Point-of-care Devices for Aerospace Application Standards
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Aerospace POCD Technology Background and Objectives
Point-of-care devices (POCDs) have revolutionized healthcare delivery by enabling rapid diagnostic testing at or near the patient's location. In aerospace environments, these devices face unique challenges due to extreme conditions including microgravity, radiation exposure, pressure variations, and limited resources. The evolution of aerospace POCDs traces back to the early space missions where basic health monitoring tools were employed, progressing to today's sophisticated systems capable of performing complex diagnostics in space.
The technological trajectory of aerospace POCDs has been shaped by NASA's Human Research Program and international space agencies' collaborative efforts to address health risks during long-duration missions. Recent advancements in miniaturization, microfluidics, and sensor technologies have significantly enhanced the capabilities of these devices, making them more suitable for the constraints of aerospace environments.
The primary objective of aerospace POCD technology development is to create robust, reliable diagnostic systems that can function autonomously in extreme conditions while maintaining clinical accuracy comparable to Earth-based laboratory standards. These devices must address the unique physiological changes astronauts experience, including bone density loss, muscle atrophy, cardiovascular alterations, and immune system dysregulation.
Current technological goals focus on developing integrated systems that combine multiple diagnostic capabilities in compact, low-power devices with minimal consumables. These systems must be capable of detecting and monitoring various biomarkers related to radiation exposure, infectious diseases, and physiological adaptations to space environments. Additionally, they must interface seamlessly with telemedicine systems to enable remote consultation with medical experts on Earth.
The development of standardized protocols and validation methodologies specific to aerospace applications represents another critical objective. These standards must account for the unique operational constraints and environmental factors that can affect device performance and measurement accuracy in space.
Looking forward, the field is moving toward predictive health monitoring capabilities, incorporating artificial intelligence and machine learning to anticipate medical issues before they become critical. This proactive approach is essential for future long-duration missions to the Moon, Mars, and beyond, where immediate return to Earth for medical treatment is not feasible.
The convergence of aerospace requirements with terrestrial medical device innovations presents opportunities for cross-pollination of technologies, potentially benefiting both space exploration and Earth-based healthcare delivery in remote or resource-limited settings.
The technological trajectory of aerospace POCDs has been shaped by NASA's Human Research Program and international space agencies' collaborative efforts to address health risks during long-duration missions. Recent advancements in miniaturization, microfluidics, and sensor technologies have significantly enhanced the capabilities of these devices, making them more suitable for the constraints of aerospace environments.
The primary objective of aerospace POCD technology development is to create robust, reliable diagnostic systems that can function autonomously in extreme conditions while maintaining clinical accuracy comparable to Earth-based laboratory standards. These devices must address the unique physiological changes astronauts experience, including bone density loss, muscle atrophy, cardiovascular alterations, and immune system dysregulation.
Current technological goals focus on developing integrated systems that combine multiple diagnostic capabilities in compact, low-power devices with minimal consumables. These systems must be capable of detecting and monitoring various biomarkers related to radiation exposure, infectious diseases, and physiological adaptations to space environments. Additionally, they must interface seamlessly with telemedicine systems to enable remote consultation with medical experts on Earth.
The development of standardized protocols and validation methodologies specific to aerospace applications represents another critical objective. These standards must account for the unique operational constraints and environmental factors that can affect device performance and measurement accuracy in space.
Looking forward, the field is moving toward predictive health monitoring capabilities, incorporating artificial intelligence and machine learning to anticipate medical issues before they become critical. This proactive approach is essential for future long-duration missions to the Moon, Mars, and beyond, where immediate return to Earth for medical treatment is not feasible.
The convergence of aerospace requirements with terrestrial medical device innovations presents opportunities for cross-pollination of technologies, potentially benefiting both space exploration and Earth-based healthcare delivery in remote or resource-limited settings.
Market Analysis for Aerospace Medical Diagnostics
The aerospace medical diagnostics market is experiencing significant growth, driven by the increasing frequency of space missions and the expanding commercial space industry. Currently valued at approximately $2.3 billion, this specialized segment is projected to grow at a compound annual growth rate of 6.8% through 2030, reflecting the rising demand for advanced medical monitoring capabilities in extreme environments.
