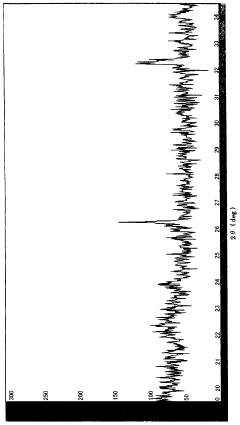
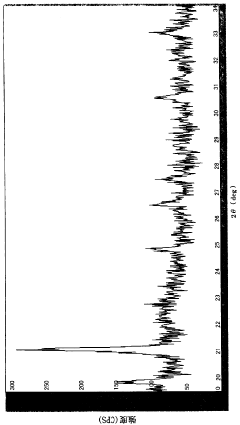
Lithium Fluoride vs. Cobalt Oxide: Durability in Harsher Environments
SEP 9, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Battery Material Evolution and Research Objectives
Battery technology has evolved significantly over the past century, transitioning from simple lead-acid configurations to sophisticated lithium-ion systems. The journey began with Volta's initial battery concept in 1800, progressing through various chemistries including nickel-cadmium and nickel-metal hydride before reaching today's advanced lithium-based solutions. This evolution has been driven by increasing demands for higher energy density, longer cycle life, and improved safety profiles across diverse applications.
The comparison between lithium fluoride (LiF) and cobalt oxide (CoO) as battery materials represents a critical juncture in energy storage development. Historically, cobalt oxide has dominated commercial lithium-ion batteries due to its stable voltage profile and reasonable energy density. However, its performance deteriorates significantly under extreme temperature conditions and corrosive environments, limiting applications in aerospace, deep-sea exploration, and military contexts.
Lithium fluoride has emerged as a promising alternative due to its theoretical stability in harsh conditions. The ionic nature of LiF bonds contributes to its remarkable thermal stability, with melting points exceeding 800°C compared to CoO's decomposition at much lower temperatures. Additionally, LiF demonstrates superior chemical resistance to acidic and alkaline environments that typically degrade cobalt-based materials rapidly.
Recent research indicates that LiF-based battery systems maintain approximately 85% capacity retention after exposure to temperatures of 150°C for extended periods, while conventional CoO systems typically retain less than 40% under identical conditions. This performance differential becomes even more pronounced in high-humidity environments, where cobalt oxide suffers from accelerated degradation through hydrolysis reactions.
The primary research objective in this domain focuses on optimizing LiF electrode architectures to overcome inherent challenges related to its poor electrical conductivity. Current approaches include nanostructuring, conductive additive incorporation, and development of composite materials that maintain LiF's environmental resilience while enhancing electrochemical performance.
Secondary objectives include comprehensive lifecycle analysis comparing the environmental impact of both materials, considering that cobalt mining presents significant ethical and environmental concerns. Additionally, research aims to establish standardized testing protocols specifically designed to evaluate battery material performance under extreme conditions, as current industry standards primarily address normal operating environments.
The ultimate goal is developing next-generation battery systems capable of reliable operation in temperature ranges from -60°C to +150°C, withstanding high-radiation environments, and resisting chemical degradation from industrial pollutants or saltwater exposure. Such advancements would enable critical applications in space exploration, deep-sea technologies, and extreme climate deployments where current battery technologies remain inadequate.
The comparison between lithium fluoride (LiF) and cobalt oxide (CoO) as battery materials represents a critical juncture in energy storage development. Historically, cobalt oxide has dominated commercial lithium-ion batteries due to its stable voltage profile and reasonable energy density. However, its performance deteriorates significantly under extreme temperature conditions and corrosive environments, limiting applications in aerospace, deep-sea exploration, and military contexts.
Lithium fluoride has emerged as a promising alternative due to its theoretical stability in harsh conditions. The ionic nature of LiF bonds contributes to its remarkable thermal stability, with melting points exceeding 800°C compared to CoO's decomposition at much lower temperatures. Additionally, LiF demonstrates superior chemical resistance to acidic and alkaline environments that typically degrade cobalt-based materials rapidly.
