How to Optimize Lithium Fluoride Efficiency in Batteries
SEP 12, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
LiF Battery Technology Background and Objectives
Lithium Fluoride (LiF) has emerged as a critical component in advanced battery technologies, particularly in the development of high-energy-density lithium-based batteries. The evolution of LiF in battery applications can be traced back to the early 2000s when researchers began exploring its potential as a solid electrolyte interface (SEI) component. Over the past decade, LiF has gained significant attention due to its unique properties that contribute to battery stability, longevity, and performance.
The technological trajectory of LiF in batteries has been marked by several breakthrough moments, including its identification as a natural SEI component in conventional lithium-ion batteries and subsequent deliberate incorporation as an engineered additive. Recent research has demonstrated that LiF can significantly enhance the cycling stability of high-capacity electrode materials, particularly those involving conversion or alloying reactions with lithium.
Current technological trends indicate a growing focus on LiF as a critical component in next-generation battery systems, including solid-state batteries, lithium-sulfur batteries, and lithium-metal batteries. The material's high electrochemical stability, wide electrochemical window, and beneficial mechanical properties make it particularly valuable for addressing key challenges in these advanced battery technologies.
The primary technical objectives for optimizing LiF efficiency in batteries include enhancing its ionic conductivity, improving its distribution within electrode structures, controlling its formation mechanisms, and developing cost-effective synthesis methods. Achieving these objectives requires interdisciplinary approaches combining materials science, electrochemistry, and advanced manufacturing techniques.
A significant challenge lies in understanding and controlling the formation of LiF during battery operation, as its properties and effectiveness are highly dependent on its morphology, crystallinity, and spatial distribution. Researchers aim to develop precise methods to engineer these characteristics for optimal battery performance.
Long-term technological goals include the development of LiF-rich composite materials that can simultaneously address multiple battery performance metrics, including energy density, power capability, cycle life, and safety. Additionally, there is growing interest in leveraging LiF's properties to enable practical lithium-metal anodes, which could potentially double the energy density of current lithium-ion batteries.
The optimization of LiF efficiency represents a critical pathway toward addressing the increasing demands for higher energy density, longer-lasting, and safer energy storage solutions required for applications ranging from portable electronics to electric vehicles and grid-scale energy storage systems.
The technological trajectory of LiF in batteries has been marked by several breakthrough moments, including its identification as a natural SEI component in conventional lithium-ion batteries and subsequent deliberate incorporation as an engineered additive. Recent research has demonstrated that LiF can significantly enhance the cycling stability of high-capacity electrode materials, particularly those involving conversion or alloying reactions with lithium.
Current technological trends indicate a growing focus on LiF as a critical component in next-generation battery systems, including solid-state batteries, lithium-sulfur batteries, and lithium-metal batteries. The material's high electrochemical stability, wide electrochemical window, and beneficial mechanical properties make it particularly valuable for addressing key challenges in these advanced battery technologies.
The primary technical objectives for optimizing LiF efficiency in batteries include enhancing its ionic conductivity, improving its distribution within electrode structures, controlling its formation mechanisms, and developing cost-effective synthesis methods. Achieving these objectives requires interdisciplinary approaches combining materials science, electrochemistry, and advanced manufacturing techniques.
A significant challenge lies in understanding and controlling the formation of LiF during battery operation, as its properties and effectiveness are highly dependent on its morphology, crystallinity, and spatial distribution. Researchers aim to develop precise methods to engineer these characteristics for optimal battery performance.
Long-term technological goals include the development of LiF-rich composite materials that can simultaneously address multiple battery performance metrics, including energy density, power capability, cycle life, and safety. Additionally, there is growing interest in leveraging LiF's properties to enable practical lithium-metal anodes, which could potentially double the energy density of current lithium-ion batteries.
The optimization of LiF efficiency represents a critical pathway toward addressing the increasing demands for higher energy density, longer-lasting, and safer energy storage solutions required for applications ranging from portable electronics to electric vehicles and grid-scale energy storage systems.
Market Analysis for LiF-Enhanced Battery Solutions
The global market for lithium-ion batteries has experienced unprecedented growth, with a compound annual growth rate exceeding 20% since 2015. This expansion is primarily driven by the electric vehicle (EV) revolution, renewable energy storage systems, and the proliferation of portable electronic devices. Within this landscape, Lithium Fluoride (LiF) has emerged as a critical component for enhancing battery performance, particularly as a solid electrolyte interphase (SEI) film former and cathode coating material.
Market research indicates that the demand for high-performance batteries with extended cycle life, improved safety profiles, and faster charging capabilities continues to accelerate across multiple sectors. The automotive segment represents the largest market opportunity, with premium EV manufacturers willing to pay significant premiums for battery technologies that deliver superior range and longevity. Tesla, BMW, and Volkswagen have all announced strategic initiatives focused on next-generation battery chemistries, with LiF-enhanced solutions featuring prominently in their roadmaps.
