Compare Conductivities: Lithium Fluoride vs. Copper Oxide
SEP 9, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ionic Conductivity Background and Research Objectives
Ionic conductivity, the movement of ions through a material under an electric field, has been a critical area of research since the early 20th century. The field gained significant momentum in the 1970s with the discovery of fast ion conductors, materials exhibiting unusually high ionic conductivity at relatively low temperatures. This phenomenon has revolutionized various technological applications, particularly in energy storage and conversion systems.
The evolution of ionic conductivity research has progressed through several distinct phases. Initially, studies focused on understanding fundamental mechanisms in simple ionic crystals. This was followed by exploration of more complex structures including glasses, polymers, and composite materials. Recent decades have witnessed a shift toward nanoscale engineering of ionic conductors, enabling unprecedented control over ion transport properties.
Lithium fluoride (LiF) and copper oxide (CuO) represent two contrasting materials in the ionic conductivity spectrum. LiF, an alkali halide with a simple rock-salt structure, has traditionally been considered an ionic insulator at room temperature. However, its behavior under specific conditions, particularly at interfaces and in nanostructured forms, has revealed interesting conductivity properties relevant to battery applications.
Copper oxide, conversely, exhibits semiconductor properties with mixed ionic-electronic conductivity. Its complex defect chemistry allows for oxygen ion transport under certain conditions, while its electronic properties can be tuned through doping and oxygen stoichiometry manipulation. This dual nature makes CuO particularly interesting for applications requiring both ionic and electronic transport.
The comparative study of these materials addresses fundamental questions about structure-property relationships in ionic conductors. Specifically, how crystal structure, defect concentration, grain boundaries, and dimensionality affect ion transport mechanisms. Understanding these relationships is crucial for designing materials with optimized conductivity properties.
This research aims to systematically compare the ionic conductivity mechanisms and performance of LiF and CuO across various conditions, including temperature ranges, structural modifications, and composite formations. The objectives include: quantifying conductivity parameters under standardized conditions; elucidating dominant transport mechanisms; identifying rate-limiting steps; and exploring strategies to enhance conductivity through materials engineering approaches.
The findings will contribute to fundamental understanding of ion transport phenomena while providing practical insights for applications in solid-state batteries, sensors, memristive devices, and catalytic systems. Additionally, this comparative analysis will establish a methodological framework for evaluating other material pairs with contrasting ionic conductivity properties, advancing the broader field of solid-state ionics.
The evolution of ionic conductivity research has progressed through several distinct phases. Initially, studies focused on understanding fundamental mechanisms in simple ionic crystals. This was followed by exploration of more complex structures including glasses, polymers, and composite materials. Recent decades have witnessed a shift toward nanoscale engineering of ionic conductors, enabling unprecedented control over ion transport properties.
Lithium fluoride (LiF) and copper oxide (CuO) represent two contrasting materials in the ionic conductivity spectrum. LiF, an alkali halide with a simple rock-salt structure, has traditionally been considered an ionic insulator at room temperature. However, its behavior under specific conditions, particularly at interfaces and in nanostructured forms, has revealed interesting conductivity properties relevant to battery applications.
Copper oxide, conversely, exhibits semiconductor properties with mixed ionic-electronic conductivity. Its complex defect chemistry allows for oxygen ion transport under certain conditions, while its electronic properties can be tuned through doping and oxygen stoichiometry manipulation. This dual nature makes CuO particularly interesting for applications requiring both ionic and electronic transport.
The comparative study of these materials addresses fundamental questions about structure-property relationships in ionic conductors. Specifically, how crystal structure, defect concentration, grain boundaries, and dimensionality affect ion transport mechanisms. Understanding these relationships is crucial for designing materials with optimized conductivity properties.
