How to Reduce Lithium Fluoride Dissolution Under Moist Conditions
SEP 12, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
LiF Dissolution Challenges and Research Objectives
Lithium fluoride (LiF) has emerged as a critical component in various advanced technologies, particularly in battery systems, optical applications, and nuclear energy. The dissolution behavior of LiF under moist conditions presents significant challenges that impact the performance, durability, and safety of these applications. This technical research aims to comprehensively investigate the mechanisms behind LiF dissolution in humid environments and develop innovative solutions to mitigate this issue.
The dissolution of LiF in moisture-rich environments occurs through complex physicochemical processes involving hydrolysis reactions, surface interactions, and ion transport phenomena. When exposed to water molecules, LiF undergoes partial dissociation, releasing lithium and fluoride ions that can migrate away from their original lattice positions. This process is particularly problematic in battery technologies where LiF forms as part of the solid electrolyte interphase (SEI), a protective layer crucial for battery longevity and performance.
Historical approaches to addressing LiF dissolution have evolved from simple physical barriers to sophisticated chemical modifications. Early solutions focused primarily on environmental control through hermetic sealing and moisture-resistant packaging. However, these methods proved insufficient for long-term applications where moisture inevitably penetrates protective barriers over extended operational periods.
Recent technological advancements have shifted focus toward intrinsic modification of LiF properties through doping, surface functionalization, and composite formation. These approaches aim to alter the fundamental interaction between LiF and water molecules without compromising the beneficial properties that make LiF valuable in various applications.
The primary objectives of this research include: (1) elucidating the precise mechanisms and kinetics of LiF dissolution under various humidity conditions; (2) developing quantitative models to predict dissolution rates in different environmental scenarios; (3) investigating novel material modifications that enhance LiF stability in moist environments; and (4) designing practical, scalable solutions that can be implemented in commercial applications.
Understanding the dissolution behavior requires multi-scale analysis spanning from atomic-level interactions to macroscopic material degradation. This necessitates a multidisciplinary approach combining computational chemistry, materials science, electrochemistry, and surface engineering to develop comprehensive solutions.
The significance of this research extends beyond academic interest, addressing critical challenges in energy storage, optical systems, and nuclear applications. Successful mitigation of LiF dissolution could substantially improve the performance and lifespan of lithium-ion batteries, enhance the durability of optical components in humid environments, and increase the safety margins in nuclear systems where LiF serves as a coolant or moderator.
The dissolution of LiF in moisture-rich environments occurs through complex physicochemical processes involving hydrolysis reactions, surface interactions, and ion transport phenomena. When exposed to water molecules, LiF undergoes partial dissociation, releasing lithium and fluoride ions that can migrate away from their original lattice positions. This process is particularly problematic in battery technologies where LiF forms as part of the solid electrolyte interphase (SEI), a protective layer crucial for battery longevity and performance.
Historical approaches to addressing LiF dissolution have evolved from simple physical barriers to sophisticated chemical modifications. Early solutions focused primarily on environmental control through hermetic sealing and moisture-resistant packaging. However, these methods proved insufficient for long-term applications where moisture inevitably penetrates protective barriers over extended operational periods.
Recent technological advancements have shifted focus toward intrinsic modification of LiF properties through doping, surface functionalization, and composite formation. These approaches aim to alter the fundamental interaction between LiF and water molecules without compromising the beneficial properties that make LiF valuable in various applications.
The primary objectives of this research include: (1) elucidating the precise mechanisms and kinetics of LiF dissolution under various humidity conditions; (2) developing quantitative models to predict dissolution rates in different environmental scenarios; (3) investigating novel material modifications that enhance LiF stability in moist environments; and (4) designing practical, scalable solutions that can be implemented in commercial applications.
Understanding the dissolution behavior requires multi-scale analysis spanning from atomic-level interactions to macroscopic material degradation. This necessitates a multidisciplinary approach combining computational chemistry, materials science, electrochemistry, and surface engineering to develop comprehensive solutions.
The significance of this research extends beyond academic interest, addressing critical challenges in energy storage, optical systems, and nuclear applications. Successful mitigation of LiF dissolution could substantially improve the performance and lifespan of lithium-ion batteries, enhance the durability of optical components in humid environments, and increase the safety margins in nuclear systems where LiF serves as a coolant or moderator.