Consumer electronics manufacturers constitute another substantial market segment, with Apple, Samsung, and Xiaomi actively pursuing battery innovations that enable slimmer device profiles while maintaining or improving battery life. The integration of LiF-based technologies in this sector could potentially command price premiums of 15-30% over conventional solutions, depending on performance improvements.
The grid-scale energy storage market presents a growing opportunity for LiF-enhanced batteries, particularly in regions with high renewable energy penetration. Countries like Germany, Australia, and California (USA) have implemented aggressive energy storage targets, creating substantial demand for batteries with improved cycle life and safety characteristics—both areas where LiF technologies excel.
Regional analysis reveals Asia-Pacific as the dominant manufacturing hub for LiF-enhanced battery components, with China, South Korea, and Japan collectively controlling approximately 85% of production capacity. However, recent geopolitical tensions and supply chain vulnerabilities have accelerated efforts to establish alternative manufacturing bases in North America and Europe, creating new market entry opportunities.
Price sensitivity varies significantly across application segments. While consumer electronics manufacturers demonstrate high price elasticity, the EV and grid storage segments show greater willingness to absorb premium costs for performance advantages. Current market pricing for high-purity LiF suitable for battery applications ranges between $15-25 per kilogram, with specialized nano-LiF formulations commanding substantially higher prices.
Market forecasts project the global market for LiF-enhanced battery solutions to grow at a CAGR of 25-30% through 2030, outpacing the broader battery market. This accelerated growth trajectory reflects increasing recognition of LiF's role in addressing key performance limitations in current lithium-ion technologies and enabling next-generation battery architectures.
Market research indicates that the demand for high-performance batteries with extended cycle life, improved safety profiles, and faster charging capabilities continues to accelerate across multiple sectors. The automotive segment represents the largest market opportunity, with premium EV manufacturers willing to pay significant premiums for battery technologies that deliver superior range and longevity. Tesla, BMW, and Volkswagen have all announced strategic initiatives focused on next-generation battery chemistries, with LiF-enhanced solutions featuring prominently in their roadmaps.
Consumer electronics manufacturers constitute another substantial market segment, with Apple, Samsung, and Xiaomi actively pursuing battery innovations that enable slimmer device profiles while maintaining or improving battery life. The integration of LiF-based technologies in this sector could potentially command price premiums of 15-30% over conventional solutions, depending on performance improvements.
The grid-scale energy storage market presents a growing opportunity for LiF-enhanced batteries, particularly in regions with high renewable energy penetration. Countries like Germany, Australia, and California (USA) have implemented aggressive energy storage targets, creating substantial demand for batteries with improved cycle life and safety characteristics—both areas where LiF technologies excel.
Regional analysis reveals Asia-Pacific as the dominant manufacturing hub for LiF-enhanced battery components, with China, South Korea, and Japan collectively controlling approximately 85% of production capacity. However, recent geopolitical tensions and supply chain vulnerabilities have accelerated efforts to establish alternative manufacturing bases in North America and Europe, creating new market entry opportunities.
Price sensitivity varies significantly across application segments. While consumer electronics manufacturers demonstrate high price elasticity, the EV and grid storage segments show greater willingness to absorb premium costs for performance advantages. Current market pricing for high-purity LiF suitable for battery applications ranges between $15-25 per kilogram, with specialized nano-LiF formulations commanding substantially higher prices.
Market forecasts project the global market for LiF-enhanced battery solutions to grow at a CAGR of 25-30% through 2030, outpacing the broader battery market. This accelerated growth trajectory reflects increasing recognition of LiF's role in addressing key performance limitations in current lithium-ion technologies and enabling next-generation battery architectures.
Current Challenges in LiF Battery Integration
Despite the promising theoretical energy density of lithium fluoride (LiF) in battery applications, its integration into practical battery systems faces significant challenges. The primary obstacle lies in LiF's inherently poor ionic and electronic conductivity, which severely limits its electrochemical performance in battery environments. At room temperature, LiF exhibits an ionic conductivity of approximately 10^-13 S/cm, several orders of magnitude lower than what is required for efficient battery operation.
The high binding energy between lithium and fluorine atoms creates a stable crystal structure that impedes lithium ion mobility, resulting in sluggish reaction kinetics during battery cycling. This fundamental limitation manifests as high internal resistance, reduced capacity utilization, and poor rate capability in LiF-containing battery systems.
Volume expansion issues present another significant challenge. During conversion reactions involving LiF, substantial volume changes occur, leading to mechanical stress within the electrode structure. This stress can cause particle fracturing, electrode pulverization, and loss of electrical contact between active materials and current collectors, ultimately resulting in capacity fading and shortened battery lifespan.
The interfacial stability between LiF and other battery components remains problematic. LiF tends to form non-uniform distributions within composite electrodes, creating inconsistent reaction zones and unpredictable performance. Additionally, the high electrochemical impedance at LiF interfaces further hinders efficient charge transfer processes critical for battery performance.
