Point-of-care Devices and Digital Health Integration Strategies
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
POC Devices and Digital Health Background & Objectives
Point-of-care (POC) devices have evolved significantly over the past two decades, transforming from simple glucose meters to sophisticated multi-parameter diagnostic platforms. This evolution has been driven by advances in microfluidics, biosensors, miniaturization technologies, and wireless connectivity. The integration of these devices with digital health ecosystems represents a paradigm shift in healthcare delivery, enabling real-time diagnostics, remote monitoring, and personalized medicine approaches previously unattainable in traditional healthcare settings.
The global healthcare landscape faces mounting challenges including aging populations, rising chronic disease prevalence, healthcare access disparities, and escalating costs. POC testing coupled with digital health integration offers a promising solution by decentralizing diagnostic capabilities while maintaining clinical accuracy and enhancing data utilization. This technological convergence aims to bridge critical gaps in healthcare delivery while improving patient outcomes and system efficiency.
Historical development of POC technologies has progressed through several distinct phases: from basic lateral flow assays and electrochemical sensors to current microfluidic lab-on-chip platforms and smartphone-enabled diagnostic systems. Parallel advancements in digital health infrastructure, including electronic health records, telehealth platforms, and health information exchanges, have created an environment ripe for meaningful integration with diagnostic technologies.
The primary objective of this technical research is to comprehensively evaluate current POC device technologies and identify optimal strategies for their integration with digital health ecosystems. Specific goals include assessing technical capabilities and limitations of contemporary POC platforms, analyzing connectivity standards and interoperability challenges, evaluating data security frameworks, and identifying emerging technologies that may accelerate integration efforts.
This research also aims to establish a technological roadmap for POC-digital health integration that addresses key technical barriers while maximizing clinical utility. By examining both established solutions and emerging innovations, we seek to identify architectures that enable seamless data flow from diagnostic devices to clinical decision support systems while maintaining regulatory compliance and ensuring data integrity.
The scope encompasses molecular diagnostics, immunoassays, clinical chemistry, hematology, and other diagnostic modalities in POC format, with particular emphasis on technologies demonstrating potential for meaningful digital integration. This investigation will provide critical insights to guide strategic R&D investments and inform product development priorities in this rapidly evolving technological landscape.
The global healthcare landscape faces mounting challenges including aging populations, rising chronic disease prevalence, healthcare access disparities, and escalating costs. POC testing coupled with digital health integration offers a promising solution by decentralizing diagnostic capabilities while maintaining clinical accuracy and enhancing data utilization. This technological convergence aims to bridge critical gaps in healthcare delivery while improving patient outcomes and system efficiency.
Historical development of POC technologies has progressed through several distinct phases: from basic lateral flow assays and electrochemical sensors to current microfluidic lab-on-chip platforms and smartphone-enabled diagnostic systems. Parallel advancements in digital health infrastructure, including electronic health records, telehealth platforms, and health information exchanges, have created an environment ripe for meaningful integration with diagnostic technologies.
The primary objective of this technical research is to comprehensively evaluate current POC device technologies and identify optimal strategies for their integration with digital health ecosystems. Specific goals include assessing technical capabilities and limitations of contemporary POC platforms, analyzing connectivity standards and interoperability challenges, evaluating data security frameworks, and identifying emerging technologies that may accelerate integration efforts.
This research also aims to establish a technological roadmap for POC-digital health integration that addresses key technical barriers while maximizing clinical utility. By examining both established solutions and emerging innovations, we seek to identify architectures that enable seamless data flow from diagnostic devices to clinical decision support systems while maintaining regulatory compliance and ensuring data integrity.
The scope encompasses molecular diagnostics, immunoassays, clinical chemistry, hematology, and other diagnostic modalities in POC format, with particular emphasis on technologies demonstrating potential for meaningful digital integration. This investigation will provide critical insights to guide strategic R&D investments and inform product development priorities in this rapidly evolving technological landscape.
Market Analysis for Integrated POC Solutions
The global Point-of-Care (POC) diagnostics market is experiencing robust growth, valued at approximately $29.5 billion in 2022 and projected to reach $50.6 billion by 2027, representing a compound annual growth rate (CAGR) of 11.4%. This growth is primarily driven by the increasing prevalence of chronic and infectious diseases, the aging global population, and the rising demand for rapid and accurate diagnostic solutions.