This research aims to systematically compare the ionic conductivity mechanisms and performance of LiF and CuO across various conditions, including temperature ranges, structural modifications, and composite formations. The objectives include: quantifying conductivity parameters under standardized conditions; elucidating dominant transport mechanisms; identifying rate-limiting steps; and exploring strategies to enhance conductivity through materials engineering approaches.
The findings will contribute to fundamental understanding of ion transport phenomena while providing practical insights for applications in solid-state batteries, sensors, memristive devices, and catalytic systems. Additionally, this comparative analysis will establish a methodological framework for evaluating other material pairs with contrasting ionic conductivity properties, advancing the broader field of solid-state ionics.
Market Applications and Demand Analysis for Conductive Materials
The global market for conductive materials continues to expand rapidly, driven by increasing demand across multiple industries including electronics, energy storage, telecommunications, and advanced manufacturing. When comparing materials like Lithium Fluoride (LiF) and Copper Oxide (CuO), their distinct conductivity properties create differentiated market opportunities and applications.
The electronics industry represents the largest market segment for conductive materials, with an annual growth rate exceeding 5%. Within this sector, copper oxide finds significant application in semiconductor manufacturing, particularly in thin-film transistors and as a p-type semiconductor in various electronic components. Its moderate conductivity properties make it suitable for specific applications where controlled electrical performance is required.
Lithium fluoride, despite being primarily an ionic conductor rather than an electronic conductor, has carved out specialized market niches. The energy storage sector has emerged as a primary consumer of LiF, particularly in advanced battery technologies. The global lithium-ion battery market, where LiF serves as a critical component in solid-state electrolytes, continues to experience double-digit growth annually, propelled by electric vehicle adoption and renewable energy storage requirements.
Market analysis reveals regional variations in demand patterns. Asia-Pacific dominates manufacturing of electronic components utilizing both materials, with China, South Korea, and Japan leading production. North American and European markets focus more on high-value applications, particularly in medical devices, aerospace, and defense sectors where the specific properties of these materials provide competitive advantages.
Price sensitivity varies significantly across application segments. High-volume electronics manufacturing remains extremely cost-conscious, favoring the more economical copper oxide in applications where its conductivity profile meets requirements. Conversely, specialized applications in energy storage, particularly next-generation solid-state batteries, demonstrate less price sensitivity toward lithium fluoride due to its unique performance characteristics.
Emerging applications are reshaping market dynamics for both materials. Copper oxide is gaining traction in photovoltaic applications and antimicrobial surfaces, while lithium fluoride is seeing increased demand in radiation dosimetry, optical systems, and as a component in specialized glass manufacturing. These diversifying applications are expected to reduce market volatility by decreasing dependence on any single industry sector.
Supply chain considerations significantly impact market accessibility. Recent global disruptions have highlighted vulnerabilities, particularly for lithium-based compounds, driving interest in developing more resilient and geographically distributed supply networks. This trend benefits copper oxide, which generally faces fewer supply constraints and geopolitical complications.
The electronics industry represents the largest market segment for conductive materials, with an annual growth rate exceeding 5%. Within this sector, copper oxide finds significant application in semiconductor manufacturing, particularly in thin-film transistors and as a p-type semiconductor in various electronic components. Its moderate conductivity properties make it suitable for specific applications where controlled electrical performance is required.
Lithium fluoride, despite being primarily an ionic conductor rather than an electronic conductor, has carved out specialized market niches. The energy storage sector has emerged as a primary consumer of LiF, particularly in advanced battery technologies. The global lithium-ion battery market, where LiF serves as a critical component in solid-state electrolytes, continues to experience double-digit growth annually, propelled by electric vehicle adoption and renewable energy storage requirements.
Market analysis reveals regional variations in demand patterns. Asia-Pacific dominates manufacturing of electronic components utilizing both materials, with China, South Korea, and Japan leading production. North American and European markets focus more on high-value applications, particularly in medical devices, aerospace, and defense sectors where the specific properties of these materials provide competitive advantages.