The market for point-of-care (POC) devices specifically designed for aerospace applications represents a critical subsector, estimated at $580 million in 2023. This segment addresses the unique challenges of providing medical care during space missions, including microgravity conditions, radiation exposure, and limited resources. The demand is primarily driven by government space agencies such as NASA, ESA, and ROSCOSMOS, which collectively account for 65% of current market consumption.
Commercial space companies are emerging as significant market players, with entities like SpaceX, Blue Origin, and Virgin Galactic increasingly investing in medical diagnostic capabilities for their crewed missions. Industry analysts predict that commercial space tourism will create a new market segment worth potentially $150 million annually by 2028 for specialized medical diagnostic equipment.
Geographically, North America dominates the aerospace medical diagnostics market with a 48% share, followed by Europe (27%) and Asia-Pacific (18%). This distribution closely mirrors the concentration of aerospace research and development activities globally. However, emerging space programs in India, China, and the United Arab Emirates are expected to shift this balance over the next decade.
The customer base for aerospace POC devices exhibits unique requirements compared to traditional medical markets. Key purchasing factors include extreme miniaturization (devices typically must be 60-80% smaller than hospital equivalents), radiation hardening, operation in variable gravity conditions, and minimal power consumption. These specialized requirements create significant barriers to entry but also command premium pricing, with aerospace-grade POC devices typically priced 3-5 times higher than their terrestrial counterparts.
Market research indicates growing demand for integrated diagnostic platforms that can monitor multiple physiological parameters simultaneously while maintaining minimal size, weight, and power requirements. Blood analysis, cardiac monitoring, and radiation exposure assessment represent the three highest-value diagnostic categories, collectively accounting for 72% of current market revenue.
The regulatory landscape presents both challenges and opportunities, with aerospace medical devices subject to rigorous certification processes through agencies like NASA's Space Medicine Division and the FAA's Office of Aerospace Medicine. These certification requirements, while stringent, help establish clear standards that can guide product development and market entry strategies.
The market for point-of-care (POC) devices specifically designed for aerospace applications represents a critical subsector, estimated at $580 million in 2023. This segment addresses the unique challenges of providing medical care during space missions, including microgravity conditions, radiation exposure, and limited resources. The demand is primarily driven by government space agencies such as NASA, ESA, and ROSCOSMOS, which collectively account for 65% of current market consumption.
Commercial space companies are emerging as significant market players, with entities like SpaceX, Blue Origin, and Virgin Galactic increasingly investing in medical diagnostic capabilities for their crewed missions. Industry analysts predict that commercial space tourism will create a new market segment worth potentially $150 million annually by 2028 for specialized medical diagnostic equipment.
Geographically, North America dominates the aerospace medical diagnostics market with a 48% share, followed by Europe (27%) and Asia-Pacific (18%). This distribution closely mirrors the concentration of aerospace research and development activities globally. However, emerging space programs in India, China, and the United Arab Emirates are expected to shift this balance over the next decade.
The customer base for aerospace POC devices exhibits unique requirements compared to traditional medical markets. Key purchasing factors include extreme miniaturization (devices typically must be 60-80% smaller than hospital equivalents), radiation hardening, operation in variable gravity conditions, and minimal power consumption. These specialized requirements create significant barriers to entry but also command premium pricing, with aerospace-grade POC devices typically priced 3-5 times higher than their terrestrial counterparts.
Market research indicates growing demand for integrated diagnostic platforms that can monitor multiple physiological parameters simultaneously while maintaining minimal size, weight, and power requirements. Blood analysis, cardiac monitoring, and radiation exposure assessment represent the three highest-value diagnostic categories, collectively accounting for 72% of current market revenue.
The regulatory landscape presents both challenges and opportunities, with aerospace medical devices subject to rigorous certification processes through agencies like NASA's Space Medicine Division and the FAA's Office of Aerospace Medicine. These certification requirements, while stringent, help establish clear standards that can guide product development and market entry strategies.
Current Challenges in Space Medicine Diagnostics
Space medicine diagnostics faces unprecedented challenges in the aerospace environment, where traditional Earth-based medical technologies prove inadequate. The microgravity environment significantly alters human physiology, affecting fluid distribution, bone density, muscle mass, and immune function. These physiological changes necessitate specialized diagnostic approaches that can accurately assess astronaut health during extended missions.