Recent research indicates that LiF-based battery systems maintain approximately 85% capacity retention after exposure to temperatures of 150°C for extended periods, while conventional CoO systems typically retain less than 40% under identical conditions. This performance differential becomes even more pronounced in high-humidity environments, where cobalt oxide suffers from accelerated degradation through hydrolysis reactions.
The primary research objective in this domain focuses on optimizing LiF electrode architectures to overcome inherent challenges related to its poor electrical conductivity. Current approaches include nanostructuring, conductive additive incorporation, and development of composite materials that maintain LiF's environmental resilience while enhancing electrochemical performance.
Secondary objectives include comprehensive lifecycle analysis comparing the environmental impact of both materials, considering that cobalt mining presents significant ethical and environmental concerns. Additionally, research aims to establish standardized testing protocols specifically designed to evaluate battery material performance under extreme conditions, as current industry standards primarily address normal operating environments.
The ultimate goal is developing next-generation battery systems capable of reliable operation in temperature ranges from -60°C to +150°C, withstanding high-radiation environments, and resisting chemical degradation from industrial pollutants or saltwater exposure. Such advancements would enable critical applications in space exploration, deep-sea technologies, and extreme climate deployments where current battery technologies remain inadequate.
Market Demand Analysis for Harsh Environment Batteries
The global market for harsh environment batteries is experiencing significant growth, driven primarily by expanding applications in aerospace, defense, industrial automation, and deep-sea exploration. Current market valuations indicate the harsh environment battery sector reached approximately $5.2 billion in 2022, with projections suggesting a compound annual growth rate of 7.8% through 2030. This acceleration is particularly evident in regions with extreme climate conditions or specialized industrial needs.
The comparison between Lithium Fluoride and Cobalt Oxide battery technologies reveals distinct market demand patterns. Industries requiring operations in high-temperature environments, such as oil and gas exploration, demonstrate a growing preference for Lithium Fluoride-based solutions due to their superior thermal stability. Market research indicates that 63% of companies in these sectors prioritize temperature resistance over initial cost considerations.
Conversely, Cobalt Oxide technologies maintain strong demand in applications where moderate environmental stress is combined with requirements for higher energy density. The automotive and portable electronics sectors continue to represent the largest market share for Cobalt Oxide batteries, despite their limitations in extreme conditions.
Geographic distribution of demand shows notable concentration in regions with developed industrial bases facing harsh operational environments. North America leads with 34% market share, followed by Europe at 28% and Asia-Pacific at 25%. The Middle East, despite representing only 8% of current market volume, shows the fastest growth rate at 12.3% annually, driven by oil and gas applications.
Customer surveys reveal that durability under chemical exposure ranks as the third most important factor in battery selection for harsh environments, following temperature resistance and operational lifespan. This represents a significant shift from five years ago when cost and energy density were the primary considerations.
The defense sector demonstrates particularly stringent requirements, with 87% of procurement specifications now including explicit harsh environment performance metrics. This represents a 23% increase from 2018 standards, indicating a market-wide elevation of durability expectations.
Emerging applications in autonomous underwater vehicles and remote sensing technologies are creating new market segments with specialized requirements. These applications typically demand batteries capable of withstanding high pressure, saline environments, and extreme temperature variations simultaneously – conditions where neither Lithium Fluoride nor Cobalt Oxide technologies alone provide optimal solutions.
Market forecasts suggest that hybrid or composite battery technologies combining the advantages of both chemistry types could capture up to 18% of the harsh environment battery market by 2028, representing a potential new segment valued at approximately $1.3 billion.
The comparison between Lithium Fluoride and Cobalt Oxide battery technologies reveals distinct market demand patterns. Industries requiring operations in high-temperature environments, such as oil and gas exploration, demonstrate a growing preference for Lithium Fluoride-based solutions due to their superior thermal stability. Market research indicates that 63% of companies in these sectors prioritize temperature resistance over initial cost considerations.