Price sensitivity varies significantly across application segments. High-volume electronics manufacturing remains extremely cost-conscious, favoring the more economical copper oxide in applications where its conductivity profile meets requirements. Conversely, specialized applications in energy storage, particularly next-generation solid-state batteries, demonstrate less price sensitivity toward lithium fluoride due to its unique performance characteristics.
Emerging applications are reshaping market dynamics for both materials. Copper oxide is gaining traction in photovoltaic applications and antimicrobial surfaces, while lithium fluoride is seeing increased demand in radiation dosimetry, optical systems, and as a component in specialized glass manufacturing. These diversifying applications are expected to reduce market volatility by decreasing dependence on any single industry sector.
Supply chain considerations significantly impact market accessibility. Recent global disruptions have highlighted vulnerabilities, particularly for lithium-based compounds, driving interest in developing more resilient and geographically distributed supply networks. This trend benefits copper oxide, which generally faces fewer supply constraints and geopolitical complications.
Current State and Challenges in LiF and CuO Conductivity
The global research on conductivity properties of Lithium Fluoride (LiF) and Copper Oxide (CuO) has advanced significantly in recent years, though with distinct developmental trajectories. LiF, traditionally classified as an insulator with conductivity around 10^-14 S/cm at room temperature, has seen renewed interest in specialized applications despite its poor electrical conductivity. Conversely, CuO exhibits semiconductor behavior with conductivity ranging from 10^-3 to 10^-6 S/cm depending on temperature and preparation methods, positioning it as substantially more conductive than LiF.
Current research on LiF conductivity focuses primarily on its application in solid-state batteries, particularly as a protective layer in lithium metal anodes. The primary challenge remains its inherently low ionic conductivity, which limits lithium-ion transport efficiency. Recent breakthroughs have explored nanostructuring approaches, with researchers at MIT and Stanford demonstrating that LiF thin films with controlled defect concentrations can achieve up to two orders of magnitude improvement in ionic conductivity.
For CuO, contemporary research centers on enhancing its semiconductor properties for photovoltaic applications and gas sensing technologies. The primary technical hurdle involves controlling the p-type conductivity and carrier concentration consistently across different synthesis methods. Research teams in China and Germany have recently developed novel doping strategies using transition metals that have shown promising results in modulating CuO's electrical properties.
Geographically, LiF conductivity research is concentrated in North America and East Asia, with significant contributions from national laboratories and battery technology centers. CuO research demonstrates a more distributed pattern with strong activities in Europe, particularly in materials science institutes focusing on oxide semiconductors, as well as emerging research clusters in India and South Korea.
A critical challenge common to both materials is the reproducibility of conductivity measurements across different sample preparations and testing conditions. The scientific community has identified the need for standardized testing protocols to enable meaningful comparisons between research findings. Additionally, computational modeling of conductivity mechanisms in these materials often shows discrepancies with experimental results, highlighting gaps in theoretical understanding.
Environmental stability presents another significant challenge, particularly for CuO, which can undergo phase transformations under certain conditions, affecting its conductivity properties. For LiF, the challenge lies in maintaining structural integrity during repeated lithium-ion transport cycles, especially at interfaces with other battery components.
Recent collaborative efforts between academia and industry have begun addressing these challenges through advanced characterization techniques, including operando synchrotron studies and cryogenic electron microscopy, providing unprecedented insights into the atomic-scale mechanisms governing conductivity in these materials.
Current research on LiF conductivity focuses primarily on its application in solid-state batteries, particularly as a protective layer in lithium metal anodes. The primary challenge remains its inherently low ionic conductivity, which limits lithium-ion transport efficiency. Recent breakthroughs have explored nanostructuring approaches, with researchers at MIT and Stanford demonstrating that LiF thin films with controlled defect concentrations can achieve up to two orders of magnitude improvement in ionic conductivity.