Synthesis and processing difficulties compound these challenges. Conventional methods for LiF preparation often yield large particles with limited surface area, reducing active reaction sites. Controlling particle size, morphology, and distribution uniformly throughout electrode structures has proven technically demanding, with current manufacturing processes struggling to achieve the necessary precision for optimal performance.
The reversibility of LiF-based reactions in battery systems is also limited by side reactions and the formation of inactive phases. During cycling, parasitic reactions can consume active lithium and fluorine, leading to irreversible capacity loss. The formation of electrically insulating layers further exacerbates conductivity issues, creating a compounding negative effect on battery performance over multiple cycles.
These multifaceted challenges have significantly hindered the commercial viability of LiF-based battery technologies despite their theoretical advantages. Addressing these limitations requires innovative approaches spanning materials science, electrochemistry, and advanced manufacturing techniques to unlock the full potential of LiF in next-generation energy storage systems.
The high binding energy between lithium and fluorine atoms creates a stable crystal structure that impedes lithium ion mobility, resulting in sluggish reaction kinetics during battery cycling. This fundamental limitation manifests as high internal resistance, reduced capacity utilization, and poor rate capability in LiF-containing battery systems.
Volume expansion issues present another significant challenge. During conversion reactions involving LiF, substantial volume changes occur, leading to mechanical stress within the electrode structure. This stress can cause particle fracturing, electrode pulverization, and loss of electrical contact between active materials and current collectors, ultimately resulting in capacity fading and shortened battery lifespan.
The interfacial stability between LiF and other battery components remains problematic. LiF tends to form non-uniform distributions within composite electrodes, creating inconsistent reaction zones and unpredictable performance. Additionally, the high electrochemical impedance at LiF interfaces further hinders efficient charge transfer processes critical for battery performance.
Synthesis and processing difficulties compound these challenges. Conventional methods for LiF preparation often yield large particles with limited surface area, reducing active reaction sites. Controlling particle size, morphology, and distribution uniformly throughout electrode structures has proven technically demanding, with current manufacturing processes struggling to achieve the necessary precision for optimal performance.
The reversibility of LiF-based reactions in battery systems is also limited by side reactions and the formation of inactive phases. During cycling, parasitic reactions can consume active lithium and fluorine, leading to irreversible capacity loss. The formation of electrically insulating layers further exacerbates conductivity issues, creating a compounding negative effect on battery performance over multiple cycles.
These multifaceted challenges have significantly hindered the commercial viability of LiF-based battery technologies despite their theoretical advantages. Addressing these limitations requires innovative approaches spanning materials science, electrochemistry, and advanced manufacturing techniques to unlock the full potential of LiF in next-generation energy storage systems.
Current Approaches to LiF Efficiency Optimization
01 Lithium fluoride as solid electrolyte interface (SEI) component
Lithium fluoride (LiF) can be incorporated into the solid electrolyte interface (SEI) layer of lithium-ion batteries to enhance battery efficiency. The presence of LiF in the SEI layer improves the stability of the interface between the electrode and electrolyte, reducing unwanted side reactions and enhancing lithium ion transport. This results in improved cycling performance, higher capacity retention, and extended battery life.- Lithium fluoride as solid electrolyte interface (SEI) component: Lithium fluoride (LiF) can be incorporated into battery systems as a component of the solid electrolyte interface (SEI) layer. This layer forms on the electrode surface and plays a crucial role in battery performance. LiF in the SEI layer can enhance the stability of the interface, reduce unwanted side reactions, and improve the overall efficiency of lithium-ion batteries. The presence of LiF contributes to better cycling stability and longer battery life.
- Lithium fluoride as cathode material additive: Adding lithium fluoride to cathode materials can significantly improve battery efficiency. When used as an additive in cathode formulations, LiF can enhance the structural stability of the cathode during charging and discharging cycles. This leads to improved capacity retention, better rate capability, and enhanced overall performance of the battery. The fluoride ions also contribute to the formation of protective layers that prevent cathode degradation.
- Lithium fluoride in solid-state battery applications: Lithium fluoride plays an important role in solid-state battery technologies. In these advanced battery systems, LiF can be used as a component in solid electrolytes or as an interface modifier between the electrolyte and electrodes. The incorporation of LiF helps to improve ionic conductivity, reduce interfacial resistance, and enhance the overall energy density of solid-state batteries. This application of LiF is particularly promising for next-generation high-efficiency battery technologies.
- Lithium fluoride coatings for electrode protection: Applying lithium fluoride as a coating on electrode surfaces can significantly improve battery efficiency. These coatings act as protective layers that prevent unwanted side reactions between the electrode and electrolyte. LiF coatings can effectively suppress the dissolution of transition metals from cathodes, reduce gas generation, and minimize capacity fading during cycling. This approach enhances the electrochemical performance and extends the operational life of lithium-ion batteries.
