Point-of-care Devices and Real-time Data Integration
SEP 19, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
POC Device Evolution and Objectives
Point-of-care (POC) testing has evolved significantly over the past three decades, transforming from basic glucose meters and pregnancy tests to sophisticated multiparameter analytical platforms. The evolution began in the 1980s with simple qualitative tests, progressing through the 1990s with the introduction of quantitative measurements, and accelerating in the 2000s with the integration of digital technologies and connectivity features.
The primary objective of modern POC devices is to deliver laboratory-quality diagnostic results at or near the patient location, enabling immediate clinical decisions without the delays associated with central laboratory testing. This immediacy is particularly crucial in emergency settings, remote healthcare facilities, and resource-limited environments where traditional laboratory infrastructure is unavailable.
Recent technological advancements have dramatically expanded POC capabilities, incorporating microfluidics, biosensors, miniaturized optics, and advanced signal processing algorithms. These innovations have enabled the development of portable devices capable of performing multiple tests simultaneously with improved sensitivity and specificity. The integration of wireless connectivity, cloud computing, and artificial intelligence has further enhanced the utility of POC devices by enabling real-time data transmission, analysis, and interpretation.
The COVID-19 pandemic served as a catalyst for POC innovation, accelerating the development and deployment of rapid diagnostic tests and highlighting the critical importance of decentralized testing capabilities. This global health crisis demonstrated the value of POC testing in disease surveillance, patient triage, and pandemic response management.
Current technological objectives for POC devices focus on several key areas: improving analytical performance to match central laboratory standards; enhancing connectivity for seamless integration with electronic health records; reducing cost and complexity to enable broader adoption; and developing multiplexed testing capabilities to simultaneously detect multiple analytes from a single sample.
Looking forward, the convergence of POC testing with real-time data integration represents a paradigm shift in healthcare delivery. The ability to generate actionable diagnostic information at the point of patient contact and immediately incorporate this data into clinical decision support systems promises to improve patient outcomes, reduce healthcare costs, and enable more personalized treatment approaches. This integration also supports the broader transition toward value-based healthcare by providing timely information for clinical decision-making and facilitating continuous monitoring of treatment effectiveness.
The primary objective of modern POC devices is to deliver laboratory-quality diagnostic results at or near the patient location, enabling immediate clinical decisions without the delays associated with central laboratory testing. This immediacy is particularly crucial in emergency settings, remote healthcare facilities, and resource-limited environments where traditional laboratory infrastructure is unavailable.
Recent technological advancements have dramatically expanded POC capabilities, incorporating microfluidics, biosensors, miniaturized optics, and advanced signal processing algorithms. These innovations have enabled the development of portable devices capable of performing multiple tests simultaneously with improved sensitivity and specificity. The integration of wireless connectivity, cloud computing, and artificial intelligence has further enhanced the utility of POC devices by enabling real-time data transmission, analysis, and interpretation.
The COVID-19 pandemic served as a catalyst for POC innovation, accelerating the development and deployment of rapid diagnostic tests and highlighting the critical importance of decentralized testing capabilities. This global health crisis demonstrated the value of POC testing in disease surveillance, patient triage, and pandemic response management.
Current technological objectives for POC devices focus on several key areas: improving analytical performance to match central laboratory standards; enhancing connectivity for seamless integration with electronic health records; reducing cost and complexity to enable broader adoption; and developing multiplexed testing capabilities to simultaneously detect multiple analytes from a single sample.
Looking forward, the convergence of POC testing with real-time data integration represents a paradigm shift in healthcare delivery. The ability to generate actionable diagnostic information at the point of patient contact and immediately incorporate this data into clinical decision support systems promises to improve patient outcomes, reduce healthcare costs, and enable more personalized treatment approaches. This integration also supports the broader transition toward value-based healthcare by providing timely information for clinical decision-making and facilitating continuous monitoring of treatment effectiveness.