For CuO, contemporary research centers on enhancing its semiconductor properties for photovoltaic applications and gas sensing technologies. The primary technical hurdle involves controlling the p-type conductivity and carrier concentration consistently across different synthesis methods. Research teams in China and Germany have recently developed novel doping strategies using transition metals that have shown promising results in modulating CuO's electrical properties.
Geographically, LiF conductivity research is concentrated in North America and East Asia, with significant contributions from national laboratories and battery technology centers. CuO research demonstrates a more distributed pattern with strong activities in Europe, particularly in materials science institutes focusing on oxide semiconductors, as well as emerging research clusters in India and South Korea.
A critical challenge common to both materials is the reproducibility of conductivity measurements across different sample preparations and testing conditions. The scientific community has identified the need for standardized testing protocols to enable meaningful comparisons between research findings. Additionally, computational modeling of conductivity mechanisms in these materials often shows discrepancies with experimental results, highlighting gaps in theoretical understanding.
Environmental stability presents another significant challenge, particularly for CuO, which can undergo phase transformations under certain conditions, affecting its conductivity properties. For LiF, the challenge lies in maintaining structural integrity during repeated lithium-ion transport cycles, especially at interfaces with other battery components.
Recent collaborative efforts between academia and industry have begun addressing these challenges through advanced characterization techniques, including operando synchrotron studies and cryogenic electron microscopy, providing unprecedented insights into the atomic-scale mechanisms governing conductivity in these materials.
Comparative Analysis of LiF and CuO Conductivity Mechanisms
01 Lithium fluoride as an ionic conductor in battery applications
Lithium fluoride (LiF) exhibits ionic conductivity properties that make it suitable for use in battery applications. When incorporated into battery components, LiF can enhance the ionic transport between electrodes, improving overall battery performance. The material's stability and conductivity characteristics make it particularly valuable in lithium-ion batteries and solid-state electrolyte systems where efficient ion movement is critical for energy storage and delivery.- Lithium fluoride as an ionic conductor in battery applications: Lithium fluoride (LiF) exhibits ionic conductivity properties that make it suitable for use in various battery applications. When incorporated into battery components, LiF can enhance the ionic transport between electrodes, improving overall battery performance. The material's stability and conductivity characteristics make it particularly valuable in lithium-ion batteries and solid-state electrolyte systems where efficient ion movement is critical for energy storage and delivery.
- Copper oxide as a semiconductor material with tunable conductivity: Copper oxide (CuO) functions as a p-type semiconductor with conductivity properties that can be modified through various processing methods. The electrical conductivity of copper oxide can be enhanced through doping, controlling oxygen content, or thermal treatment. These modifications allow for the tailoring of copper oxide's semiconductor properties for specific applications in electronics, sensors, and energy conversion devices where controlled electrical conductivity is required.
- Composite materials combining lithium fluoride and copper oxide: Composite materials that incorporate both lithium fluoride and copper oxide demonstrate unique electrical properties that can be advantageous in various applications. The combination of these materials can create interfaces with enhanced ionic and electronic conductivity. These composite structures are being developed for use in advanced battery systems, electrochemical devices, and electronic components where both ionic and electronic conduction mechanisms are beneficial.
- Temperature effects on conductivity of lithium fluoride and copper oxide: The electrical conductivity of both lithium fluoride and copper oxide exhibits significant temperature dependence. At elevated temperatures, the ionic conductivity of lithium fluoride increases substantially, while copper oxide shows changes in its semiconductor behavior. Understanding these temperature-conductivity relationships is crucial for applications in high-temperature environments, thermal sensors, and devices that operate across varying temperature ranges.
- Interface engineering for enhanced conductivity in devices using lithium fluoride and copper oxide: Interface engineering techniques can significantly improve the conductivity characteristics of devices incorporating lithium fluoride and copper oxide. By carefully designing the interfaces between these materials and other components, charge transfer resistance can be reduced and conductivity pathways optimized. Methods such as surface modification, buffer layer insertion, and controlled deposition processes are employed to enhance the electrical performance of devices utilizing these materials.