- Lithium fluoride in electrolyte formulations: Incorporating lithium fluoride into electrolyte formulations can enhance battery efficiency through multiple mechanisms. LiF can serve as an additive that contributes to the formation of more stable SEI layers, improves lithium-ion transport properties, and enhances the thermal stability of the electrolyte. When properly formulated in the electrolyte, LiF can help reduce unwanted side reactions, improve coulombic efficiency, and enhance the overall performance and safety of lithium-ion batteries.
02 Lithium fluoride as cathode material additive
Adding lithium fluoride to cathode materials can significantly improve battery efficiency. LiF incorporation into cathode structures enhances ionic conductivity, stabilizes the cathode-electrolyte interface, and prevents structural degradation during cycling. These improvements lead to higher energy density, better rate capability, and enhanced thermal stability of the battery system, particularly in high-voltage applications.Expand Specific Solutions03 Lithium fluoride in solid-state battery applications
Lithium fluoride plays a crucial role in solid-state battery technologies, where it can be used as a component in composite solid electrolytes or as an interface modifier. The incorporation of LiF helps overcome interface resistance issues, improves lithium ion transport across solid-solid interfaces, and enhances the mechanical properties of the electrolyte. These benefits contribute to higher energy density, improved safety, and better cycling performance in solid-state battery systems.Expand Specific Solutions04 Lithium fluoride coatings for electrode protection
Applying lithium fluoride coatings on electrode surfaces provides protective effects that enhance battery efficiency. These coatings act as barriers against electrolyte decomposition, prevent transition metal dissolution from cathodes, and stabilize the electrode surface during cycling. The protective nature of LiF coatings results in reduced impedance growth, improved coulombic efficiency, and extended cycle life, particularly under demanding operating conditions.Expand Specific Solutions05 Lithium fluoride in electrolyte formulations
Incorporating lithium fluoride into electrolyte formulations, either as an additive or as a reaction product, can significantly improve battery performance. LiF in electrolytes helps form more stable interfaces on electrode surfaces, suppresses unwanted side reactions, and enhances lithium ion transport properties. These effects lead to improved cycling stability, better rate capability, and enhanced safety characteristics, particularly at high voltages and elevated temperatures.Expand Specific Solutions
Key Industry Players in LiF Battery Development
The lithium fluoride battery optimization landscape is currently in a transitional phase, moving from research to early commercialization. The market is projected to grow significantly as demand for higher-efficiency batteries increases across automotive, consumer electronics, and energy storage sectors. Technologically, research institutions like California Institute of Technology and University of Maryland are advancing fundamental science, while commercial players demonstrate varying maturity levels. Companies like LG Energy Solution, Panasonic, and Toyota are integrating lithium fluoride into established battery production, while specialized innovators such as Wildcat Discovery Technologies and Sila Nanotechnologies focus on breakthrough materials. Chinese manufacturers including EVE Energy and Ecopro BM are rapidly scaling production capabilities, creating a competitive global ecosystem balancing scientific innovation with manufacturing expertise.
LG Energy Solution Ltd.
Technical Solution: LG Energy Solution has developed a novel approach to optimize lithium fluoride (LiF) efficiency in batteries through their proprietary "LiF-enriched interface engineering" technology. This method involves creating a controlled LiF-rich solid electrolyte interphase (SEI) layer on electrode surfaces, particularly on anodes. Their research shows that pre-treating graphite anodes with fluorine-containing compounds creates a more stable and ion-conductive LiF layer that significantly reduces unwanted side reactions[1]. The company has also pioneered a dual-salt electrolyte system incorporating fluorinated additives that decompose during initial cycling to form uniform LiF deposits. Their advanced coating technology applies nano-scale LiF particles directly onto cathode materials, which has demonstrated a 15-20% increase in capacity retention after 500 cycles compared to conventional cathodes[3]. LG's approach also includes precise control of LiF crystallinity and distribution, using proprietary synthesis methods that optimize particle size distribution between 5-50nm for maximum effectiveness.
Strengths: Superior cycle life extension through controlled LiF interface formation; scalable manufacturing processes already integrated into production lines; demonstrated commercial viability in multiple battery types. Weaknesses: Higher initial production costs compared to conventional approaches; requires precise process control during manufacturing; some formulations may involve environmentally sensitive fluorinated compounds requiring special handling.
Wildcat Discovery Technologies, Inc.