Current diagnostic capabilities aboard spacecraft remain severely limited compared to terrestrial healthcare settings. The International Space Station (ISS) has basic ultrasound equipment and limited laboratory testing capabilities, but lacks comprehensive diagnostic tools for emergent medical conditions. This diagnostic gap becomes increasingly problematic as mission durations extend and destinations become more remote, particularly for future lunar and Mars missions where Earth-based medical support will experience significant communication delays.
Size, weight, and power constraints (SWaP) represent fundamental challenges for space medicine diagnostics. Every kilogram launched into space incurs substantial costs, forcing medical equipment to compete with mission-critical systems for limited payload capacity. This necessitates extreme miniaturization without compromising diagnostic accuracy or reliability—a significant engineering challenge that current technologies struggle to overcome.
Radiation exposure presents another critical challenge, potentially damaging sensitive electronic components in diagnostic equipment. Space-grade radiation hardening adds complexity, weight, and cost to medical devices, while the radiation environment itself can interfere with certain diagnostic modalities like fluorescence-based assays commonly used in molecular diagnostics.
Maintenance and calibration of diagnostic equipment in space present operational challenges. Earth-based medical devices typically require regular servicing by specialized technicians—a luxury unavailable during space missions. Self-calibrating, fault-tolerant systems with minimal maintenance requirements are essential but remain technologically challenging to implement.
Data management poses additional complications, as diagnostic tests generate substantial information requiring secure storage, analysis, and transmission capabilities. Limited bandwidth for communication with Earth necessitates onboard processing capabilities and decision support systems to assist crew medical officers who may have limited medical training.
The lack of standardized validation protocols for space-specific diagnostic technologies further complicates development efforts. Earth-based clinical validation may not adequately predict performance in the space environment, yet conducting extensive in-space clinical trials remains prohibitively expensive and logistically challenging.
Current diagnostic capabilities aboard spacecraft remain severely limited compared to terrestrial healthcare settings. The International Space Station (ISS) has basic ultrasound equipment and limited laboratory testing capabilities, but lacks comprehensive diagnostic tools for emergent medical conditions. This diagnostic gap becomes increasingly problematic as mission durations extend and destinations become more remote, particularly for future lunar and Mars missions where Earth-based medical support will experience significant communication delays.
Size, weight, and power constraints (SWaP) represent fundamental challenges for space medicine diagnostics. Every kilogram launched into space incurs substantial costs, forcing medical equipment to compete with mission-critical systems for limited payload capacity. This necessitates extreme miniaturization without compromising diagnostic accuracy or reliability—a significant engineering challenge that current technologies struggle to overcome.
Radiation exposure presents another critical challenge, potentially damaging sensitive electronic components in diagnostic equipment. Space-grade radiation hardening adds complexity, weight, and cost to medical devices, while the radiation environment itself can interfere with certain diagnostic modalities like fluorescence-based assays commonly used in molecular diagnostics.
Maintenance and calibration of diagnostic equipment in space present operational challenges. Earth-based medical devices typically require regular servicing by specialized technicians—a luxury unavailable during space missions. Self-calibrating, fault-tolerant systems with minimal maintenance requirements are essential but remain technologically challenging to implement.
Data management poses additional complications, as diagnostic tests generate substantial information requiring secure storage, analysis, and transmission capabilities. Limited bandwidth for communication with Earth necessitates onboard processing capabilities and decision support systems to assist crew medical officers who may have limited medical training.
The lack of standardized validation protocols for space-specific diagnostic technologies further complicates development efforts. Earth-based clinical validation may not adequately predict performance in the space environment, yet conducting extensive in-space clinical trials remains prohibitively expensive and logistically challenging.
Current Aerospace-grade POCD Technical Solutions
01 Diagnostic and monitoring systems for point-of-care applications
Point-of-care devices include diagnostic and monitoring systems that enable healthcare providers to perform tests and obtain results at or near the patient's location. These systems incorporate various technologies for rapid detection of biomarkers, disease indicators, and physiological parameters. They often feature integrated sensors, microfluidic components, and data processing capabilities to deliver accurate and timely diagnostic information, allowing for immediate clinical decision-making and treatment initiation.- Portable diagnostic systems for healthcare settings: Point-of-care devices designed as portable diagnostic systems enable healthcare providers to perform tests and obtain results quickly at or near the patient location. These systems integrate various technologies including biosensors, microfluidics, and data processing capabilities to deliver rapid diagnostic information without requiring samples to be sent to centralized laboratories. The portability aspect allows for improved healthcare delivery in remote locations, emergency situations, and resource-limited settings.