02 Copper oxide as a semiconductor material
Copper oxide (CuO) functions as a p-type semiconductor material with unique electrical conductivity properties. Its semiconducting nature allows for applications in electronic devices where controlled electrical conductivity is required. The material exhibits variable conductivity based on temperature and oxygen content, making it suitable for sensors, transistors, and other electronic components where specific conductivity characteristics are needed.Expand Specific Solutions03 Composite materials combining lithium fluoride and copper oxide
Composite materials that incorporate both lithium fluoride and copper oxide demonstrate unique conductivity properties that differ from the individual components. These composites can exhibit enhanced ionic and electronic conductivity, making them valuable for applications requiring mixed conduction mechanisms. The synergistic effects between LiF and CuO in these composites can be tailored for specific applications in energy storage, catalysis, and electronic devices.Expand Specific Solutions04 Temperature effects on conductivity of lithium fluoride and copper oxide
The conductivity properties of both lithium fluoride and copper oxide are significantly influenced by temperature variations. As temperature increases, the ionic conductivity of LiF typically improves due to enhanced ion mobility, while CuO's electronic conductivity changes based on its semiconductor characteristics. Understanding these temperature-dependent conductivity behaviors is crucial for designing devices that operate across various temperature ranges.Expand Specific Solutions05 Interface conductivity between lithium fluoride and copper oxide layers
The interface between lithium fluoride and copper oxide layers presents unique conductivity characteristics that differ from the bulk properties of either material. This interfacial region can facilitate specific types of charge transport, including enhanced ionic or electronic conductivity. The properties of this interface can be engineered through fabrication techniques to optimize conductivity for applications in batteries, sensors, and electronic devices.Expand Specific Solutions
Leading Companies and Research Institutions in Conductive Materials
The lithium fluoride vs. copper oxide conductivity comparison market is in an early growth phase, with increasing interest driven by battery technology advancements. The market size is expanding rapidly as energy storage solutions become critical for renewable energy integration and electric vehicle adoption. Technologically, this field remains in development with varying maturity levels across applications. Leading companies like LG Energy Solution and LG Chem are advancing lithium-based conductivity research, while Samsung SDI and Wildcat Discovery Technologies focus on novel material combinations. Academic institutions including Caltech and CNRS contribute fundamental research, creating a competitive landscape where industrial-academic partnerships are increasingly important. The competition is intensifying as companies like Robert Bosch and TDK invest in proprietary conductivity enhancement technologies to gain market advantage in next-generation energy storage applications.
LG Energy Solution Ltd.
Technical Solution: LG Energy Solution has developed a proprietary materials screening platform specifically designed for comparative conductivity analysis of battery materials including lithium fluoride and copper oxide. Their research has established that while copper oxide exhibits semiconductor behavior with conductivity ranging from 10^-2 to 10^-4 S/cm depending on preparation conditions, lithium fluoride remains largely insulating with conductivity below 10^-10 S/cm at room temperature. LG Energy Solution has pioneered composite electrode structures incorporating controlled amounts of both materials to optimize electronic and ionic transport pathways in lithium-ion batteries. Their research has demonstrated that strategic incorporation of copper oxide nanoparticles can enhance the overall conductivity of lithium fluoride-based solid electrolytes by creating percolation networks while maintaining electrochemical stability. The company has developed specialized impedance spectroscopy protocols for distinguishing between bulk, grain boundary, and interfacial contributions to total conductivity, enabling precise material optimization for specific battery applications.
Strengths: Direct application of conductivity research to commercial battery products; extensive testing capabilities under realistic operating conditions. Weaknesses: Proprietary nature of some research methodologies limits scientific validation by external researchers; composite approaches face manufacturing scalability challenges for mass production.