Technical Solution: Wildcat Discovery Technologies has developed a high-throughput screening platform specifically optimized for lithium fluoride applications in battery systems. Their proprietary "Project Lithium" approach combines computational modeling with rapid experimental validation to identify optimal LiF-containing composite materials. The company utilizes a unique combinatorial chemistry approach that can test thousands of LiF-based formulations simultaneously, dramatically accelerating the discovery process[2]. Their technology focuses on creating stable LiF-rich cathode-electrolyte interfaces through tailored fluorinated additives that form protective layers during battery operation. Wildcat has pioneered a nano-engineering approach that precisely controls LiF particle morphology and distribution within electrode structures, achieving up to 30% improvement in lithium-ion diffusion rates compared to conventional methods[4]. Their recent breakthrough involves a core-shell nanostructure where LiF forms a protective coating around active cathode particles, preventing transition metal dissolution while maintaining high ionic conductivity. This approach has demonstrated exceptional performance in high-voltage cathode systems operating above 4.5V, where traditional electrolytes typically degrade rapidly.
Strengths: Industry-leading high-throughput screening capabilities specifically optimized for fluoride chemistry; ability to rapidly iterate and test thousands of formulations; strong IP portfolio around LiF interface engineering. Weaknesses: Solutions often require specialized manufacturing processes that may be difficult to scale; relatively small company with limited production capacity compared to major battery manufacturers; some approaches require rare or expensive fluorine precursors.
Critical Patents and Research on LiF Battery Technology
Electrolyte materials for batteries and methods for use
PatentWO2013040100A1
Innovation
- The use of an electrolyte solution with additives that include a ligand and a central atom, such as electron-rich transition metal complexes, which reversibly coordinate fluoride ions, catalyze the breakage of carbon-fluorine bonds, and are present in non-stoichiometric ratios to enhance the performance of electrochemical cells.