- Integration with healthcare information systems: Point-of-care devices that integrate with electronic health records (EHR) and healthcare information systems enable seamless data transfer and management. These devices can automatically upload test results to patient records, facilitate clinical decision support, and enable remote monitoring capabilities. The integration improves workflow efficiency, reduces transcription errors, and allows for better coordination of care across different healthcare settings and providers.
- Multiplex testing capabilities: Advanced point-of-care devices incorporate multiplex testing capabilities that allow for simultaneous detection of multiple analytes or biomarkers from a single patient sample. These systems utilize various detection methods including optical, electrochemical, and molecular techniques to provide comprehensive diagnostic information. Multiplex testing reduces sample volume requirements, decreases time to results, and enables more efficient patient management by providing multiple clinically relevant parameters at once.
- Mobile health and telemedicine applications: Point-of-care devices designed for mobile health applications leverage smartphone technology, wireless connectivity, and cloud computing to extend diagnostic capabilities beyond traditional healthcare settings. These devices can be operated by patients at home or by healthcare workers in community settings, with results transmitted to healthcare providers for interpretation and follow-up. The integration with telemedicine platforms enables remote consultation and monitoring, improving access to healthcare services particularly for underserved populations.
- Quality control and regulatory compliance features: Modern point-of-care devices incorporate sophisticated quality control mechanisms and regulatory compliance features to ensure accuracy and reliability of test results. These include internal calibration systems, automated quality checks, user authentication protocols, and audit trail capabilities. Such features help maintain testing standards across different operators and environments, facilitate regulatory compliance documentation, and support quality assurance programs essential for clinical decision-making based on point-of-care test results.
02 Healthcare data management and integration platforms
These point-of-care solutions focus on the management, integration, and analysis of healthcare data collected from various sources. They provide platforms for storing, processing, and sharing patient information securely across healthcare systems. These technologies enable seamless integration with electronic health records, facilitate remote monitoring capabilities, and support clinical decision-making through advanced analytics and artificial intelligence algorithms, ultimately improving care coordination and patient outcomes.Expand Specific Solutions03 Mobile and portable healthcare devices
This category encompasses portable and mobile point-of-care technologies designed for use in various settings, including remote locations, emergency situations, and home care environments. These devices are characterized by their compact size, battery operation, wireless connectivity, and user-friendly interfaces. They enable healthcare delivery outside traditional clinical settings, supporting telemedicine initiatives, community-based care, and self-monitoring by patients, thereby expanding healthcare access and improving patient engagement.Expand Specific Solutions04 Patient management and workflow optimization systems
These point-of-care solutions focus on optimizing healthcare workflows, patient management, and resource allocation. They include systems for patient registration, scheduling, triage, and tracking throughout the care continuum. By streamlining administrative processes, automating routine tasks, and providing real-time visibility into patient status and resource availability, these technologies help healthcare providers improve operational efficiency, reduce wait times, and enhance the overall quality of care delivery.Expand Specific Solutions05 Specialized point-of-care testing devices for specific medical conditions
This category includes point-of-care devices specifically designed for diagnosing and monitoring particular medical conditions or disease states. These specialized devices incorporate targeted testing methodologies, disease-specific biomarkers, and condition-appropriate sampling techniques. They may focus on infectious diseases, chronic conditions, cardiac health, respiratory function, or other specific health concerns, providing healthcare providers with rapid, condition-specific diagnostic information to guide immediate treatment decisions.Expand Specific Solutions
Leading Aerospace POCD Manufacturers and Stakeholders
The point-of-care devices market for aerospace applications is currently in an early growth phase, characterized by increasing integration of medical technology into aerospace environments. The global market is estimated to reach approximately $300-350 million by 2025, driven by growing demand for real-time health monitoring in space missions and commercial aviation. From a technological maturity perspective, companies like Abbott Point of Care and Siemens Healthcare Diagnostics lead with established portable diagnostic platforms, while aerospace specialists including Northrop Grumman, Boeing, and Thales are adapting these technologies to meet rigorous aerospace standards. Novel Microdevices and Zeteo Tech represent emerging innovators developing specialized microfluidic and mass spectrometry solutions specifically engineered for the unique constraints of aerospace environments, including microgravity conditions, radiation exposure, and limited resource settings.