Market Analysis for Real-time Healthcare Solutions
The global market for Point-of-Care (POC) devices integrated with real-time data capabilities is experiencing unprecedented growth, driven by increasing demand for immediate diagnostic results and the need for efficient healthcare delivery systems. Current market valuations indicate that the POC testing market reached approximately 29 billion USD in 2022, with projections suggesting a compound annual growth rate of 11.4% through 2030. The real-time data integration segment within this market is growing even faster, at nearly 14% annually.
Healthcare providers worldwide are increasingly adopting these technologies to reduce hospital readmissions, decrease emergency department visits, and improve patient outcomes. A significant market driver is the rising prevalence of chronic diseases such as diabetes, cardiovascular conditions, and respiratory disorders, which require continuous monitoring and immediate intervention. The global diabetes monitoring device market alone contributes substantially to this growth, with continuous glucose monitoring systems seeing adoption rates increasing by over 25% annually in developed markets.
Regional analysis reveals North America currently dominates the market with approximately 42% share, followed by Europe at 28% and Asia-Pacific at 22%. However, the highest growth rates are being observed in emerging economies, particularly in India and China, where healthcare infrastructure development and increasing healthcare expenditure are creating new market opportunities.
Consumer demand patterns show a clear shift toward connected health solutions that offer seamless integration with smartphones and cloud platforms. Nearly 67% of patients surveyed across major markets express preference for healthcare solutions that provide real-time feedback and remote monitoring capabilities. This consumer preference is reshaping product development strategies across the industry.
The reimbursement landscape is evolving favorably for POC technologies, with many insurance providers now covering remote patient monitoring and telehealth services. This shift in payment models has accelerated following the COVID-19 pandemic, which served as a catalyst for telehealth adoption and remote monitoring solutions.
Market segmentation analysis reveals that hospital-based POC testing currently accounts for the largest market share at 38%, followed by clinics and physician offices at 29%, and home-based testing at 24%. However, the home-based segment is experiencing the fastest growth rate as healthcare systems worldwide embrace decentralized care models.
Key market challenges include interoperability issues between different healthcare IT systems, data security concerns, and varying regulatory requirements across regions. Despite these challenges, the market outlook remains highly positive, with technological advancements in miniaturization, connectivity, and AI-driven analytics continuing to expand the application scope and market potential of real-time healthcare solutions.
Healthcare providers worldwide are increasingly adopting these technologies to reduce hospital readmissions, decrease emergency department visits, and improve patient outcomes. A significant market driver is the rising prevalence of chronic diseases such as diabetes, cardiovascular conditions, and respiratory disorders, which require continuous monitoring and immediate intervention. The global diabetes monitoring device market alone contributes substantially to this growth, with continuous glucose monitoring systems seeing adoption rates increasing by over 25% annually in developed markets.
Regional analysis reveals North America currently dominates the market with approximately 42% share, followed by Europe at 28% and Asia-Pacific at 22%. However, the highest growth rates are being observed in emerging economies, particularly in India and China, where healthcare infrastructure development and increasing healthcare expenditure are creating new market opportunities.
Consumer demand patterns show a clear shift toward connected health solutions that offer seamless integration with smartphones and cloud platforms. Nearly 67% of patients surveyed across major markets express preference for healthcare solutions that provide real-time feedback and remote monitoring capabilities. This consumer preference is reshaping product development strategies across the industry.
The reimbursement landscape is evolving favorably for POC technologies, with many insurance providers now covering remote patient monitoring and telehealth services. This shift in payment models has accelerated following the COVID-19 pandemic, which served as a catalyst for telehealth adoption and remote monitoring solutions.
Market segmentation analysis reveals that hospital-based POC testing currently accounts for the largest market share at 38%, followed by clinics and physician offices at 29%, and home-based testing at 24%. However, the home-based segment is experiencing the fastest growth rate as healthcare systems worldwide embrace decentralized care models.