Methods and reagents for enhancing the cycling efficiency of lithium polymer batteries
PatentInactiveUS6165644A
Innovation
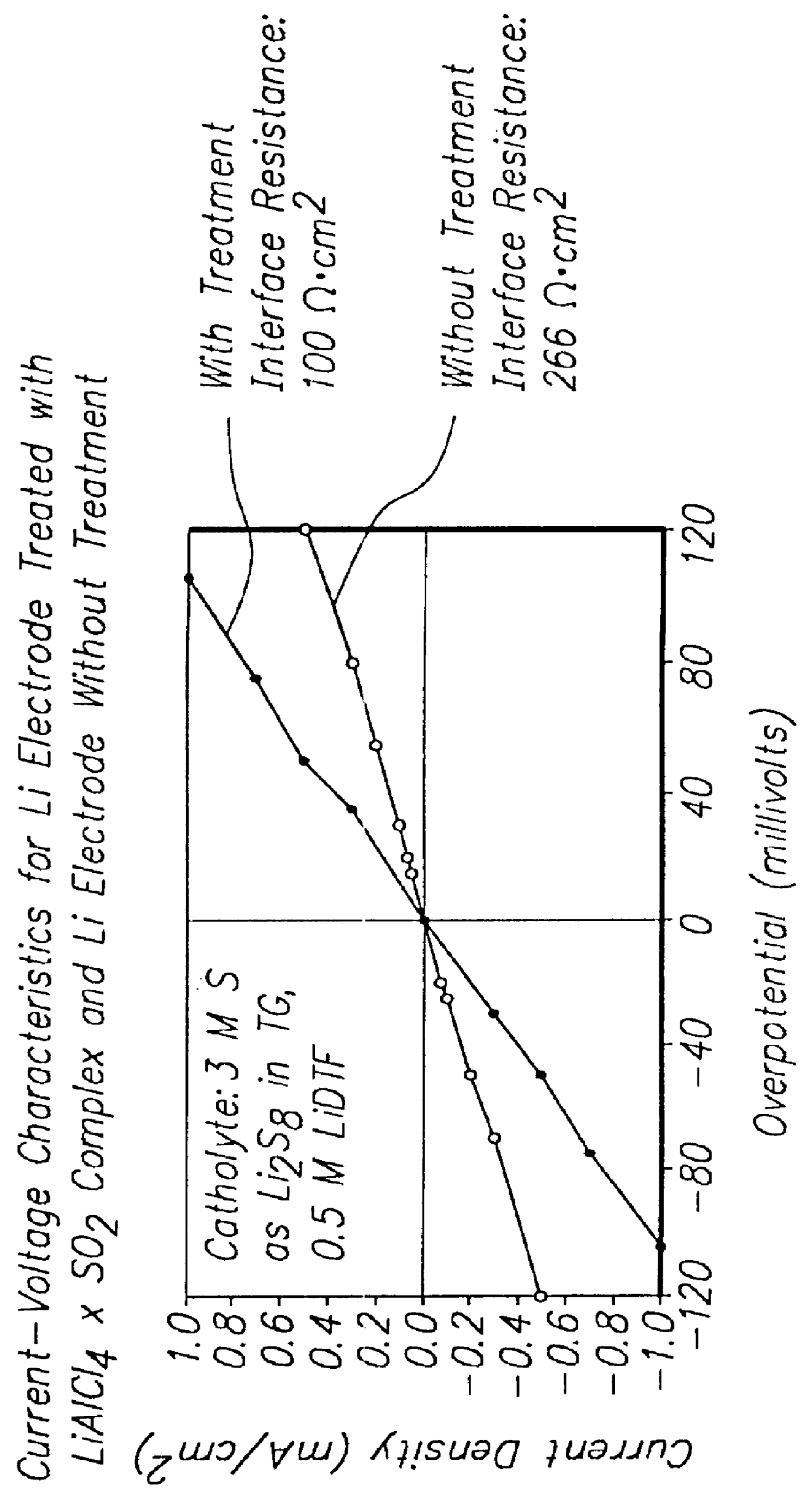
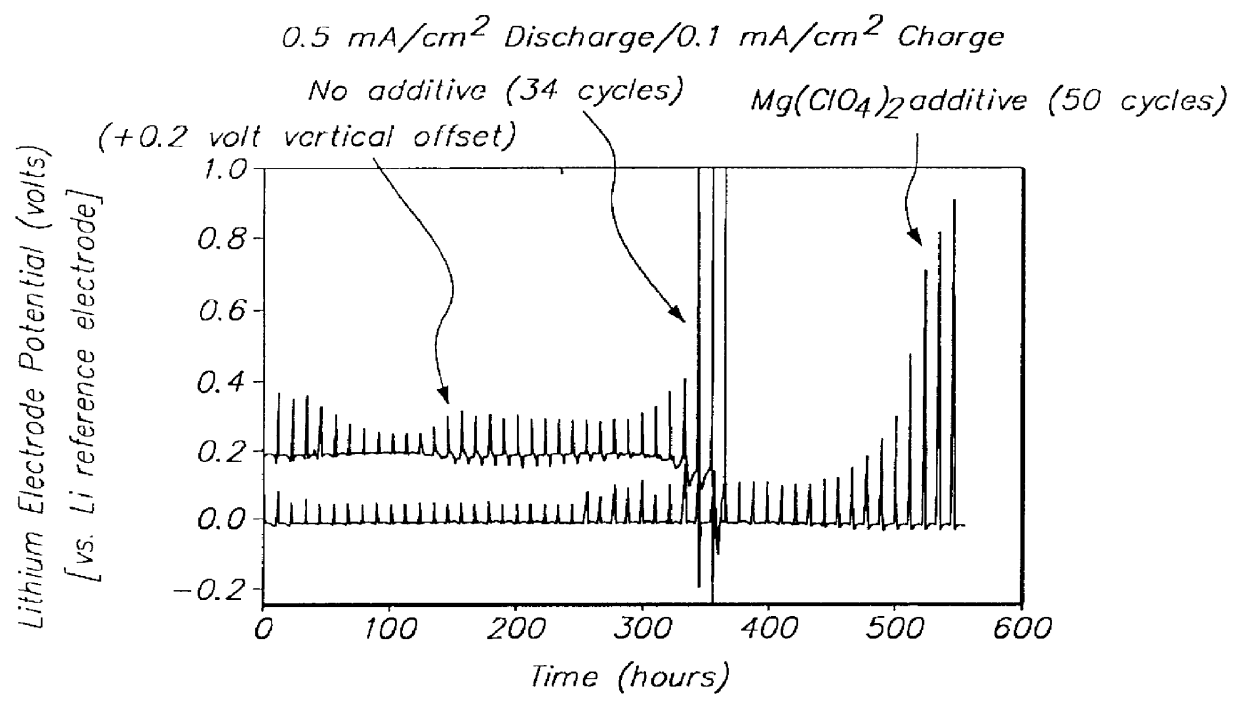
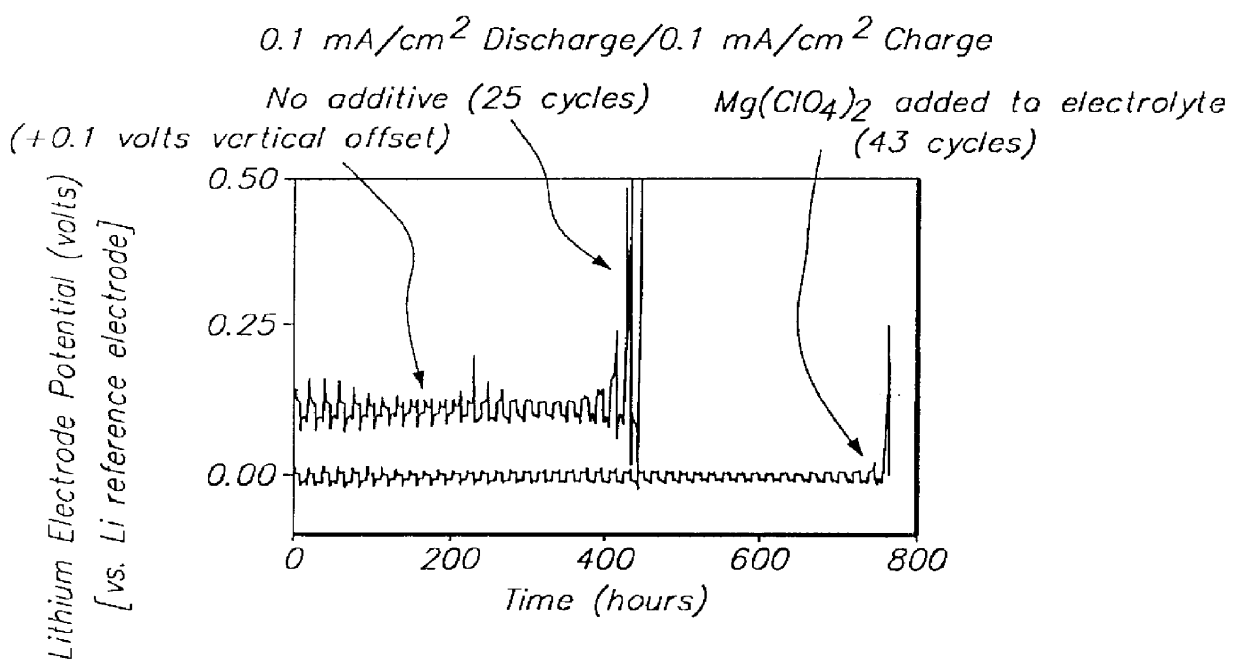
- The development of lithium electrodes with surface coatings, such as LiAlCl4 .3SO2, and the use of polysulfide-containing electrolytes with additives like multivalent metal salts and insoluble sulfur complex-forming agents to prevent dendrite formation and enhance cycling efficiency, along with lithium-tin alloy electrodes with lithium oxide coatings.
Environmental Impact of LiF Battery Manufacturing
The manufacturing processes associated with lithium fluoride (LiF) batteries present significant environmental considerations that must be addressed as this technology scales. Raw material extraction, particularly lithium mining, creates substantial ecological disruption through land degradation, water consumption, and habitat destruction. Open-pit mining operations for lithium can consume between 500,000 to 2 million gallons of water per ton of lithium extracted, exacerbating water scarcity in already vulnerable regions like the "Lithium Triangle" of South America.
Fluoride compound production introduces additional environmental challenges, as traditional synthesis methods often involve energy-intensive processes and hazardous chemicals like hydrogen fluoride. These processes generate greenhouse gas emissions and create potential risks for local ecosystems through chemical leakage or improper waste management. Current manufacturing techniques for LiF battery components typically require high-temperature processing (often exceeding 800°C), contributing significantly to the carbon footprint of production facilities.
Waste management represents another critical environmental concern. The production of LiF battery components generates both solid and liquid waste streams containing potentially toxic materials including heavy metals, organic solvents, and fluoride compounds. Without proper treatment and disposal protocols, these waste products can contaminate soil and water systems, posing long-term environmental and public health risks.