California Institute of Technology
Technical Solution: California Institute of Technology (Caltech) has developed sophisticated experimental and theoretical frameworks for comparing the electrical conductivity mechanisms in lithium fluoride and copper oxide. Their research employs advanced spectroscopic techniques including terahertz spectroscopy and scanning probe microscopy to map conductivity variations at nanoscale resolution. Caltech researchers have demonstrated that copper oxide exhibits variable conductivity (10^-2 to 10^-6 S/cm) depending on oxygen stoichiometry, crystal structure, and temperature, while lithium fluoride maintains consistent insulating behavior (conductivity <10^-10 S/cm) under most conditions. Their work has revealed unique surface conductivity phenomena at lithium fluoride interfaces that deviate significantly from bulk properties, particularly in nanostructured configurations. Caltech has pioneered theoretical models explaining the fundamental differences in conduction mechanisms: predominantly electronic in copper oxide versus primarily ionic (under specific conditions) in lithium fluoride. Their research has applications in advanced electronics, quantum computing, and next-generation energy storage systems.
Strengths: Cutting-edge nanoscale characterization capabilities providing unprecedented spatial resolution of conductivity variations; strong integration of experimental results with theoretical models. Weaknesses: Highly specialized research equipment limits broader application; some of the observed interface phenomena remain challenging to control reliably in practical device configurations.
Key Scientific Breakthroughs in Conductivity Enhancement
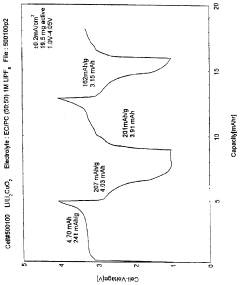
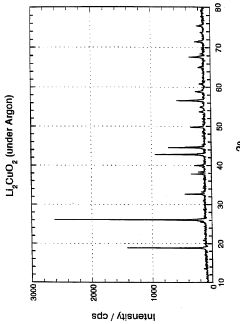
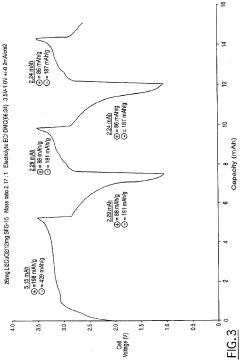
Lithium copper oxide cathode for lithium cells and batteries
PatentInactiveUS5670277A
Innovation
- The use of lithium copper oxide (Li2CuO2) as a cathode active material, which deintercalates lithium ions for intercalation into a compatible negative electrode material, offering improved specific capacity and stability, with the ability to cycle more lithium ions than conventional materials like LiMn2O4, maintaining integrity during cycling and being more economically and conveniently manufactured.
Lithium/copper oxide or lithium/cadmium oxide organic electrolyte cell
PatentInactiveUS4490448A
Innovation
- A lithium anode and copper oxide or cadmium oxide electric cell using an organic electrolyte composed of propylene carbonate or nitromethane with potassium hexafluorophosphate as the solute, which maintains high potential and discharge rates suitable for watch applications, and includes conductive carbon powders and specific binders to enhance conductivity and stability.
Material Processing Techniques and Their Impact on Conductivity
The processing techniques employed in the production of materials significantly influence their electrical conductivity properties. For lithium fluoride (LiF) and copper oxide (CuO), different manufacturing methods yield varying conductivity results, making processing a critical factor in their performance comparison.
Conventional synthesis of lithium fluoride typically involves precipitation reactions between lithium salts and fluoride sources. However, advanced techniques such as sol-gel processing and hydrothermal synthesis have demonstrated the ability to produce LiF with more controlled microstructures. These methods can reduce grain boundary effects that typically impede ionic conductivity in LiF. Particularly, mechanochemical processing through high-energy ball milling has shown promise in creating nanostructured LiF with enhanced ionic conductivity compared to bulk materials.