Key market challenges include interoperability issues between different healthcare IT systems, data security concerns, and varying regulatory requirements across regions. Despite these challenges, the market outlook remains highly positive, with technological advancements in miniaturization, connectivity, and AI-driven analytics continuing to expand the application scope and market potential of real-time healthcare solutions.
Technical Barriers in POC Data Integration
Despite significant advancements in Point-of-Care (POC) technology, several technical barriers continue to impede seamless data integration from these devices into healthcare systems. The heterogeneity of POC devices presents a fundamental challenge, with various manufacturers employing proprietary data formats, communication protocols, and APIs. This lack of standardization creates significant interoperability issues when attempting to consolidate data from multiple devices into a unified system.
Connectivity limitations further exacerbate integration challenges. Many POC devices operate in environments with unreliable internet access or bandwidth constraints. The intermittent connectivity results in asynchronous data transmission, creating complications for real-time monitoring systems that require continuous data streams. Additionally, legacy POC devices often lack modern communication capabilities entirely, necessitating manual data entry or custom hardware interfaces.
Data security and privacy requirements introduce another layer of complexity. Healthcare data transmission must comply with regulations like HIPAA, GDPR, and other regional healthcare data protection frameworks. Implementing robust encryption, authentication, and access control mechanisms across diverse POC devices while maintaining usability for healthcare professionals remains technically challenging, particularly for resource-constrained devices.
The quality and reliability of POC-generated data present significant technical hurdles. Ensuring data accuracy, precision, and reliability across different environmental conditions requires sophisticated calibration and validation mechanisms. Many POC devices lack built-in data validation capabilities, potentially leading to erroneous clinical decisions if invalid data enters the healthcare system undetected.
Resource constraints of POC devices themselves create integration barriers. Limited processing power, memory, and battery life restrict the implementation of complex data processing, encryption, or communication protocols directly on the devices. These constraints often necessitate compromises in functionality or require additional gateway hardware to facilitate integration.
Scalability challenges emerge when deploying POC solutions across large healthcare networks. The technical infrastructure must accommodate thousands of devices generating continuous data streams while maintaining performance, reliability, and security. Current healthcare IT systems frequently struggle with this volume and velocity of data, particularly when real-time analytics are required.
Lastly, the absence of comprehensive technical standards specifically addressing POC data integration remains a significant barrier. While standards like HL7 FHIR provide frameworks for healthcare data exchange, they often require adaptation for the unique constraints and requirements of POC environments. The development and adoption of specialized standards for POC data integration continue to lag behind the rapid proliferation of these devices in clinical settings.
Connectivity limitations further exacerbate integration challenges. Many POC devices operate in environments with unreliable internet access or bandwidth constraints. The intermittent connectivity results in asynchronous data transmission, creating complications for real-time monitoring systems that require continuous data streams. Additionally, legacy POC devices often lack modern communication capabilities entirely, necessitating manual data entry or custom hardware interfaces.
Data security and privacy requirements introduce another layer of complexity. Healthcare data transmission must comply with regulations like HIPAA, GDPR, and other regional healthcare data protection frameworks. Implementing robust encryption, authentication, and access control mechanisms across diverse POC devices while maintaining usability for healthcare professionals remains technically challenging, particularly for resource-constrained devices.
The quality and reliability of POC-generated data present significant technical hurdles. Ensuring data accuracy, precision, and reliability across different environmental conditions requires sophisticated calibration and validation mechanisms. Many POC devices lack built-in data validation capabilities, potentially leading to erroneous clinical decisions if invalid data enters the healthcare system undetected.
Resource constraints of POC devices themselves create integration barriers. Limited processing power, memory, and battery life restrict the implementation of complex data processing, encryption, or communication protocols directly on the devices. These constraints often necessitate compromises in functionality or require additional gateway hardware to facilitate integration.