The integration of POC devices with digital health platforms represents a particularly dynamic segment within this market. This integrated approach combines the immediacy of POC testing with the connectivity and data management capabilities of digital health systems, creating comprehensive healthcare solutions that extend beyond traditional diagnostic boundaries.
North America currently dominates the integrated POC solutions market, accounting for roughly 40% of global market share. This leadership position stems from advanced healthcare infrastructure, favorable reimbursement policies, and high adoption rates of digital health technologies. The Asia-Pacific region, however, is emerging as the fastest-growing market with a CAGR exceeding 13%, fueled by improving healthcare access, increasing healthcare expenditure, and rapid technological adoption in countries like China and India.
By application segment, infectious disease testing represents the largest market share (32%) for integrated POC solutions, followed by cardiac markers (18%) and glucose monitoring (15%). The COVID-19 pandemic significantly accelerated market growth, with an unprecedented surge in demand for rapid diagnostic capabilities combined with digital reporting and tracking systems.
Hospital outpatient departments and emergency rooms constitute the primary end-users of integrated POC solutions (45% market share), followed by physician offices and urgent care centers (30%). However, the home healthcare segment is witnessing the fastest growth rate at 14.2% annually, reflecting the increasing consumer preference for self-monitoring and telehealth services.
Key market drivers include the push toward value-based healthcare models, growing patient preference for decentralized testing, and healthcare providers' need for efficient diagnostic workflows. The integration of artificial intelligence and machine learning capabilities with POC devices is creating new market opportunities, enabling predictive analytics and personalized medicine approaches.
Barriers to market expansion include concerns regarding data security and privacy, interoperability challenges between different systems, and varying regulatory frameworks across regions. Additionally, reimbursement uncertainties for integrated digital health solutions remain a significant obstacle in many markets, particularly for novel applications that don't fit traditional payment models.
The integration of POC devices with digital health platforms represents a particularly dynamic segment within this market. This integrated approach combines the immediacy of POC testing with the connectivity and data management capabilities of digital health systems, creating comprehensive healthcare solutions that extend beyond traditional diagnostic boundaries.
North America currently dominates the integrated POC solutions market, accounting for roughly 40% of global market share. This leadership position stems from advanced healthcare infrastructure, favorable reimbursement policies, and high adoption rates of digital health technologies. The Asia-Pacific region, however, is emerging as the fastest-growing market with a CAGR exceeding 13%, fueled by improving healthcare access, increasing healthcare expenditure, and rapid technological adoption in countries like China and India.
By application segment, infectious disease testing represents the largest market share (32%) for integrated POC solutions, followed by cardiac markers (18%) and glucose monitoring (15%). The COVID-19 pandemic significantly accelerated market growth, with an unprecedented surge in demand for rapid diagnostic capabilities combined with digital reporting and tracking systems.
Hospital outpatient departments and emergency rooms constitute the primary end-users of integrated POC solutions (45% market share), followed by physician offices and urgent care centers (30%). However, the home healthcare segment is witnessing the fastest growth rate at 14.2% annually, reflecting the increasing consumer preference for self-monitoring and telehealth services.
Key market drivers include the push toward value-based healthcare models, growing patient preference for decentralized testing, and healthcare providers' need for efficient diagnostic workflows. The integration of artificial intelligence and machine learning capabilities with POC devices is creating new market opportunities, enabling predictive analytics and personalized medicine approaches.
Barriers to market expansion include concerns regarding data security and privacy, interoperability challenges between different systems, and varying regulatory frameworks across regions. Additionally, reimbursement uncertainties for integrated digital health solutions remain a significant obstacle in many markets, particularly for novel applications that don't fit traditional payment models.
Technical Barriers in POC-Digital Health Integration
Despite significant advancements in both point-of-care (POC) technologies and digital health platforms, their integration faces substantial technical barriers that impede widespread implementation. The fundamental challenge lies in the heterogeneity of POC devices, which employ diverse communication protocols, data formats, and operating systems. This lack of standardization creates significant interoperability issues when attempting to connect these devices with digital health ecosystems.
Data security and privacy concerns represent another critical barrier. POC devices often collect sensitive patient information that must be transmitted securely to digital platforms. The implementation of robust encryption methods, secure authentication protocols, and compliance with regulations such as HIPAA and GDPR adds layers of complexity to integration efforts, particularly for smaller manufacturers with limited resources.