Abbott Point of Care, Inc.
Technical Solution: Abbott Point of Care has developed the i-STAT system, a handheld blood analyzer specifically adapted for aerospace applications. This portable clinical analyzer provides real-time, lab-quality results for multiple critical care parameters within minutes using small blood samples. The aerospace-certified version meets DO-160 environmental testing standards for avionics equipment and has been modified to function reliably in pressurized cabins, under microgravity conditions, and with electromagnetic compatibility requirements specific to aircraft systems. The i-STAT cartridge technology uses microfluidics and biosensor technology to perform multiple tests on a single disposable cartridge, minimizing waste and sample volume requirements. Abbott has implemented specific calibration protocols that account for altitude changes and cabin pressure variations, ensuring accurate results regardless of flight conditions. The system includes specialized data management capabilities that interface with aerospace medical systems while maintaining HIPAA compliance and security protocols required for sensitive medical data in flight operations.
Strengths: Compact, lightweight design optimized for space-constrained environments; rapid results delivery (2-3 minutes) critical for in-flight medical emergencies; robust validation for aerospace conditions including pressure, temperature, and vibration extremes. Weaknesses: Higher cost compared to standard medical equipment; requires specific training for aerospace medical personnel; limited test menu compared to full laboratory capabilities.
Northrop Grumman Systems Corp.
Technical Solution: Northrop Grumman has engineered the Advanced Health Monitoring System (AHMS), a comprehensive point-of-care solution designed specifically for aerospace applications. The AHMS integrates multiple biosensors and diagnostic capabilities into a ruggedized platform that meets MIL-STD-810 environmental standards and DO-160 avionics requirements. The system employs a modular architecture allowing for customization based on mission requirements, from short-duration flights to extended space missions. Core technologies include non-invasive vital signs monitoring, microfluidic-based blood analysis, and radiation exposure assessment capabilities. Northrop Grumman's solution incorporates AI-driven predictive analytics that can identify potential health issues before they become critical, particularly valuable for long-duration missions where medical evacuation is not feasible. The system features secure data transmission protocols compliant with both military encryption standards and NASA's space communications architecture, enabling real-time telemedicine support from ground-based medical teams. The AHMS is designed with autonomous operation capabilities, requiring minimal crew intervention while providing comprehensive health status information to both onboard personnel and mission control.
Strengths: Military-grade durability and reliability in extreme environments; integrated approach combining multiple diagnostic modalities in one system; advanced predictive capabilities for early intervention; secure communications architecture meeting defense standards. Weaknesses: Significant weight and power requirements compared to civilian solutions; high acquisition and maintenance costs; complex system requiring specialized training for operators.
Key Patents and Innovations in Space-compatible Diagnostics
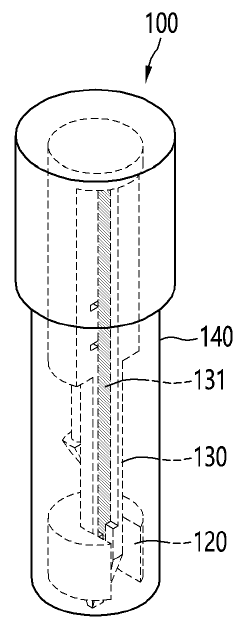
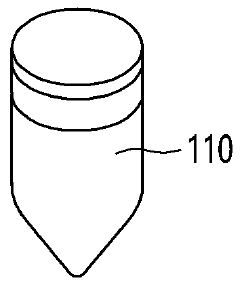
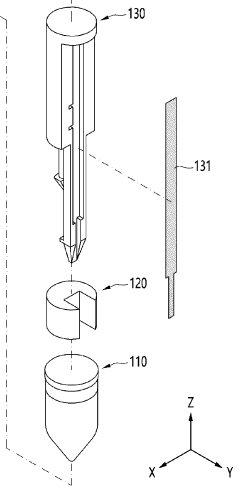
Point-of-care testing device
PatentWO2024085330A1
Innovation
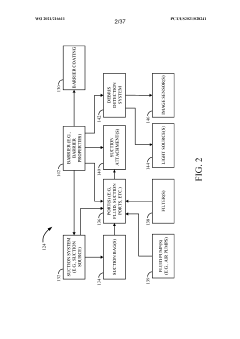
- A point-of-care diagnostic testing device is designed with a sample reservoir, buffer reservoir, and a strip holder that deploys a test strip without exposing the sample to the outside, using a guide to align the reservoirs and reduce external contamination, and incorporates a miniaturized heat block for isothermal nucleic acid amplification.