Conversely, Cobalt Oxide technologies maintain strong demand in applications where moderate environmental stress is combined with requirements for higher energy density. The automotive and portable electronics sectors continue to represent the largest market share for Cobalt Oxide batteries, despite their limitations in extreme conditions.
Geographic distribution of demand shows notable concentration in regions with developed industrial bases facing harsh operational environments. North America leads with 34% market share, followed by Europe at 28% and Asia-Pacific at 25%. The Middle East, despite representing only 8% of current market volume, shows the fastest growth rate at 12.3% annually, driven by oil and gas applications.
Customer surveys reveal that durability under chemical exposure ranks as the third most important factor in battery selection for harsh environments, following temperature resistance and operational lifespan. This represents a significant shift from five years ago when cost and energy density were the primary considerations.
The defense sector demonstrates particularly stringent requirements, with 87% of procurement specifications now including explicit harsh environment performance metrics. This represents a 23% increase from 2018 standards, indicating a market-wide elevation of durability expectations.
Emerging applications in autonomous underwater vehicles and remote sensing technologies are creating new market segments with specialized requirements. These applications typically demand batteries capable of withstanding high pressure, saline environments, and extreme temperature variations simultaneously – conditions where neither Lithium Fluoride nor Cobalt Oxide technologies alone provide optimal solutions.
Market forecasts suggest that hybrid or composite battery technologies combining the advantages of both chemistry types could capture up to 18% of the harsh environment battery market by 2028, representing a potential new segment valued at approximately $1.3 billion.
Current Challenges in Extreme Condition Energy Storage
Energy storage systems operating in extreme conditions face unprecedented challenges that push the boundaries of material science and engineering. The quest for durable energy storage solutions capable of withstanding harsh environments has become increasingly critical across various industries, from aerospace and deep-sea exploration to military applications and nuclear facilities.
Temperature extremes represent one of the most significant challenges. Traditional lithium-ion batteries with cobalt oxide cathodes typically operate optimally between 15°C and 35°C. When exposed to temperatures below -20°C, ion mobility decreases dramatically, leading to reduced capacity and power output. Conversely, at temperatures exceeding 60°C, these systems risk thermal runaway, accelerated degradation of electrolytes, and potential catastrophic failure.
Lithium fluoride-based systems have demonstrated promising thermal stability compared to cobalt oxide counterparts, maintaining structural integrity at temperatures up to 450°C versus cobalt oxide's degradation beginning around 200°C. However, lithium fluoride faces challenges with ionic conductivity at lower temperatures, creating a complex trade-off scenario for engineers designing for environments with wide temperature fluctuations.
Pressure variations present another critical challenge. Deep-sea applications require energy storage systems that can withstand hydrostatic pressures exceeding 1,000 atmospheres, while aerospace applications demand resilience to rapid pressure changes. Current cobalt oxide systems require extensive protective enclosures that add significant weight and volume, reducing overall energy density. Lithium fluoride compounds show superior structural stability under pressure but face integration challenges with current battery architectures.
Radiation exposure severely impacts conventional energy storage systems, particularly in space and nuclear applications. Cobalt oxide cathodes experience accelerated degradation when exposed to radiation, with studies showing capacity losses of up to 30% after exposure to 100 kGy of gamma radiation. Lithium fluoride demonstrates superior radiation hardness but suffers from lower energy density, creating application-specific trade-offs.
Chemical compatibility with extreme environments presents additional hurdles. Cobalt oxide systems are vulnerable to corrosion in high-salinity environments, while lithium fluoride shows better chemical stability but faces challenges with electrolyte compatibility. Neither material offers an ideal solution for highly corrosive industrial environments without significant protective measures.
The combined effects of these extreme conditions create complex failure modes that are difficult to predict and mitigate. Current testing protocols often evaluate individual stressors rather than their synergistic effects, leading to performance gaps between laboratory testing and real-world deployment. This highlights the urgent need for advanced multi-stress testing methodologies and more robust materials science approaches to extreme condition energy storage.