Scalability challenges emerge when deploying POC solutions across large healthcare networks. The technical infrastructure must accommodate thousands of devices generating continuous data streams while maintaining performance, reliability, and security. Current healthcare IT systems frequently struggle with this volume and velocity of data, particularly when real-time analytics are required.
Lastly, the absence of comprehensive technical standards specifically addressing POC data integration remains a significant barrier. While standards like HL7 FHIR provide frameworks for healthcare data exchange, they often require adaptation for the unique constraints and requirements of POC environments. The development and adoption of specialized standards for POC data integration continue to lag behind the rapid proliferation of these devices in clinical settings.
Current Integration Architectures for POC Data
01 Integration of medical data from point-of-care devices
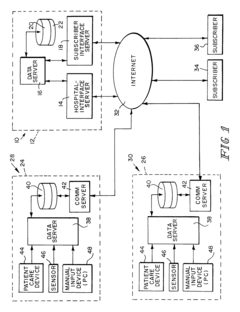
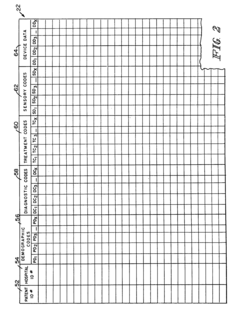
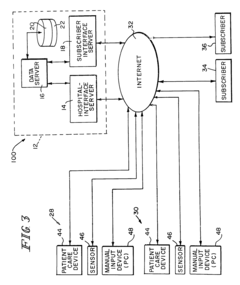
Systems that enable the collection and integration of medical data from various point-of-care devices into centralized healthcare information systems. These solutions facilitate real-time data capture from diagnostic equipment, allowing healthcare providers to access patient information immediately for timely decision-making. The integration frameworks support standardized data formats and protocols to ensure seamless communication between different devices and electronic health record systems.- Integration of medical data from point-of-care devices: Systems that enable the collection and integration of medical data from various point-of-care devices into centralized healthcare information systems. These solutions facilitate real-time access to patient data across different healthcare settings, allowing for improved clinical decision-making and patient monitoring. The integration platforms support various data formats and communication protocols to ensure seamless data flow from diverse medical devices.
- Cloud-based platforms for real-time healthcare data management: Cloud computing solutions specifically designed for healthcare data integration from point-of-care devices. These platforms provide secure, scalable infrastructure for storing, processing, and analyzing medical data in real-time. They enable healthcare providers to access patient information remotely, facilitate telemedicine services, and support collaborative care models while maintaining data privacy and compliance with healthcare regulations.
- Mobile health applications for point-of-care data collection: Mobile applications designed to interface with point-of-care devices and facilitate real-time data collection and transmission. These solutions enable healthcare providers to capture patient data at the bedside or in remote settings using smartphones or tablets. The applications often include features for data visualization, preliminary analysis, and secure transmission to electronic health record systems, enhancing the efficiency of healthcare delivery.
- Interoperability standards for medical device data integration: Technical standards and protocols that enable seamless communication and data exchange between point-of-care devices and healthcare information systems. These interoperability frameworks define common data formats, communication protocols, and security requirements to ensure that medical devices from different manufacturers can effectively share information. Implementation of these standards facilitates real-time data integration across diverse healthcare environments.
- AI-powered analytics for point-of-care data interpretation: Artificial intelligence and machine learning solutions that analyze real-time data from point-of-care devices to provide clinical decision support. These systems can identify patterns, predict adverse events, and generate alerts based on integrated patient data. By processing large volumes of medical information in real-time, these technologies help healthcare providers make more informed decisions at the point of care, potentially improving patient outcomes.
