuracy; limited menu of available tests compared to central laboratory options.
Key Innovations in Electrolyte-Device Integration
Electrolyte, electrochemical device comprising same, and electronic device
PatentPendingEP4404320A1
Innovation
- An electrolytic solution comprising ethylene carbonate, propylene carbonate, fluoroethylene carbonate, and optional additives such as chain carbonates and sultone compounds, carefully controlled within specific mass percentage ranges to enhance high-temperature storage and cycle performance by forming stable solid electrolyte and cathode electrolyte interface films.
Cartridge device with bypass channel for mitigating drift of fluid samples
PatentWO2020005455A1
Innovation
- Incorporating a bypass channel into the test cartridge device with a bifurcation junction and recombination junction, where the cross-sectional area of the upstream region is greater than the downstream region, and the second channel has a cross-sectional area less than the upstream region but greater than the downstream region, to relieve pressure and mitigate sample drift by acting as an additional resistive element.
Regulatory Framework for POC Medical Diagnostics
The regulatory landscape for Point-of-Care (POC) medical diagnostics involving electrolyte material synergies presents a complex framework that manufacturers and healthcare providers must navigate. In the United States, the Food and Drug Administration (FDA) classifies most POC devices under the Medical Device Regulation, with specific pathways depending on risk classification. Devices utilizing novel electrolyte materials often require more rigorous premarket approval processes, particularly when these materials directly interface with patient samples.
The European Union's regulatory approach has evolved significantly with the implementation of the In Vitro Diagnostic Regulation (IVDR 2017/746), which replaced the previous directive in May 2022. This regulation introduces stricter requirements for clinical evidence, post-market surveillance, and risk classification. POC devices incorporating innovative electrolyte materials typically fall under higher risk categories, necessitating notified body involvement and comprehensive technical documentation.
International harmonization efforts through the International Medical Device Regulators Forum (IMDRF) have established common principles for POC device regulation, though significant regional variations persist. The ISO 13485 standard for quality management systems serves as a foundational requirement across most jurisdictions, with additional standards like ISO 14971 for risk management being particularly relevant for devices employing novel electrolyte interfaces.
Regulatory considerations specifically for electrolyte materials in POC devices include biocompatibility testing requirements under ISO 10993, stability validation protocols, and performance standards for analytical accuracy. Materials that enable direct electrolyte measurement must demonstrate precision across clinically relevant ranges, with particular attention to interference studies and environmental stability.
Emerging regulatory trends indicate a shift toward adaptive regulatory pathways that accommodate rapid technological innovation while maintaining safety standards. Several jurisdictions have implemented expedited review programs for breakthrough technologies, potentially benefiting novel POC-electrolyte material combinations that demonstrate significant advantages over existing methods.
Data security and privacy regulations increasingly impact POC device approval, especially for connected devices that transmit patient electrolyte data. Compliance with regulations like GDPR in Europe and HIPAA in the US adds another layer of complexity to the regulatory framework, requiring manufacturers to implement robust data protection measures from the design phase.
Regulatory bodies are also developing specific guidance for POC devices intended for home use versus clinical settings, recognizing the different risk profiles and user capabilities. This distinction becomes particularly important for electrolyte monitoring devices that may be used in diverse environments with varying levels of professional oversight.
The European Union's regulatory approach has evolved significantly with the implementation of the In Vitro Diagnostic Regulation (IVDR 2017/746), which replaced the previous directive in May 2022. This regulation introduces stricter requirements for clinical evidence, post-market surveillance, and risk classification. POC devices incorporating innovative electrolyte materials typically fall under higher risk categories, necessitating notified body involvement and comprehensive technical documentation.
International harmonization efforts through the International Medical Device Regulators Forum (IMDRF) have established common principles for POC device regulation, though significant regional variations persist. The ISO 13485 standard for quality management systems serves as a foundational requirement across most jurisdictions, with additional standards like ISO 14971 for risk management being particularly relevant for devices employing novel electrolyte interfaces.
Regulatory considerations specifically for electrolyte materials in POC devices include biocompatibility testing requirements under ISO 10993, stability validation protocols, and performance standards for analytical accuracy. Materials that enable direct electrolyte measurement must demonstrate precision across clinically relevant ranges, with particular attention to interference studies and environmental stability.
Emerging regulatory trends indicate a shift toward adaptive regulatory pathways that accommodate rapid technological innovation while maintaining safety standards. Several jurisdictions have implemented expedited review programs for breakthrough technologies, potentially benefiting novel POC-electrolyte material combinations that demonstrate significant advantages over existing methods.