Connectivity limitations pose persistent challenges, especially in resource-constrained settings. Many POC devices operate in environments with unreliable internet access, requiring sophisticated data buffering and synchronization mechanisms. The technical solutions must accommodate both online and offline functionality while maintaining data integrity across transmission states.
Power management emerges as a significant constraint for portable POC devices. The energy demands of continuous wireless communication can dramatically reduce battery life, necessitating advanced power optimization techniques. This creates a technical trade-off between connectivity frequency and device longevity that integration strategies must address.
Data validation and quality assurance mechanisms represent sophisticated technical hurdles. Digital health platforms require reliable, accurate data for clinical decision support, yet POC devices vary considerably in their internal validation processes. Developing robust algorithms that can detect measurement anomalies across diverse device types requires significant computational expertise.
User interface harmonization between physical POC devices and digital platforms presents complex design challenges. Creating seamless workflows that transition between tangible devices and digital interfaces demands sophisticated UX engineering to maintain clinical efficiency and minimize user friction.
Scalability and version management constitute ongoing technical barriers. As POC devices receive firmware updates and digital platforms evolve, maintaining compatibility requires sophisticated version control systems. The technical architecture must accommodate both legacy devices and emerging technologies within a unified framework.
Computational resource limitations on POC devices restrict the implementation of advanced analytics at the edge. While cloud-based processing offers a solution, it introduces latency issues that may be problematic for time-sensitive clinical applications, creating a technical balancing act between local and remote processing capabilities.
Data security and privacy concerns represent another critical barrier. POC devices often collect sensitive patient information that must be transmitted securely to digital platforms. The implementation of robust encryption methods, secure authentication protocols, and compliance with regulations such as HIPAA and GDPR adds layers of complexity to integration efforts, particularly for smaller manufacturers with limited resources.
Connectivity limitations pose persistent challenges, especially in resource-constrained settings. Many POC devices operate in environments with unreliable internet access, requiring sophisticated data buffering and synchronization mechanisms. The technical solutions must accommodate both online and offline functionality while maintaining data integrity across transmission states.
Power management emerges as a significant constraint for portable POC devices. The energy demands of continuous wireless communication can dramatically reduce battery life, necessitating advanced power optimization techniques. This creates a technical trade-off between connectivity frequency and device longevity that integration strategies must address.
Data validation and quality assurance mechanisms represent sophisticated technical hurdles. Digital health platforms require reliable, accurate data for clinical decision support, yet POC devices vary considerably in their internal validation processes. Developing robust algorithms that can detect measurement anomalies across diverse device types requires significant computational expertise.
User interface harmonization between physical POC devices and digital platforms presents complex design challenges. Creating seamless workflows that transition between tangible devices and digital interfaces demands sophisticated UX engineering to maintain clinical efficiency and minimize user friction.
Scalability and version management constitute ongoing technical barriers. As POC devices receive firmware updates and digital platforms evolve, maintaining compatibility requires sophisticated version control systems. The technical architecture must accommodate both legacy devices and emerging technologies within a unified framework.
Computational resource limitations on POC devices restrict the implementation of advanced analytics at the edge. While cloud-based processing offers a solution, it introduces latency issues that may be problematic for time-sensitive clinical applications, creating a technical balancing act between local and remote processing capabilities.
Current Integration Architectures and Protocols
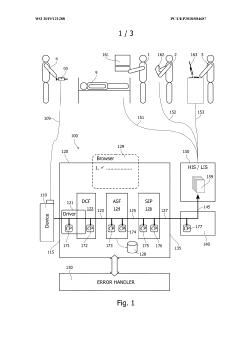
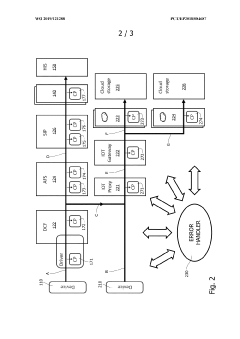
01 Integration of medical data systems for point-of-care devices
Integration of various medical data systems allows for seamless connectivity between point-of-care devices and healthcare information systems. This enables real-time data sharing, improved clinical decision-making, and enhanced patient care coordination. The integration facilitates automatic data transfer from diagnostic devices to electronic health records, reducing manual entry errors and improving workflow efficiency in healthcare settings.- Integration of medical data systems for point-of-care devices: Point-of-care devices can be integrated with medical data systems to enable seamless data collection, storage, and analysis. This integration allows healthcare providers to access patient information in real-time, improving clinical decision-making and patient care. The systems can incorporate electronic health records, laboratory results, and other clinical data to provide comprehensive patient information at the point of care.