02 Cloud-based platforms for real-time healthcare data management
Cloud computing platforms specifically designed for healthcare applications that enable real-time collection, storage, and analysis of point-of-care device data. These solutions provide secure, scalable infrastructure for managing large volumes of patient data from distributed sources. The platforms incorporate features such as data encryption, access controls, and compliance with healthcare regulations while supporting real-time synchronization across multiple facilities and devices.Expand Specific Solutions03 Mobile health monitoring and data transmission systems
Mobile-based solutions that enable continuous patient monitoring through portable point-of-care devices with real-time data transmission capabilities. These systems utilize smartphones, tablets, and wearable devices to collect vital signs and other health metrics, transmitting them instantly to healthcare providers. The technology incorporates wireless communication protocols, mobile applications, and edge computing to ensure reliable data transfer even in areas with limited connectivity.Expand Specific Solutions04 Interoperability frameworks for healthcare data exchange
Standardized frameworks and protocols designed to enable seamless data exchange between different point-of-care devices, healthcare information systems, and electronic health records. These interoperability solutions address challenges related to data format inconsistencies, terminology differences, and system incompatibilities. By implementing common data models, API standards, and exchange protocols, these frameworks facilitate real-time integration of diagnostic results and patient information across the healthcare ecosystem.Expand Specific Solutions05 AI-enhanced analytics for point-of-care data processing
Artificial intelligence and machine learning systems that process and analyze data from point-of-care devices in real-time to support clinical decision-making. These solutions can identify patterns, predict outcomes, and generate alerts based on integrated patient data. The AI algorithms can process multiple data streams simultaneously, extracting meaningful insights from complex medical information and presenting them to healthcare providers through intuitive dashboards and visualization tools.Expand Specific Solutions
Industry Leaders in POC Device Ecosystem
The point-of-care devices and real-time data integration market is currently in a growth phase, with an expanding global market driven by increasing demand for immediate diagnostic results and integrated healthcare solutions. Key players include established medical technology companies like Siemens Healthcare Diagnostics, Abbott Point of Care, and Philips, alongside specialized innovators such as Capsule Technologies. The technology landscape shows varying maturity levels, with companies like Baxter International and Becton Dickinson offering comprehensive solutions, while Mindray and FUJIFILM SonoSite focus on portable diagnostics. Integration capabilities are being enhanced by technology partners like IBM and Genesys Cloud Services, creating an ecosystem where clinical data flows seamlessly between point-of-care devices and healthcare information systems.
Abbott Point of Care, Inc.
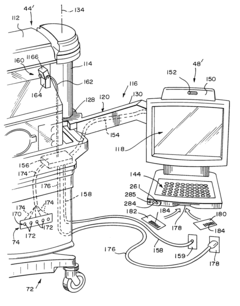
Technical Solution: Abbott's i-STAT system represents a pioneering approach to point-of-care testing with real-time data integration capabilities. The system combines handheld analyzers with cartridge-based testing for blood analysis, delivering laboratory-quality results in minutes at the patient's bedside. Their technology integrates wireless connectivity protocols that enable immediate transmission of test results to electronic health records (EHR) systems through their RALS-Plus data management solution. The i-STAT Alinity platform further enhances this capability with improved connectivity features, allowing healthcare providers to access patient data remotely and make timely clinical decisions. Abbott has implemented edge computing techniques to process data locally on devices before transmission, reducing latency and bandwidth requirements while ensuring data security through end-to-end encryption protocols. Their system architecture supports bidirectional communication, enabling not only result transmission but also receipt of updated reference ranges and quality control parameters from central laboratories.
Strengths: Comprehensive integration with hospital information systems provides seamless workflow and reduces transcription errors. The portable nature of devices allows for testing in various clinical settings including emergency departments, operating rooms, and intensive care units. Weaknesses: The system requires significant initial investment in infrastructure and training. Connectivity issues in certain healthcare environments with limited wireless coverage can disrupt real-time data transmission.
Shenzhen Mindray Bio-Medical Electronics Co., Ltd.