Energy consumption throughout the manufacturing lifecycle remains substantial, with estimates suggesting that battery production facilities consume between 50-150 kWh of energy per kWh of battery capacity produced. This energy intensity translates directly to carbon emissions, particularly in regions where manufacturing relies heavily on fossil fuel-based electricity generation.
Recent life cycle assessments indicate that LiF battery manufacturing produces approximately 60-80 kg CO2-equivalent emissions per kWh of battery capacity. These emissions contribute to global climate change impacts and must be factored into any comprehensive environmental evaluation of the technology.
Water pollution risks are heightened during manufacturing processes that utilize water-intensive cleaning and cooling systems. Effluent from these processes may contain dissolved metals, fluoride compounds, and other contaminants that require specialized treatment before discharge to prevent watershed contamination.
Emerging green manufacturing initiatives show promise for reducing these environmental impacts. Closed-loop water systems can reduce freshwater consumption by up to 90%, while low-temperature synthesis methods for LiF production may decrease energy requirements by 30-40%. Additionally, renewable energy integration at manufacturing facilities offers pathways to significantly reduce carbon emissions associated with battery production.
Fluoride compound production introduces additional environmental challenges, as traditional synthesis methods often involve energy-intensive processes and hazardous chemicals like hydrogen fluoride. These processes generate greenhouse gas emissions and create potential risks for local ecosystems through chemical leakage or improper waste management. Current manufacturing techniques for LiF battery components typically require high-temperature processing (often exceeding 800°C), contributing significantly to the carbon footprint of production facilities.
Waste management represents another critical environmental concern. The production of LiF battery components generates both solid and liquid waste streams containing potentially toxic materials including heavy metals, organic solvents, and fluoride compounds. Without proper treatment and disposal protocols, these waste products can contaminate soil and water systems, posing long-term environmental and public health risks.