- Connectivity solutions for point-of-care diagnostic devices: Various connectivity solutions enable point-of-care diagnostic devices to communicate with healthcare information systems. These solutions include wireless technologies, cloud-based platforms, and interoperability protocols that facilitate the transmission of test results and patient data. By implementing these connectivity solutions, point-of-care devices can be effectively integrated into clinical workflows, enhancing diagnostic capabilities and treatment decisions.
- Mobile health integration with point-of-care testing: Mobile health technologies can be integrated with point-of-care testing devices to extend healthcare delivery beyond traditional clinical settings. This integration enables remote monitoring, telemedicine consultations, and home-based testing with real-time data transmission to healthcare providers. The combination of mobile platforms and point-of-care devices creates opportunities for more accessible and efficient healthcare services, particularly in underserved or remote areas.
- Interoperability standards for point-of-care device integration: Interoperability standards are essential for successful integration of point-of-care devices into healthcare systems. These standards define protocols for data exchange, device communication, and system interfaces, ensuring that different devices and systems can work together seamlessly. Implementation of these standards facilitates the integration of point-of-care devices from various manufacturers into existing healthcare IT infrastructure, improving workflow efficiency and data consistency.
- Clinical workflow optimization for integrated point-of-care solutions: Integration of point-of-care devices requires optimization of clinical workflows to maximize efficiency and effectiveness. This involves redesigning processes to incorporate point-of-care testing into patient care pathways, automating data capture and documentation, and providing decision support tools at the point of care. Properly optimized workflows ensure that the benefits of integrated point-of-care devices are fully realized, leading to improved patient outcomes and healthcare provider satisfaction.
02 Mobile point-of-care diagnostic platforms
Mobile platforms for point-of-care diagnostics incorporate portable testing devices with wireless connectivity capabilities. These solutions enable healthcare providers to perform diagnostic tests at the patient's location and immediately transmit results to central healthcare systems. The mobile integration supports remote patient monitoring, telemedicine applications, and extends healthcare access to underserved or rural populations.Expand Specific Solutions03 Interoperability standards for medical device integration
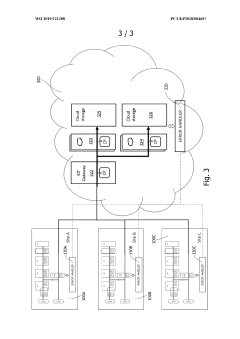
Implementation of interoperability standards ensures that point-of-care devices from different manufacturers can communicate effectively within healthcare ecosystems. These standards define protocols for data exchange, device connectivity, and system integration, allowing for plug-and-play functionality. Standardized interfaces reduce implementation costs, simplify system maintenance, and enable healthcare facilities to integrate diverse diagnostic equipment into unified networks.Expand Specific Solutions04 Cloud-based integration platforms for healthcare devices
Cloud-based integration platforms provide centralized infrastructure for connecting point-of-care devices across healthcare networks. These solutions offer scalable data storage, advanced analytics capabilities, and secure access to diagnostic information from multiple locations. Cloud integration enables remote device management, software updates, and facilitates collaborative care models where specialists can access diagnostic results regardless of physical location.Expand Specific Solutions05 Security and privacy frameworks for integrated medical devices
Security and privacy frameworks protect sensitive patient data when integrating point-of-care devices into healthcare networks. These frameworks implement encryption, authentication mechanisms, and access controls to safeguard information during transmission and storage. Comprehensive security approaches address regulatory compliance requirements while maintaining data integrity and confidentiality across integrated diagnostic systems.Expand Specific Solutions
Leading Companies in POC and Digital Health Ecosystem
The point-of-care devices and digital health integration market is currently in a growth phase, characterized by rapid technological advancement and expanding applications. The global market size is projected to reach approximately $85 billion by 2027, growing at a CAGR of 15-20%. From a technological maturity perspective, the landscape shows varying degrees of development. Established players like Siemens Healthineers, Becton Dickinson, and Baxter International have robust product portfolios with FDA-approved solutions, while newer entrants such as Cognoa and In Diagnostics are introducing innovative AI-driven diagnostic platforms. IBM is leveraging its data analytics capabilities to enhance integration between point-of-care devices and healthcare systems, while companies like Radiometer and Drägerwerk focus on specialized diagnostic equipment with digital connectivity features. The convergence of medical device manufacturing expertise and digital health capabilities is driving strategic partnerships across the ecosystem.