Technical Solution: Mindray has developed the M-IoT (Medical Internet of Things) platform that serves as the foundation for their point-of-care device integration strategy. Their approach focuses on creating a unified clinical data environment that connects bedside monitors, infusion systems, anesthesia workstations, and laboratory devices through a centralized management system. The BeneVision Distributed Monitoring System represents their flagship solution, enabling seamless data flow between point-of-care devices and hospital information systems. Mindray employs a modular architecture that allows healthcare facilities to scale their integration capabilities according to specific needs and budgets. Their technology utilizes edge computing principles to process critical data locally on devices while transmitting aggregated information to central servers, optimizing bandwidth usage and ensuring continuity during network disruptions. The platform supports standard protocols including HL7, DICOM, and IHE profiles to facilitate interoperability with existing hospital systems. Mindray has also implemented machine learning algorithms that analyze device usage patterns to predict maintenance needs and optimize resource allocation across healthcare facilities.
Strengths: Cost-effective integration solutions make advanced connectivity accessible to healthcare facilities with varying budget constraints. Modular approach allows for incremental implementation without requiring complete system overhauls. Weaknesses: Less established presence in Western markets compared to competitors may result in more limited third-party integration options. Documentation and support resources may not be as comprehensive as those offered by larger multinational competitors.
Key Patents in Real-time Health Data Processing
Apparatus and method for patient point-of-care data management
PatentInactiveUS7038588B2
Innovation
- A healthcare data system that collects and distributes patient point-of-care data by using a central database to store data from multiple healthcare facilities, allowing authorized users to access online, real-time or near-real-time data through a network, including the Internet, with features like token-based access control and integration of sensors and devices for data collection.
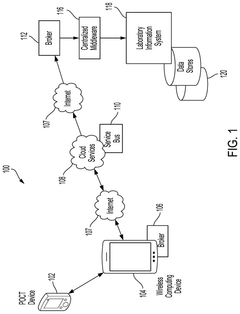
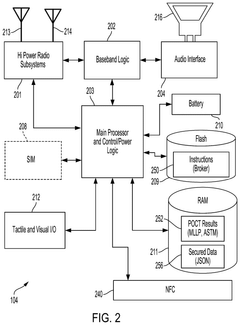
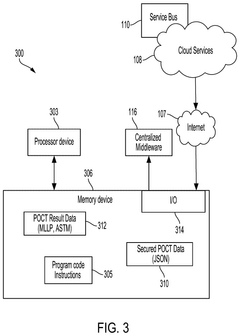
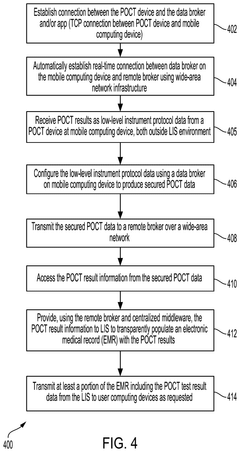
Transparent Secure Link For Point-of-Care Devices
PatentPendingUS20250056215A1
Innovation
- A system that enables a transparent secure link between remote POCT devices and laboratory information systems (LIS) or hospital information systems (HIS) using a mobile computing device with a data broker to configure and secure POCT data for transmission over a wide-area network infrastructure.
Healthcare Regulatory Compliance Framework
The regulatory landscape for Point-of-Care (POC) devices and real-time data integration systems is complex and multifaceted, requiring careful navigation to ensure compliance while enabling innovation. Healthcare regulatory frameworks vary significantly across regions, with the FDA in the United States, the EMA in Europe, and the NMPA in China each maintaining distinct requirements for medical device approval and data management.
POC devices face particularly stringent regulatory scrutiny due to their direct impact on patient care decisions. The FDA's regulatory pathway typically classifies these devices based on risk levels, with many real-time diagnostic tools falling under Class II (moderate risk) or Class III (high risk) categories, necessitating premarket approval or 510(k) clearance. The European MDR (Medical Device Regulation) similarly imposes comprehensive requirements, with additional emphasis on post-market surveillance and unique device identification.