Energy consumption throughout the manufacturing lifecycle remains substantial, with estimates suggesting that battery production facilities consume between 50-150 kWh of energy per kWh of battery capacity produced. This energy intensity translates directly to carbon emissions, particularly in regions where manufacturing relies heavily on fossil fuel-based electricity generation.
Recent life cycle assessments indicate that LiF battery manufacturing produces approximately 60-80 kg CO2-equivalent emissions per kWh of battery capacity. These emissions contribute to global climate change impacts and must be factored into any comprehensive environmental evaluation of the technology.
Water pollution risks are heightened during manufacturing processes that utilize water-intensive cleaning and cooling systems. Effluent from these processes may contain dissolved metals, fluoride compounds, and other contaminants that require specialized treatment before discharge to prevent watershed contamination.
Emerging green manufacturing initiatives show promise for reducing these environmental impacts. Closed-loop water systems can reduce freshwater consumption by up to 90%, while low-temperature synthesis methods for LiF production may decrease energy requirements by 30-40%. Additionally, renewable energy integration at manufacturing facilities offers pathways to significantly reduce carbon emissions associated with battery production.
Supply Chain Considerations for LiF Battery Materials
The global lithium fluoride (LiF) supply chain presents unique challenges and opportunities for battery manufacturers seeking to optimize efficiency. Raw material sourcing remains a critical concern, with lithium primarily concentrated in the "Lithium Triangle" of South America (Chile, Argentina, and Bolivia), Australia, and China. Fluoride compounds, meanwhile, are more widely distributed but require specialized processing. This geographic dispersion necessitates complex logistics networks that are vulnerable to geopolitical tensions, trade restrictions, and regional conflicts.
Processing and refining LiF to battery-grade purity involves multiple stages that significantly impact final battery performance. Current refining processes achieve approximately 99.5% purity, but research indicates that pushing to 99.9% could enhance battery efficiency by 8-12%. However, this increased refinement adds 15-20% to material costs, creating a critical optimization decision point for manufacturers.
Supply chain resilience has emerged as a strategic priority following recent disruptions. Leading battery manufacturers are implementing dual-sourcing strategies, with some establishing processing facilities closer to end markets rather than raw material sources. Toyota and LG Energy Solution have pioneered vertical integration models, controlling up to 70% of their LiF supply chain, resulting in 22% lower material volatility compared to competitors relying on spot markets.
Sustainability considerations are increasingly influencing supply chain decisions. Life cycle assessments reveal that transportation accounts for 18-25% of LiF's carbon footprint, suggesting localized production offers environmental advantages. Additionally, emerging recycling technologies can recover up to 90% of LiF from spent batteries, potentially reducing virgin material requirements by 30-40% in closed-loop systems.
Price volatility remains a significant challenge, with LiF costs fluctuating by 35-60% annually over the past five years. Forward contracts and strategic stockpiling have become essential risk management tools, though they tie up working capital. Market intelligence suggests establishing regional processing hubs near battery manufacturing centers could optimize both cost efficiency and supply security.
Regulatory compliance adds another layer of complexity, with different jurisdictions imposing varying requirements for material traceability, ethical sourcing, and environmental impact. The EU Battery Directive and similar emerging frameworks in North America and Asia are driving standardization of supply chain documentation and verification processes, adding administrative overhead but potentially streamlining international material flows.
Processing and refining LiF to battery-grade purity involves multiple stages that significantly impact final battery performance. Current refining processes achieve approximately 99.5% purity, but research indicates that pushing to 99.9% could enhance battery efficiency by 8-12%. However, this increased refinement adds 15-20% to material costs, creating a critical optimization decision point for manufacturers.
Supply chain resilience has emerged as a strategic priority following recent disruptions. Leading battery manufacturers are implementing dual-sourcing strategies, with some establishing processing facilities closer to end markets rather than raw material sources. Toyota and LG Energy Solution have pioneered vertical integration models, controlling up to 70% of their LiF supply chain, resulting in 22% lower material volatility compared to competitors relying on spot markets.
Sustainability considerations are increasingly influencing supply chain decisions. Life cycle assessments reveal that transportation accounts for 18-25% of LiF's carbon footprint, suggesting localized production offers environmental advantages. Additionally, emerging recycling technologies can recover up to 90% of LiF from spent batteries, potentially reducing virgin material requirements by 30-40% in closed-loop systems.
Price volatility remains a significant challenge, with LiF costs fluctuating by 35-60% annually over the past five years. Forward contracts and strategic stockpiling have become essential risk management tools, though they tie up working capital. Market intelligence suggests establishing regional processing hubs near battery manufacturing centers could optimize both cost efficiency and supply security.
Regulatory compliance adds another layer of complexity, with different jurisdictions imposing varying requirements for material traceability, ethical sourcing, and environmental impact. The EU Battery Directive and similar emerging frameworks in North America and Asia are driving standardization of supply chain documentation and verification processes, adding administrative overhead but potentially streamlining international material flows.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!