International Business Machines Corp.
Technical Solution: IBM has developed a comprehensive digital health integration platform leveraging their Watson Health technology to connect point-of-care devices with enterprise healthcare systems. Their strategy employs edge computing architecture to process data from medical devices locally before secure transmission to cloud infrastructure, reducing latency for time-critical applications. IBM's approach incorporates blockchain technology for secure, immutable record-keeping of patient data across distributed healthcare networks. The company has implemented FHIR-based APIs to facilitate standardized data exchange between point-of-care devices and electronic health record systems. Their digital health integration strategy emphasizes AI-powered analytics that can identify patterns in patient data collected from various point-of-care devices, enabling predictive insights and clinical decision support. IBM has also developed specialized IoT management tools for healthcare organizations to monitor device fleets, manage software updates, and ensure compliance with security protocols across distributed care settings. The platform includes natural language processing capabilities to transform unstructured clinical notes into structured data that can be integrated with quantitative measurements from point-of-care devices.
Strengths: Advanced AI and analytics capabilities; robust enterprise-grade security infrastructure; extensive experience with large-scale data integration projects. Weaknesses: Solutions may be overly complex for smaller healthcare organizations; implementation typically requires significant customization; higher cost compared to more specialized healthcare IT vendors.
Becton, Dickinson & Co.
Technical Solution: BD has pioneered an integrated point-of-care testing (POCT) ecosystem through their BD Synapsys™ Informatics Solution. This platform connects various point-of-care devices to centralized laboratory and hospital information systems, enabling real-time data transmission and analysis. Their strategy focuses on middleware solutions that bridge the gap between diagnostic devices and electronic health records, ensuring seamless data flow across the healthcare continuum. BD has developed specialized connectivity solutions for microbiology, molecular diagnostics, and blood glucose monitoring at the point of care. Their approach emphasizes standardized data formats and communication protocols to facilitate integration with third-party systems. The company has also implemented remote device management capabilities, allowing healthcare IT departments to monitor device status, update software, and troubleshoot issues across distributed point-of-care testing locations. BD's digital health integration strategy includes robust quality control and regulatory compliance features to meet laboratory accreditation requirements while simplifying the management of decentralized testing programs.
Strengths: Extensive experience in laboratory informatics and connectivity; strong focus on regulatory compliance features; broad portfolio of compatible diagnostic devices. Weaknesses: Integration complexity with non-BD devices may require additional middleware; implementation requires significant IT infrastructure investment; ongoing subscription costs for cloud-based services.
Key Patents and Standards in POC-Digital Integration
System and method for processing patient-related medical data
PatentWO2019121288A1
Innovation
- A patient sample analysis system with check points and an error handler that identifies measurement results by unique identifiers, adds status entries, and localizes errors in the data path, enabling efficient error correction and ensuring timely delivery of accurate data.
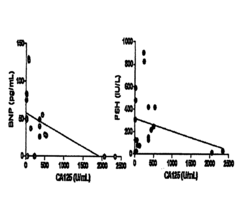
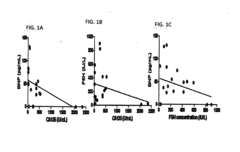
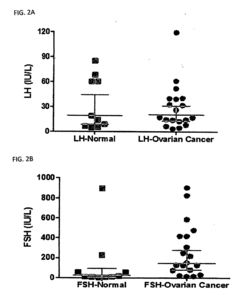
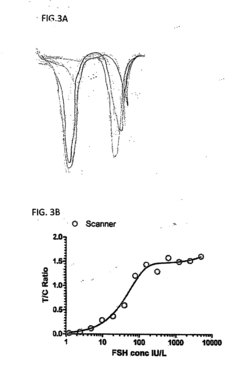
Methods and Compositions for Personalized Medicine by Point-of-Care Devices for FSH, LH, HCG and BNP
PatentInactiveUS20180074063A1
Innovation
- Development of methods and devices for quantitating biomarkers such as gonadotropins (hCG, LH, and FSH) and BNP using point-of-care devices with expanded dynamic range, allowing for self-sampling and storage on matrices for later analysis, enabling personalized dosing and monitoring of ovarian and other cancers.