Data integration systems that connect with POC devices must comply with both device regulations and data protection frameworks. HIPAA in the US, GDPR in Europe, and equivalent regulations worldwide establish strict parameters for patient data handling, transmission, and storage. Real-time data integration introduces additional compliance challenges related to data integrity, security, and consent management across dynamic healthcare environments.
Interoperability standards represent another critical regulatory consideration. Organizations such as HL7 have developed frameworks like FHIR (Fast Healthcare Interoperability Resources) that are increasingly being incorporated into regulatory requirements. The ONC's interoperability rules in the US, for instance, mandate specific standards for healthcare information exchange that POC device manufacturers must address.
Cybersecurity regulations have evolved significantly in recent years, with regulatory bodies now requiring robust security measures throughout the device lifecycle. The FDA's pre-market and post-market cybersecurity guidance documents outline expectations for threat modeling, vulnerability management, and security updates for connected medical devices, including POC systems.
Quality management systems (QMS) compliance remains foundational, with ISO 13485 serving as the international standard for medical device quality management. Manufacturers must demonstrate adherence to these standards through comprehensive documentation, risk management processes, and regular audits. For real-time data integration, additional quality considerations apply to software validation, algorithm verification, and continuous monitoring systems.
Regulatory harmonization efforts, such as the International Medical Device Regulators Forum (IMDRF), are working to standardize requirements across jurisdictions, potentially simplifying the compliance process for global POC device deployment. However, significant regional variations persist, requiring manufacturers to maintain market-specific regulatory strategies.
POC devices face particularly stringent regulatory scrutiny due to their direct impact on patient care decisions. The FDA's regulatory pathway typically classifies these devices based on risk levels, with many real-time diagnostic tools falling under Class II (moderate risk) or Class III (high risk) categories, necessitating premarket approval or 510(k) clearance. The European MDR (Medical Device Regulation) similarly imposes comprehensive requirements, with additional emphasis on post-market surveillance and unique device identification.
Data integration systems that connect with POC devices must comply with both device regulations and data protection frameworks. HIPAA in the US, GDPR in Europe, and equivalent regulations worldwide establish strict parameters for patient data handling, transmission, and storage. Real-time data integration introduces additional compliance challenges related to data integrity, security, and consent management across dynamic healthcare environments.
Interoperability standards represent another critical regulatory consideration. Organizations such as HL7 have developed frameworks like FHIR (Fast Healthcare Interoperability Resources) that are increasingly being incorporated into regulatory requirements. The ONC's interoperability rules in the US, for instance, mandate specific standards for healthcare information exchange that POC device manufacturers must address.
Cybersecurity regulations have evolved significantly in recent years, with regulatory bodies now requiring robust security measures throughout the device lifecycle. The FDA's pre-market and post-market cybersecurity guidance documents outline expectations for threat modeling, vulnerability management, and security updates for connected medical devices, including POC systems.
Quality management systems (QMS) compliance remains foundational, with ISO 13485 serving as the international standard for medical device quality management. Manufacturers must demonstrate adherence to these standards through comprehensive documentation, risk management processes, and regular audits. For real-time data integration, additional quality considerations apply to software validation, algorithm verification, and continuous monitoring systems.
Regulatory harmonization efforts, such as the International Medical Device Regulators Forum (IMDRF), are working to standardize requirements across jurisdictions, potentially simplifying the compliance process for global POC device deployment. However, significant regional variations persist, requiring manufacturers to maintain market-specific regulatory strategies.
Data Security and Patient Privacy Considerations
The integration of point-of-care devices with real-time data systems introduces significant challenges regarding data security and patient privacy. As healthcare data becomes increasingly digitized and accessible, protecting sensitive patient information has become paramount. Current regulatory frameworks, including HIPAA in the United States and GDPR in Europe, mandate strict compliance with data protection standards, requiring point-of-care systems to implement robust security measures throughout the data lifecycle.