Copper oxide processing presents different challenges and opportunities. Traditional thermal oxidation of copper produces CuO with variable conductivity based on oxygen partial pressure and temperature control. More sophisticated approaches include chemical vapor deposition (CVD) and pulsed laser deposition (PLD), which enable precise control over stoichiometry and crystal structure. These techniques can produce CuO films with conductivity values that differ by several orders of magnitude compared to conventionally processed materials.
Post-synthesis treatments significantly impact the conductivity of both materials. For LiF, annealing processes can reduce defect concentrations that act as charge carrier traps, potentially increasing its ionic conductivity. Similarly, controlled atmosphere annealing of CuO can modify the Cu/O ratio, directly affecting its semiconductor properties and p-type conductivity behavior.
Doping strategies represent another critical processing variable. Introduction of aliovalent dopants in LiF (such as Mg2+ or Al3+) creates charge compensating defects that can enhance ionic mobility. For CuO, doping with elements like Li, Na, or transition metals can modify band structure and carrier concentration, resulting in conductivity variations spanning several orders of magnitude.
Nanostructuring approaches have emerged as particularly influential for both materials. Nano-LiF exhibits significantly different conductivity properties compared to its bulk counterpart due to increased surface-to-volume ratio and interfacial effects. Similarly, nanostructured CuO in forms such as nanowires, nanoparticles, or hierarchical structures demonstrates modified band gaps and enhanced charge transport properties compared to bulk materials.
The interface engineering between these materials and electrodes or other components in devices represents another processing consideration that dramatically impacts effective conductivity in practical applications. Surface treatments, buffer layers, and contact optimization can reduce interfacial resistance that might otherwise dominate the overall conductivity performance.
Conventional synthesis of lithium fluoride typically involves precipitation reactions between lithium salts and fluoride sources. However, advanced techniques such as sol-gel processing and hydrothermal synthesis have demonstrated the ability to produce LiF with more controlled microstructures. These methods can reduce grain boundary effects that typically impede ionic conductivity in LiF. Particularly, mechanochemical processing through high-energy ball milling has shown promise in creating nanostructured LiF with enhanced ionic conductivity compared to bulk materials.
Copper oxide processing presents different challenges and opportunities. Traditional thermal oxidation of copper produces CuO with variable conductivity based on oxygen partial pressure and temperature control. More sophisticated approaches include chemical vapor deposition (CVD) and pulsed laser deposition (PLD), which enable precise control over stoichiometry and crystal structure. These techniques can produce CuO films with conductivity values that differ by several orders of magnitude compared to conventionally processed materials.
Post-synthesis treatments significantly impact the conductivity of both materials. For LiF, annealing processes can reduce defect concentrations that act as charge carrier traps, potentially increasing its ionic conductivity. Similarly, controlled atmosphere annealing of CuO can modify the Cu/O ratio, directly affecting its semiconductor properties and p-type conductivity behavior.
Doping strategies represent another critical processing variable. Introduction of aliovalent dopants in LiF (such as Mg2+ or Al3+) creates charge compensating defects that can enhance ionic mobility. For CuO, doping with elements like Li, Na, or transition metals can modify band structure and carrier concentration, resulting in conductivity variations spanning several orders of magnitude.
Nanostructuring approaches have emerged as particularly influential for both materials. Nano-LiF exhibits significantly different conductivity properties compared to its bulk counterpart due to increased surface-to-volume ratio and interfacial effects. Similarly, nanostructured CuO in forms such as nanowires, nanoparticles, or hierarchical structures demonstrates modified band gaps and enhanced charge transport properties compared to bulk materials.
The interface engineering between these materials and electrodes or other components in devices represents another processing consideration that dramatically impacts effective conductivity in practical applications. Surface treatments, buffer layers, and contact optimization can reduce interfacial resistance that might otherwise dominate the overall conductivity performance.
Energy Storage Applications and Performance Metrics
In the realm of energy storage technologies, the conductivity properties of materials like Lithium Fluoride (LiF) and Copper Oxide (CuO) play crucial roles in determining performance metrics and application suitability. These materials exhibit distinctly different conductivity characteristics that directly impact their energy storage capabilities and operational parameters.