Data Security and Privacy Frameworks
The integration of point-of-care devices with digital health systems necessitates robust data security and privacy frameworks to protect sensitive patient information. Current frameworks incorporate multi-layered security approaches, including end-to-end encryption, secure authentication mechanisms, and role-based access controls. These measures ensure that patient data remains confidential throughout its lifecycle, from collection at the point-of-care device to storage in centralized health information systems.
Regulatory standards such as HIPAA in the United States, GDPR in Europe, and similar frameworks globally have established baseline requirements for handling healthcare data. These regulations mandate specific security controls, breach notification protocols, and patient rights regarding their health information. Point-of-care device manufacturers and digital health platform providers must design their systems with these regulatory requirements as foundational elements.
Emerging technologies like blockchain are being explored to enhance data integrity and auditability in point-of-care ecosystems. Blockchain implementations provide immutable records of data access and modifications, creating transparent audit trails while maintaining patient privacy. Several pilot projects have demonstrated the feasibility of blockchain-based consent management systems that give patients granular control over who can access their health data.
Federated learning approaches represent another innovative security framework gaining traction. This technique allows machine learning models to be trained across multiple decentralized edge devices containing local data samples, without exchanging the data itself. For point-of-care devices, this means valuable insights can be derived from collective data while raw patient information remains securely on local devices.
Zero-trust security architectures are increasingly being adopted in digital health integration strategies. This approach operates on the principle of "never trust, always verify," requiring continuous authentication and authorization for all users and devices accessing the system, regardless of their location relative to the network perimeter. This model is particularly relevant for distributed point-of-care devices that may operate across various healthcare settings.
Privacy-preserving computation techniques, including homomorphic encryption and secure multi-party computation, enable analysis of encrypted data without decryption. These advanced methods allow healthcare organizations to derive insights from sensitive point-of-care data while maintaining patient privacy, facilitating secure collaboration between institutions without compromising confidentiality.
As point-of-care devices become more sophisticated and interconnected, security frameworks must evolve to address emerging threats. Future frameworks will likely incorporate artificial intelligence for anomaly detection, adaptive security measures that respond to changing threat landscapes, and standardized security protocols specifically designed for medical Internet of Things (IoT) devices.
Regulatory standards such as HIPAA in the United States, GDPR in Europe, and similar frameworks globally have established baseline requirements for handling healthcare data. These regulations mandate specific security controls, breach notification protocols, and patient rights regarding their health information. Point-of-care device manufacturers and digital health platform providers must design their systems with these regulatory requirements as foundational elements.
Emerging technologies like blockchain are being explored to enhance data integrity and auditability in point-of-care ecosystems. Blockchain implementations provide immutable records of data access and modifications, creating transparent audit trails while maintaining patient privacy. Several pilot projects have demonstrated the feasibility of blockchain-based consent management systems that give patients granular control over who can access their health data.
Federated learning approaches represent another innovative security framework gaining traction. This technique allows machine learning models to be trained across multiple decentralized edge devices containing local data samples, without exchanging the data itself. For point-of-care devices, this means valuable insights can be derived from collective data while raw patient information remains securely on local devices.
Zero-trust security architectures are increasingly being adopted in digital health integration strategies. This approach operates on the principle of "never trust, always verify," requiring continuous authentication and authorization for all users and devices accessing the system, regardless of their location relative to the network perimeter. This model is particularly relevant for distributed point-of-care devices that may operate across various healthcare settings.
Privacy-preserving computation techniques, including homomorphic encryption and secure multi-party computation, enable analysis of encrypted data without decryption. These advanced methods allow healthcare organizations to derive insights from sensitive point-of-care data while maintaining patient privacy, facilitating secure collaboration between institutions without compromising confidentiality.