Systems and methods for controlling the spread of airborne materials during clinical or laboratory procedures
PatentWO2021216611A1
Innovation
- A three-dimensional barrier system is introduced that encloses the patient or laboratory sample, featuring passages for access and mechanisms to control the movement of aerosols and particulates, including suction systems and filters, to mitigate the spread of airborne substances from the interior to the exterior space, thereby protecting personnel.
Regulatory Framework for Aerospace Medical Devices
The regulatory landscape for aerospace medical devices, particularly point-of-care (POC) devices, is governed by a complex framework of international standards, national regulations, and industry-specific requirements. The Federal Aviation Administration (FAA) and the European Union Aviation Safety Agency (EASA) serve as primary regulatory bodies overseeing the certification and approval processes for medical equipment used in aerospace environments.
These regulatory bodies have established stringent requirements for POC devices, focusing on electromagnetic compatibility (EMC), pressure and temperature tolerance, vibration resistance, and radiation hardening. Medical devices intended for aerospace applications must comply with DO-160 standards, which outline environmental conditions and test procedures for airborne equipment.
The International Organization for Standardization (ISO) has developed specific standards such as ISO 13485 for quality management systems in medical devices, which applies to aerospace medical equipment. Additionally, the ISO 14971 standard for risk management is particularly relevant due to the high-risk nature of aerospace environments.
The FDA's Center for Devices and Radiological Health (CDRH) provides regulatory oversight for medical devices in the United States, including those used in aerospace applications. These devices typically require premarket approval (PMA) or 510(k) clearance, depending on their risk classification. For aerospace applications, additional testing and validation are often necessary to ensure reliability under extreme conditions.
In the European market, medical devices must conform to the Medical Device Regulation (MDR) or In Vitro Diagnostic Regulation (IVDR), with specific considerations for aerospace use. The CE marking process includes additional requirements for devices intended for use in aviation environments, particularly regarding pressure changes, electromagnetic interference, and mechanical stress resistance.
The International Air Transport Association (IATA) and the International Civil Aviation Organization (ICAO) also provide guidelines for medical equipment used during air transport, focusing on safety, security, and operational considerations. These guidelines address issues such as battery safety, device stowage, and emergency use protocols.
Regulatory compliance for aerospace medical devices requires extensive documentation, including technical files, clinical evaluation reports, and post-market surveillance plans. Manufacturers must demonstrate not only the clinical efficacy of their devices but also their ability to function reliably in the unique conditions encountered during flight.
These regulatory bodies have established stringent requirements for POC devices, focusing on electromagnetic compatibility (EMC), pressure and temperature tolerance, vibration resistance, and radiation hardening. Medical devices intended for aerospace applications must comply with DO-160 standards, which outline environmental conditions and test procedures for airborne equipment.
The International Organization for Standardization (ISO) has developed specific standards such as ISO 13485 for quality management systems in medical devices, which applies to aerospace medical equipment. Additionally, the ISO 14971 standard for risk management is particularly relevant due to the high-risk nature of aerospace environments.
The FDA's Center for Devices and Radiological Health (CDRH) provides regulatory oversight for medical devices in the United States, including those used in aerospace applications. These devices typically require premarket approval (PMA) or 510(k) clearance, depending on their risk classification. For aerospace applications, additional testing and validation are often necessary to ensure reliability under extreme conditions.
In the European market, medical devices must conform to the Medical Device Regulation (MDR) or In Vitro Diagnostic Regulation (IVDR), with specific considerations for aerospace use. The CE marking process includes additional requirements for devices intended for use in aviation environments, particularly regarding pressure changes, electromagnetic interference, and mechanical stress resistance.
The International Air Transport Association (IATA) and the International Civil Aviation Organization (ICAO) also provide guidelines for medical equipment used during air transport, focusing on safety, security, and operational considerations. These guidelines address issues such as battery safety, device stowage, and emergency use protocols.
Regulatory compliance for aerospace medical devices requires extensive documentation, including technical files, clinical evaluation reports, and post-market surveillance plans. Manufacturers must demonstrate not only the clinical efficacy of their devices but also their ability to function reliably in the unique conditions encountered during flight.