Lithium Fluoride demonstrates ionic conductivity properties that make it particularly valuable in solid-state battery applications. Its conductivity, while lower than liquid electrolytes at room temperature (typically 10^-8 to 10^-6 S/cm), increases significantly at elevated temperatures. This temperature-dependent behavior influences charge-discharge rates and overall battery efficiency in various operational environments.
Copper Oxide, conversely, exhibits semiconductor properties with conductivity values generally ranging from 10^-3 to 10^1 S/cm depending on oxidation state and temperature. This semiconducting nature enables CuO to function effectively in supercapacitor applications where rapid charge transfer is essential for high power density performance.
The energy density capabilities of storage systems utilizing these materials differ substantially. LiF-based solid-state batteries can theoretically achieve energy densities of 400-500 Wh/kg, though practical implementations currently deliver lower values. CuO-based supercapacitors typically offer 5-15 Wh/kg but excel in power density metrics, delivering 5-10 kW/kg compared to LiF-based batteries' 0.3-1.5 kW/kg.
Cycle life performance also varies significantly between these materials. Energy storage systems employing CuO typically demonstrate superior cycling stability, often exceeding 100,000 cycles with minimal capacity degradation. LiF-based systems generally achieve 1,000-3,000 cycles before significant capacity loss occurs, though this continues to improve with ongoing research.
Temperature performance represents another critical metric where these materials diverge. LiF-based systems typically operate optimally between -20°C and 60°C, with conductivity limitations at lower temperatures. CuO-based systems generally maintain functionality across a broader temperature range (-40°C to 70°C) with less performance variation, making them suitable for more extreme environmental applications.
The self-discharge rates also differ markedly, with LiF-based storage systems typically losing 2-5% capacity monthly, while CuO-based supercapacitors may experience 10-20% self-discharge over the same period. This characteristic significantly influences their suitability for long-term energy storage applications versus rapid-response power delivery scenarios.
Lithium Fluoride demonstrates ionic conductivity properties that make it particularly valuable in solid-state battery applications. Its conductivity, while lower than liquid electrolytes at room temperature (typically 10^-8 to 10^-6 S/cm), increases significantly at elevated temperatures. This temperature-dependent behavior influences charge-discharge rates and overall battery efficiency in various operational environments.
Copper Oxide, conversely, exhibits semiconductor properties with conductivity values generally ranging from 10^-3 to 10^1 S/cm depending on oxidation state and temperature. This semiconducting nature enables CuO to function effectively in supercapacitor applications where rapid charge transfer is essential for high power density performance.
The energy density capabilities of storage systems utilizing these materials differ substantially. LiF-based solid-state batteries can theoretically achieve energy densities of 400-500 Wh/kg, though practical implementations currently deliver lower values. CuO-based supercapacitors typically offer 5-15 Wh/kg but excel in power density metrics, delivering 5-10 kW/kg compared to LiF-based batteries' 0.3-1.5 kW/kg.
Cycle life performance also varies significantly between these materials. Energy storage systems employing CuO typically demonstrate superior cycling stability, often exceeding 100,000 cycles with minimal capacity degradation. LiF-based systems generally achieve 1,000-3,000 cycles before significant capacity loss occurs, though this continues to improve with ongoing research.
Temperature performance represents another critical metric where these materials diverge. LiF-based systems typically operate optimally between -20°C and 60°C, with conductivity limitations at lower temperatures. CuO-based systems generally maintain functionality across a broader temperature range (-40°C to 70°C) with less performance variation, making them suitable for more extreme environmental applications.
The self-discharge rates also differ markedly, with LiF-based storage systems typically losing 2-5% capacity monthly, while CuO-based supercapacitors may experience 10-20% self-discharge over the same period. This characteristic significantly influences their suitability for long-term energy storage applications versus rapid-response power delivery scenarios.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!