Encryption technologies represent the first line of defense in securing patient data. Advanced encryption standards (AES) and secure socket layer (SSL) protocols are widely implemented in modern point-of-care devices, ensuring data remains protected during transmission between devices and central healthcare systems. However, the real-time nature of these systems introduces unique vulnerabilities, as continuous data streams require persistent security monitoring without compromising transmission speed or clinical utility.
Authentication mechanisms have evolved significantly to address these challenges. Multi-factor authentication has become standard practice, requiring healthcare professionals to verify their identity through multiple means before accessing patient data. Biometric authentication methods, including fingerprint scanning and facial recognition, are increasingly integrated into point-of-care devices, providing enhanced security while maintaining operational efficiency in fast-paced clinical environments.
Data anonymization and de-identification techniques play a crucial role in balancing research needs with privacy concerns. These approaches allow valuable clinical data to be utilized for research and quality improvement initiatives while protecting individual patient identities. However, studies have demonstrated that re-identification remains possible with sufficient computational resources, highlighting the need for continuous advancement in anonymization algorithms.
Blockchain technology has emerged as a promising solution for maintaining immutable audit trails of data access and modifications. Several healthcare organizations have implemented blockchain-based systems to enhance transparency and accountability in data handling. These systems create tamper-proof records of who accessed what information and when, enabling more effective compliance monitoring and breach detection.
Consent management frameworks have become increasingly sophisticated to accommodate the complex data sharing requirements of integrated healthcare systems. Dynamic consent models allow patients to modify their data sharing preferences over time and across different use cases, providing greater autonomy while supporting necessary clinical information exchange. However, implementing these systems requires careful user interface design to avoid overwhelming patients with technical decisions.
The proliferation of edge computing in point-of-care devices introduces new security considerations. Processing sensitive data at the network edge reduces transmission vulnerabilities but requires robust device-level security measures. Manufacturers are increasingly implementing hardware security modules and secure boot processes to protect against physical tampering and unauthorized software modifications.
Encryption technologies represent the first line of defense in securing patient data. Advanced encryption standards (AES) and secure socket layer (SSL) protocols are widely implemented in modern point-of-care devices, ensuring data remains protected during transmission between devices and central healthcare systems. However, the real-time nature of these systems introduces unique vulnerabilities, as continuous data streams require persistent security monitoring without compromising transmission speed or clinical utility.
Authentication mechanisms have evolved significantly to address these challenges. Multi-factor authentication has become standard practice, requiring healthcare professionals to verify their identity through multiple means before accessing patient data. Biometric authentication methods, including fingerprint scanning and facial recognition, are increasingly integrated into point-of-care devices, providing enhanced security while maintaining operational efficiency in fast-paced clinical environments.
Data anonymization and de-identification techniques play a crucial role in balancing research needs with privacy concerns. These approaches allow valuable clinical data to be utilized for research and quality improvement initiatives while protecting individual patient identities. However, studies have demonstrated that re-identification remains possible with sufficient computational resources, highlighting the need for continuous advancement in anonymization algorithms.
Blockchain technology has emerged as a promising solution for maintaining immutable audit trails of data access and modifications. Several healthcare organizations have implemented blockchain-based systems to enhance transparency and accountability in data handling. These systems create tamper-proof records of who accessed what information and when, enabling more effective compliance monitoring and breach detection.
Consent management frameworks have become increasingly sophisticated to accommodate the complex data sharing requirements of integrated healthcare systems. Dynamic consent models allow patients to modify their data sharing preferences over time and across different use cases, providing greater autonomy while supporting necessary clinical information exchange. However, implementing these systems requires careful user interface design to avoid overwhelming patients with technical decisions.
The proliferation of edge computing in point-of-care devices introduces new security considerations. Processing sensitive data at the network edge reduces transmission vulnerabilities but requires robust device-level security measures. Manufacturers are increasingly implementing hardware security modules and secure boot processes to protect against physical tampering and unauthorized software modifications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!