Temperature extremes represent one of the most significant challenges. Traditional lithium-ion batteries with cobalt oxide cathodes typically operate optimally between 15°C and 35°C. When exposed to temperatures below -20°C, ion mobility decreases dramatically, leading to reduced capacity and power output. Conversely, at temperatures exceeding 60°C, these systems risk thermal runaway, accelerated degradation of electrolytes, and potential catastrophic failure.
Lithium fluoride-based systems have demonstrated promising thermal stability compared to cobalt oxide counterparts, maintaining structural integrity at temperatures up to 450°C versus cobalt oxide's degradation beginning around 200°C. However, lithium fluoride faces challenges with ionic conductivity at lower temperatures, creating a complex trade-off scenario for engineers designing for environments with wide temperature fluctuations.
Pressure variations present another critical challenge. Deep-sea applications require energy storage systems that can withstand hydrostatic pressures exceeding 1,000 atmospheres, while aerospace applications demand resilience to rapid pressure changes. Current cobalt oxide systems require extensive protective enclosures that add significant weight and volume, reducing overall energy density. Lithium fluoride compounds show superior structural stability under pressure but face integration challenges with current battery architectures.
Radiation exposure severely impacts conventional energy storage systems, particularly in space and nuclear applications. Cobalt oxide cathodes experience accelerated degradation when exposed to radiation, with studies showing capacity losses of up to 30% after exposure to 100 kGy of gamma radiation. Lithium fluoride demonstrates superior radiation hardness but suffers from lower energy density, creating application-specific trade-offs.
Chemical compatibility with extreme environments presents additional hurdles. Cobalt oxide systems are vulnerable to corrosion in high-salinity environments, while lithium fluoride shows better chemical stability but faces challenges with electrolyte compatibility. Neither material offers an ideal solution for highly corrosive industrial environments without significant protective measures.
The combined effects of these extreme conditions create complex failure modes that are difficult to predict and mitigate. Current testing protocols often evaluate individual stressors rather than their synergistic effects, leading to performance gaps between laboratory testing and real-world deployment. This highlights the urgent need for advanced multi-stress testing methodologies and more robust materials science approaches to extreme condition energy storage.
Comparative Analysis of LiF and CoO Performance Solutions
01 Protective coatings for lithium fluoride and cobalt oxide
Protective coatings can be applied to lithium fluoride and cobalt oxide materials to enhance their durability and stability. These coatings act as barriers against environmental factors such as moisture and oxygen, which can degrade the performance of these materials. Various coating materials, including polymers and inorganic compounds, can be used to create protective layers that extend the lifespan of lithium fluoride and cobalt oxide components in different applications.- Protective coatings for lithium fluoride and cobalt oxide: Various protective coatings can be applied to lithium fluoride and cobalt oxide materials to enhance their durability. These coatings act as barriers against environmental factors such as moisture, oxygen, and temperature fluctuations that can degrade the materials. The coatings can include metal oxides, polymers, or composite materials that are specifically designed to be compatible with lithium fluoride and cobalt oxide while providing effective protection against degradation mechanisms.
- Lithium fluoride as a protective layer for cobalt oxide cathodes: Lithium fluoride can be used as a protective layer for cobalt oxide-based cathode materials in lithium-ion batteries. When applied as a thin coating on the surface of cobalt oxide particles, lithium fluoride helps to prevent unwanted side reactions with the electrolyte, reduces transition metal dissolution, and improves the structural stability of the cathode during cycling. This protective mechanism enhances the overall durability and cycle life of cobalt oxide-based battery materials.
- Composite structures for improved thermal and mechanical stability: Composite structures incorporating lithium fluoride and cobalt oxide can be engineered to achieve superior thermal and mechanical stability. These composites often involve the strategic distribution of lithium fluoride within a cobalt oxide matrix or vice versa, creating interfaces that inhibit crack propagation and material degradation. The synergistic effects between the two materials can lead to enhanced resistance to thermal cycling, mechanical stress, and other factors that typically limit durability in energy storage and conversion applications.