As point-of-care devices become more sophisticated and interconnected, security frameworks must evolve to address emerging threats. Future frameworks will likely incorporate artificial intelligence for anomaly detection, adaptive security measures that respond to changing threat landscapes, and standardized security protocols specifically designed for medical Internet of Things (IoT) devices.
Interoperability Standards and Challenges
Interoperability remains the cornerstone challenge in integrating point-of-care (POC) devices with broader digital health ecosystems. The healthcare industry has developed several standards to address this challenge, with HL7 FHIR (Fast Healthcare Interoperability Resources) emerging as the dominant framework. FHIR provides a standardized API for exchanging healthcare information electronically, enabling POC devices to communicate seamlessly with electronic health records (EHRs) and other clinical systems.
Despite these advances, significant challenges persist in achieving true interoperability. Device manufacturers often implement proprietary data formats and communication protocols, creating siloed ecosystems that impede data exchange. This fragmentation is particularly problematic in clinical settings where multiple devices from different vendors must work in concert to deliver comprehensive patient care.
Technical barriers further complicate interoperability efforts. Legacy systems with outdated interfaces coexist alongside modern cloud-connected devices, creating a heterogeneous environment that requires complex integration solutions. Data mapping between different standards remains labor-intensive, with variations in terminology and data structures necessitating custom transformation logic for each integration point.
Security and privacy considerations add another layer of complexity. POC devices must adhere to stringent regulatory requirements such as HIPAA in the United States and GDPR in Europe, which mandate secure data transmission and storage. Implementing these security measures while maintaining seamless interoperability requires sophisticated authentication mechanisms and encryption protocols that can function across diverse system architectures.
Governance frameworks for interoperability standards present additional challenges. While organizations like IHE (Integrating the Healthcare Enterprise) provide implementation guides and conformance testing, adoption remains inconsistent across the industry. The lack of universal certification requirements allows manufacturers to claim "standards compliance" while implementing only partial or modified versions of established standards.
Recent initiatives like the USCDI (United States Core Data for Interoperability) aim to standardize the essential data elements that should be exchangeable across systems. Similarly, the European EHR Exchange Format seeks to harmonize health data exchange across EU member states. These efforts represent important steps toward resolving interoperability challenges, though full implementation remains a work in progress.
The economic aspects of interoperability cannot be overlooked. Implementing comprehensive interoperability solutions requires significant investment from device manufacturers, healthcare providers, and health IT vendors. Without clear financial incentives or regulatory mandates, many organizations prioritize short-term market considerations over long-term interoperability goals.
Despite these advances, significant challenges persist in achieving true interoperability. Device manufacturers often implement proprietary data formats and communication protocols, creating siloed ecosystems that impede data exchange. This fragmentation is particularly problematic in clinical settings where multiple devices from different vendors must work in concert to deliver comprehensive patient care.
Technical barriers further complicate interoperability efforts. Legacy systems with outdated interfaces coexist alongside modern cloud-connected devices, creating a heterogeneous environment that requires complex integration solutions. Data mapping between different standards remains labor-intensive, with variations in terminology and data structures necessitating custom transformation logic for each integration point.
Security and privacy considerations add another layer of complexity. POC devices must adhere to stringent regulatory requirements such as HIPAA in the United States and GDPR in Europe, which mandate secure data transmission and storage. Implementing these security measures while maintaining seamless interoperability requires sophisticated authentication mechanisms and encryption protocols that can function across diverse system architectures.
Governance frameworks for interoperability standards present additional challenges. While organizations like IHE (Integrating the Healthcare Enterprise) provide implementation guides and conformance testing, adoption remains inconsistent across the industry. The lack of universal certification requirements allows manufacturers to claim "standards compliance" while implementing only partial or modified versions of established standards.
Recent initiatives like the USCDI (United States Core Data for Interoperability) aim to standardize the essential data elements that should be exchangeable across systems. Similarly, the European EHR Exchange Format seeks to harmonize health data exchange across EU member states. These efforts represent important steps toward resolving interoperability challenges, though full implementation remains a work in progress.
The economic aspects of interoperability cannot be overlooked. Implementing comprehensive interoperability solutions requires significant investment from device manufacturers, healthcare providers, and health IT vendors. Without clear financial incentives or regulatory mandates, many organizations prioritize short-term market considerations over long-term interoperability goals.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!