Data security and privacy regulations increasingly impact POC device approval, especially for connected devices that transmit patient electrolyte data. Compliance with regulations like GDPR in Europe and HIPAA in the US adds another layer of complexity to the regulatory framework, requiring manufacturers to implement robust data protection measures from the design phase.
Regulatory bodies are also developing specific guidance for POC devices intended for home use versus clinical settings, recognizing the different risk profiles and user capabilities. This distinction becomes particularly important for electrolyte monitoring devices that may be used in diverse environments with varying levels of professional oversight.
Sustainability Aspects of Electrolyte Materials
The sustainability of electrolyte materials in point-of-care devices represents a critical consideration in the evolving healthcare technology landscape. Current electrolyte materials often rely on rare earth elements and environmentally problematic compounds, creating significant sustainability challenges throughout their lifecycle. The environmental footprint of these materials extends from resource extraction through manufacturing to disposal, with mining operations for key components frequently associated with habitat destruction, water pollution, and high energy consumption.
Manufacturing processes for specialized electrolyte materials typically involve energy-intensive procedures and hazardous chemical treatments, contributing to carbon emissions and potential environmental contamination. The limited recyclability of many current electrolyte formulations further exacerbates sustainability concerns, as these materials often end up in landfills after their relatively short operational lifespan in point-of-care devices.
Recent innovations are addressing these sustainability challenges through several promising approaches. Bio-derived electrolyte materials, synthesized from renewable resources such as cellulose derivatives and alginate compounds, offer biodegradable alternatives with comparable electrochemical performance. These materials significantly reduce end-of-life environmental impact while maintaining the necessary conductivity properties for diagnostic applications.
Water-based electrolyte systems represent another sustainable direction, eliminating the need for organic solvents that pose environmental and health risks. These aqueous formulations demonstrate increasing stability and performance metrics while dramatically reducing toxicity concerns in both manufacturing and disposal phases.
Circular economy principles are increasingly being applied to electrolyte material development, with design-for-recycling approaches gaining traction. Advanced recovery techniques now enable the separation and reuse of valuable components from spent electrolytes, creating closed-loop systems that minimize resource depletion and waste generation.
Regulatory frameworks worldwide are evolving to address sustainability concerns in medical device materials. The European Union's Restriction of Hazardous Substances (RoHS) directive and similar regulations in other regions are driving manufacturers toward greener electrolyte formulations. These regulatory pressures, combined with growing consumer demand for environmentally responsible healthcare products, are accelerating the transition to sustainable alternatives.
The economic implications of sustainable electrolyte materials extend beyond environmental benefits. While initial development costs may be higher, the long-term advantages include reduced regulatory compliance expenses, decreased waste management costs, and potential market differentiation through sustainability credentials. As production scales increase, many bio-based and environmentally friendly electrolyte materials are achieving cost parity with traditional options.
Manufacturing processes for specialized electrolyte materials typically involve energy-intensive procedures and hazardous chemical treatments, contributing to carbon emissions and potential environmental contamination. The limited recyclability of many current electrolyte formulations further exacerbates sustainability concerns, as these materials often end up in landfills after their relatively short operational lifespan in point-of-care devices.
Recent innovations are addressing these sustainability challenges through several promising approaches. Bio-derived electrolyte materials, synthesized from renewable resources such as cellulose derivatives and alginate compounds, offer biodegradable alternatives with comparable electrochemical performance. These materials significantly reduce end-of-life environmental impact while maintaining the necessary conductivity properties for diagnostic applications.
Water-based electrolyte systems represent another sustainable direction, eliminating the need for organic solvents that pose environmental and health risks. These aqueous formulations demonstrate increasing stability and performance metrics while dramatically reducing toxicity concerns in both manufacturing and disposal phases.
Circular economy principles are increasingly being applied to electrolyte material development, with design-for-recycling approaches gaining traction. Advanced recovery techniques now enable the separation and reuse of valuable components from spent electrolytes, creating closed-loop systems that minimize resource depletion and waste generation.
Regulatory frameworks worldwide are evolving to address sustainability concerns in medical device materials. The European Union's Restriction of Hazardous Substances (RoHS) directive and similar regulations in other regions are driving manufacturers toward greener electrolyte formulations. These regulatory pressures, combined with growing consumer demand for environmentally responsible healthcare products, are accelerating the transition to sustainable alternatives.
The economic implications of sustainable electrolyte materials extend beyond environmental benefits. While initial development costs may be higher, the long-term advantages include reduced regulatory compliance expenses, decreased waste management costs, and potential market differentiation through sustainability credentials. As production scales increase, many bio-based and environmentally friendly electrolyte materials are achieving cost parity with traditional options.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!