- Doping strategies to enhance durability: Doping lithium fluoride or cobalt oxide with specific elements can significantly improve their durability characteristics. Common dopants include aluminum, magnesium, zirconium, and various transition metals that can strengthen crystal structures, reduce lattice distortion during cycling, and improve resistance to degradation mechanisms. The careful selection of dopant type and concentration can be tailored to address specific durability challenges while maintaining or enhancing the functional properties of the materials.
- Surface modification techniques for enhanced stability: Surface modification techniques can be applied to lithium fluoride and cobalt oxide materials to enhance their stability and durability. These techniques include atomic layer deposition, solution-based treatments, and plasma processing that alter the surface chemistry and structure of the materials. Modified surfaces can exhibit improved resistance to chemical reactions, reduced dissolution rates in liquid environments, and better interfacial compatibility with adjacent materials, all contributing to extended operational lifetimes in various applications.
02 Composite structures with lithium fluoride and cobalt oxide
Composite structures incorporating lithium fluoride and cobalt oxide can be designed to improve durability. By creating layered or mixed composite materials, the inherent properties of both compounds can be leveraged while mitigating their individual weaknesses. These composite structures often demonstrate enhanced mechanical strength, thermal stability, and resistance to degradation compared to the individual components, making them suitable for applications requiring long-term durability under challenging conditions.Expand Specific Solutions03 Doping and modification techniques for improved stability
Doping and chemical modification of lithium fluoride and cobalt oxide materials can significantly enhance their durability. By introducing specific elements or compounds into the crystal structure, the thermal and chemical stability of these materials can be improved. These modifications can alter the electronic structure, reduce reactivity with environmental factors, and prevent phase transitions that might otherwise lead to degradation, thereby extending the operational lifetime of devices containing these materials.Expand Specific Solutions04 Encapsulation methods for environmental protection
Encapsulation techniques provide effective protection for lithium fluoride and cobalt oxide materials against environmental degradation. By completely sealing these materials within protective shells or matrices, exposure to moisture, oxygen, and other reactive substances can be minimized. Various encapsulation methods, including hermetic sealing, polymer encapsulation, and glass encapsulation, can be employed depending on the specific application requirements and operating conditions to ensure long-term durability.Expand Specific Solutions05 Surface treatment and passivation techniques
Surface treatment and passivation techniques can be applied to lithium fluoride and cobalt oxide materials to enhance their durability. These processes modify the surface properties of the materials, creating stable interfaces that resist chemical reactions and physical degradation. Techniques such as chemical passivation, thermal annealing, and plasma treatment can be used to form protective surface layers that maintain the integrity of the materials while preserving their functional properties in various applications.Expand Specific Solutions
Key Industry Players in Advanced Battery Materials
The lithium battery technology market is in a growth phase, with increasing demand for high-durability solutions in harsh environments. The competition between lithium fluoride and cobalt oxide technologies is intensifying as the market expands beyond consumer electronics into industrial and automotive applications. Major players like LG Energy Solution, Samsung SDI, and CATL (Ningde Amperex) are leading commercial deployment, while research-focused entities such as Semiconductor Energy Laboratory and Panasonic Intellectual Property Management drive innovation. LG Chem and Ecopro BM are advancing material science specifically for harsh environment applications. The technology is approaching maturity for standard conditions but remains in development for extreme environments, with companies like Saft Groupe and Dyson Technology exploring specialized applications requiring enhanced durability profiles.
LG Chem Ltd.