Radiation and Environmental Hardening Requirements
Point-of-care devices deployed in aerospace environments face exceptional challenges related to radiation exposure and harsh environmental conditions that are not typically encountered in terrestrial applications. The space radiation environment consists of galactic cosmic rays, solar particle events, and trapped radiation belts, all of which can cause single event effects, total ionizing dose damage, and displacement damage in electronic components. These radiation effects can lead to data corruption, functional interrupts, parametric degradation, and even catastrophic failures in medical devices.
For aerospace applications, point-of-care devices must comply with radiation hardening standards such as MIL-STD-883 Method 1019 for ionizing radiation tests and ASTM F1192 for proton radiation testing. Components must demonstrate resilience to total ionizing dose levels ranging from 10 krad(Si) for low Earth orbit applications to over 100 krad(Si) for deep space missions. Single event effect mitigation strategies must be implemented, including error detection and correction codes, watchdog timers, and redundant system architectures.
Beyond radiation concerns, aerospace point-of-care devices must withstand extreme temperature fluctuations (-65°C to +125°C), rapid pressure changes, and high vibration environments during launch and re-entry phases. Compliance with standards such as MIL-STD-810G for environmental engineering considerations is essential, particularly for temperature cycling, mechanical shock, and vibration resistance.
Vacuum conditions in space present additional challenges, including outgassing of materials that can contaminate sensitive optical components in diagnostic devices. Materials selection must adhere to NASA outgassing standards with total mass loss (TML) less than 1.0% and collected volatile condensable materials (CVCM) less than 0.1%. Electromagnetic compatibility requirements are also more stringent in aerospace applications, with devices needing to meet MIL-STD-461G to prevent interference with critical spacecraft systems.
Design approaches for meeting these requirements include using radiation-hardened components, implementing triple modular redundancy for critical functions, utilizing radiation-tolerant FPGAs, and employing robust packaging techniques. Shielding strategies must balance radiation protection against mass constraints, often utilizing materials like aluminum, tantalum, or composite materials strategically placed around sensitive components.
Testing protocols for aerospace point-of-care devices must include radiation beam testing at facilities capable of simulating the space radiation environment, thermal vacuum cycling, and vibration testing on shake tables that replicate launch conditions. Qualification testing typically requires demonstration of functionality after exposure to radiation doses 2-3 times higher than the expected mission dose to provide adequate margin.
For aerospace applications, point-of-care devices must comply with radiation hardening standards such as MIL-STD-883 Method 1019 for ionizing radiation tests and ASTM F1192 for proton radiation testing. Components must demonstrate resilience to total ionizing dose levels ranging from 10 krad(Si) for low Earth orbit applications to over 100 krad(Si) for deep space missions. Single event effect mitigation strategies must be implemented, including error detection and correction codes, watchdog timers, and redundant system architectures.
Beyond radiation concerns, aerospace point-of-care devices must withstand extreme temperature fluctuations (-65°C to +125°C), rapid pressure changes, and high vibration environments during launch and re-entry phases. Compliance with standards such as MIL-STD-810G for environmental engineering considerations is essential, particularly for temperature cycling, mechanical shock, and vibration resistance.
Vacuum conditions in space present additional challenges, including outgassing of materials that can contaminate sensitive optical components in diagnostic devices. Materials selection must adhere to NASA outgassing standards with total mass loss (TML) less than 1.0% and collected volatile condensable materials (CVCM) less than 0.1%. Electromagnetic compatibility requirements are also more stringent in aerospace applications, with devices needing to meet MIL-STD-461G to prevent interference with critical spacecraft systems.
Design approaches for meeting these requirements include using radiation-hardened components, implementing triple modular redundancy for critical functions, utilizing radiation-tolerant FPGAs, and employing robust packaging techniques. Shielding strategies must balance radiation protection against mass constraints, often utilizing materials like aluminum, tantalum, or composite materials strategically placed around sensitive components.
Testing protocols for aerospace point-of-care devices must include radiation beam testing at facilities capable of simulating the space radiation environment, thermal vacuum cycling, and vibration testing on shake tables that replicate launch conditions. Qualification testing typically requires demonstration of functionality after exposure to radiation doses 2-3 times higher than the expected mission dose to provide adequate margin.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!