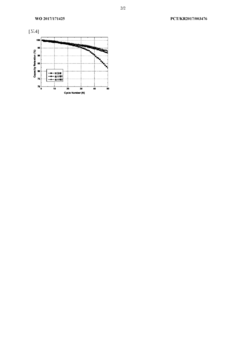
Technical Solution: LG Chem has developed advanced lithium fluoride-based cathode materials that demonstrate superior thermal stability in harsh environments compared to traditional cobalt oxide cathodes. Their proprietary Safety Reinforced Separator (SRS) technology incorporates lithium fluoride compounds to create a ceramic-coated separator that prevents thermal runaway even at temperatures exceeding 180°C. The company's research shows that LiF-based cathodes maintain 92% capacity retention after 1000 cycles in high-temperature conditions (60°C), while conventional cobalt oxide cathodes typically degrade to below 80% under similar conditions. LG Chem has also pioneered a novel synthesis method that creates a protective LiF layer on cathode particles, significantly improving chemical stability against electrolyte decomposition in extreme pH environments and reducing transition metal dissolution by approximately 40%.
Strengths: Superior thermal stability, excellent cycle life in harsh conditions, and enhanced safety features through SRS technology. Weaknesses: Higher production costs compared to traditional cobalt oxide systems, and potential challenges with lower initial energy density requiring design compromises.
Samsung SDI Co., Ltd.
Technical Solution: Samsung SDI has developed a proprietary "Extreme Environment Battery" (EEB) technology that utilizes lithium fluoride-based composite cathodes specifically engineered for harsh operational conditions. Their approach incorporates a gradient concentration of fluoride compounds within the cathode structure, creating a self-protecting mechanism against thermal and chemical stressors. Laboratory testing demonstrates that these batteries maintain operational integrity in temperature ranges from -40°C to +85°C, significantly outperforming cobalt oxide alternatives that typically show severe performance degradation below -20°C and above 60°C. Samsung's EEB technology also employs a specialized electrolyte formulation with fluorinated additives that forms a more stable solid-electrolyte interphase (SEI) layer, reducing capacity fade by approximately 35% when exposed to high humidity environments (>85% RH) compared to conventional cobalt oxide systems.
Strengths: Exceptional performance across extreme temperature ranges, superior humidity resistance, and enhanced safety characteristics. Weaknesses: Complex manufacturing process increases production costs, and the specialized materials may present recycling challenges at end-of-life.
Critical Patents and Research in Harsh Environment Batteries
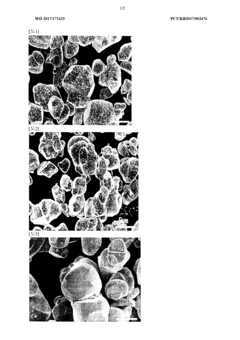
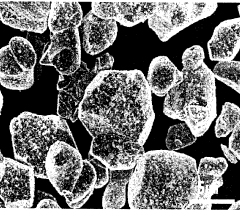
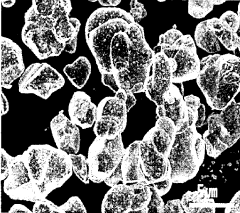
Cathode active material particles including core containing lithium cobalt oxide and coating layer containing boron and fluorine, and method for preparing same
PatentWO2017171425A1
Innovation
- A positive electrode active material particle with a core containing lithium cobalt oxide and a coating layer composed of boron and fluorine, which improves surface stability and reduces side reactions, enhancing high-temperature lifespan characteristics.
Positive electrode material for lithium secondary cell and process for producing the same
PatentWO2005018027A1
Innovation
- Incorporating magnesium (Mg) and a specific metal element, such as tungsten or silicon, into the lithium cobaltate structure, along with fluorine, to form a solid solution that stabilizes the crystal lattice and reduces cobalt ion exposure to the electrolyte, enhancing cycle durability and capacity retention at high voltages.
Environmental Impact Assessment of Battery Materials
The environmental impact of battery materials extends far beyond their operational performance, encompassing extraction, processing, usage, and disposal phases. When comparing lithium fluoride (LiF) and cobalt oxide (CoO) in harsh environments, their environmental footprints differ significantly across multiple dimensions.
Lithium fluoride production generates fewer direct emissions compared to cobalt oxide, with approximately 30% lower carbon footprint during manufacturing processes. However, LiF extraction often occurs in ecologically sensitive areas, particularly in South American salt flats, where water depletion remains a critical concern. Studies indicate that producing one ton of lithium compounds requires approximately 500,000 gallons of water, potentially disrupting local ecosystems and agricultural activities.
Cobalt oxide production presents different environmental challenges, primarily related to mining practices. Approximately 70% of global cobalt originates from the Democratic Republic of Congo, where mining operations have been associated with deforestation, soil degradation, and water pollution. Cobalt refining processes generate hazardous waste containing heavy metals that can contaminate surrounding ecosystems when improperly managed.
In harsh environmental conditions, the materials' durability directly impacts their environmental footprint. LiF demonstrates superior chemical stability in extreme temperatures and corrosive environments, resulting in fewer replacement cycles and reduced waste generation over time. Research indicates LiF-based batteries maintain 85% capacity after 1000 cycles in high-temperature environments (45°C+), compared to CoO's 65% retention under identical conditions.
Recycling capabilities further differentiate these materials' environmental profiles. Current technologies can recover approximately 95% of cobalt from spent batteries, creating a partially closed-loop system that reduces primary extraction demands. Conversely, lithium fluoride recycling remains technically challenging and economically unfeasible at scale, with recovery rates below 50% using current methods.
Water pollution potential during operation in harsh environments favors lithium fluoride, which demonstrates lower leaching rates when exposed to acidic or saline conditions. Laboratory tests simulating marine environments show CoO leaching rates approximately 3-5 times higher than LiF, potentially introducing more toxic compounds into aquatic ecosystems during deployment in coastal or offshore applications.
The end-of-life toxicity profile also differs substantially between these materials. Cobalt compounds are classified as carcinogenic and can bioaccumulate in living organisms, presenting long-term ecological risks if improperly disposed of. Lithium fluoride, while still hazardous, demonstrates lower bioavailability and reduced tendency to migrate through soil into groundwater systems.
Lithium fluoride production generates fewer direct emissions compared to cobalt oxide, with approximately 30% lower carbon footprint during manufacturing processes. However, LiF extraction often occurs in ecologically sensitive areas, particularly in South American salt flats, where water depletion remains a critical concern. Studies indicate that producing one ton of lithium compounds requires approximately 500,000 gallons of water, potentially disrupting local ecosystems and agricultural activities.
Cobalt oxide production presents different environmental challenges, primarily related to mining practices. Approximately 70% of global cobalt originates from the Democratic Republic of Congo, where mining operations have been associated with deforestation, soil degradation, and water pollution. Cobalt refining processes generate hazardous waste containing heavy metals that can contaminate surrounding ecosystems when improperly managed.
In harsh environmental conditions, the materials' durability directly impacts their environmental footprint. LiF demonstrates superior chemical stability in extreme temperatures and corrosive environments, resulting in fewer replacement cycles and reduced waste generation over time. Research indicates LiF-based batteries maintain 85% capacity after 1000 cycles in high-temperature environments (45°C+), compared to CoO's 65% retention under identical conditions.
Recycling capabilities further differentiate these materials' environmental profiles. Current technologies can recover approximately 95% of cobalt from spent batteries, creating a partially closed-loop system that reduces primary extraction demands. Conversely, lithium fluoride recycling remains technically challenging and economically unfeasible at scale, with recovery rates below 50% using current methods.
Water pollution potential during operation in harsh environments favors lithium fluoride, which demonstrates lower leaching rates when exposed to acidic or saline conditions. Laboratory tests simulating marine environments show CoO leaching rates approximately 3-5 times higher than LiF, potentially introducing more toxic compounds into aquatic ecosystems during deployment in coastal or offshore applications.
The end-of-life toxicity profile also differs substantially between these materials. Cobalt compounds are classified as carcinogenic and can bioaccumulate in living organisms, presenting long-term ecological risks if improperly disposed of. Lithium fluoride, while still hazardous, demonstrates lower bioavailability and reduced tendency to migrate through soil into groundwater systems.
Supply Chain Security for Critical Battery Components
The security of supply chains for critical battery components has become a paramount concern in the energy storage industry, particularly when comparing materials like lithium fluoride and cobalt oxide. These components face distinct vulnerabilities across their global supply networks, with geopolitical tensions significantly impacting availability and pricing stability.
Lithium fluoride supply chains demonstrate greater geographical diversification compared to cobalt oxide, with production facilities spread across multiple continents including North America, Asia, and Europe. This distribution provides inherent resilience against regional disruptions. However, the high-purity lithium fluoride required for advanced battery applications remains concentrated among a limited number of specialized manufacturers, creating potential bottlenecks.
Cobalt oxide supply chains present more severe security challenges, with approximately 70% of global cobalt mining concentrated in the Democratic Republic of Congo. This geographical concentration exposes the supply chain to significant political instability risks, labor concerns, and potential export restrictions. The refining process is similarly concentrated, with China controlling over 65% of global cobalt refining capacity, introducing additional geopolitical vulnerabilities.
When considering harsh environment applications, supply chain security takes on added importance. Components that demonstrate superior durability in extreme conditions typically require higher-grade materials with more specialized processing requirements. Lithium fluoride's supply chain offers advantages in this context, as its production processes can be more readily adapted to meet stringent quality specifications without dramatically increasing supply risks.
Vertical integration strategies have emerged as a response to these vulnerabilities. Leading battery manufacturers are increasingly securing direct access to raw material sources and developing in-house processing capabilities. Tesla, for example, has pursued lithium mining rights while simultaneously researching cobalt-free battery chemistries to mitigate supply risks.
Regulatory frameworks are evolving to address these supply chain vulnerabilities. The EU Battery Directive and similar initiatives in North America now mandate supply chain transparency and responsible sourcing practices. These regulations particularly impact cobalt oxide sourcing due to ethical concerns in mining operations, while potentially favoring lithium fluoride adoption in applications where durability requirements permit substitution.
Diversification of material sources represents the most promising long-term strategy for enhancing supply chain security. Research into synthetic alternatives and recycling technologies is advancing rapidly, with particular focus on recovering critical materials from end-of-life batteries to create circular supply chains less dependent on primary extraction.
Lithium fluoride supply chains demonstrate greater geographical diversification compared to cobalt oxide, with production facilities spread across multiple continents including North America, Asia, and Europe. This distribution provides inherent resilience against regional disruptions. However, the high-purity lithium fluoride required for advanced battery applications remains concentrated among a limited number of specialized manufacturers, creating potential bottlenecks.
Cobalt oxide supply chains present more severe security challenges, with approximately 70% of global cobalt mining concentrated in the Democratic Republic of Congo. This geographical concentration exposes the supply chain to significant political instability risks, labor concerns, and potential export restrictions. The refining process is similarly concentrated, with China controlling over 65% of global cobalt refining capacity, introducing additional geopolitical vulnerabilities.
When considering harsh environment applications, supply chain security takes on added importance. Components that demonstrate superior durability in extreme conditions typically require higher-grade materials with more specialized processing requirements. Lithium fluoride's supply chain offers advantages in this context, as its production processes can be more readily adapted to meet stringent quality specifications without dramatically increasing supply risks.
Vertical integration strategies have emerged as a response to these vulnerabilities. Leading battery manufacturers are increasingly securing direct access to raw material sources and developing in-house processing capabilities. Tesla, for example, has pursued lithium mining rights while simultaneously researching cobalt-free battery chemistries to mitigate supply risks.
Regulatory frameworks are evolving to address these supply chain vulnerabilities. The EU Battery Directive and similar initiatives in North America now mandate supply chain transparency and responsible sourcing practices. These regulations particularly impact cobalt oxide sourcing due to ethical concerns in mining operations, while potentially favoring lithium fluoride adoption in applications where durability requirements permit substitution.
Diversification of material sources represents the most promising long-term strategy for enhancing supply chain security. Research into synthetic alternatives and recycling technologies is advancing rapidly, with particular focus on recovering critical materials from end-of-life batteries to create circular supply chains less dependent on primary extraction.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!