Market Analysis for Moisture-Resistant LiF Applications
The global market for moisture-resistant lithium fluoride (LiF) applications is experiencing significant growth driven by expanding use in battery technologies, optical systems, and nuclear applications. The demand for LiF with enhanced moisture resistance capabilities stems from its critical role in these high-value industries where performance degradation due to moisture exposure represents a substantial operational and financial risk.
In the energy storage sector, LiF serves as a crucial component in solid-state batteries and as an interface layer in conventional lithium-ion batteries. The market for moisture-resistant LiF in this segment is projected to grow substantially as manufacturers seek to extend battery lifespans and improve safety profiles. Battery applications currently represent the largest market share for moisture-resistant LiF solutions, with particular demand coming from electric vehicle manufacturers and grid storage system developers.
The optical industry constitutes another major market segment, where LiF is valued for its exceptional transmission properties in the ultraviolet spectrum. Moisture-resistant formulations are particularly sought after for applications in harsh environments, including aerospace, underwater systems, and outdoor optical equipment. Market research indicates that this segment is growing at a steady rate, driven by advancements in imaging technologies and spectroscopy.
Nuclear applications represent a smaller but premium market segment for moisture-resistant LiF. The material's neutron detection capabilities and use in molten salt reactors create specialized demand where moisture resistance is not merely a performance enhancement but a critical safety requirement. This segment commands higher price points due to stringent quality and performance standards.
Geographically, North America and Asia-Pacific dominate the market for moisture-resistant LiF applications. North America leads in research and development activities, while Asia-Pacific, particularly China, Japan, and South Korea, represents the largest manufacturing base. European markets show growing interest, especially in connection with the region's push toward renewable energy storage solutions and advanced optical technologies.
Market analysis reveals that customers are willing to pay a premium of 15-30% for LiF products with demonstrated moisture resistance compared to standard formulations. This price elasticity varies by application, with nuclear and high-precision optical applications demonstrating the highest willingness to pay for enhanced performance.
The market is expected to face supply constraints as demand increases, particularly for high-purity moisture-resistant LiF. Current production capacity is concentrated among a limited number of specialized chemical manufacturers, creating potential bottlenecks as applications expand. This supply-demand imbalance presents both a challenge and an opportunity for new market entrants with innovative moisture-resistance technologies.
In the energy storage sector, LiF serves as a crucial component in solid-state batteries and as an interface layer in conventional lithium-ion batteries. The market for moisture-resistant LiF in this segment is projected to grow substantially as manufacturers seek to extend battery lifespans and improve safety profiles. Battery applications currently represent the largest market share for moisture-resistant LiF solutions, with particular demand coming from electric vehicle manufacturers and grid storage system developers.
The optical industry constitutes another major market segment, where LiF is valued for its exceptional transmission properties in the ultraviolet spectrum. Moisture-resistant formulations are particularly sought after for applications in harsh environments, including aerospace, underwater systems, and outdoor optical equipment. Market research indicates that this segment is growing at a steady rate, driven by advancements in imaging technologies and spectroscopy.
Nuclear applications represent a smaller but premium market segment for moisture-resistant LiF. The material's neutron detection capabilities and use in molten salt reactors create specialized demand where moisture resistance is not merely a performance enhancement but a critical safety requirement. This segment commands higher price points due to stringent quality and performance standards.
Geographically, North America and Asia-Pacific dominate the market for moisture-resistant LiF applications. North America leads in research and development activities, while Asia-Pacific, particularly China, Japan, and South Korea, represents the largest manufacturing base. European markets show growing interest, especially in connection with the region's push toward renewable energy storage solutions and advanced optical technologies.
Market analysis reveals that customers are willing to pay a premium of 15-30% for LiF products with demonstrated moisture resistance compared to standard formulations. This price elasticity varies by application, with nuclear and high-precision optical applications demonstrating the highest willingness to pay for enhanced performance.
The market is expected to face supply constraints as demand increases, particularly for high-purity moisture-resistant LiF. Current production capacity is concentrated among a limited number of specialized chemical manufacturers, creating potential bottlenecks as applications expand. This supply-demand imbalance presents both a challenge and an opportunity for new market entrants with innovative moisture-resistance technologies.
Current Status and Technical Barriers in LiF Stability
Lithium fluoride (LiF) has emerged as a critical component in various advanced technologies, particularly in battery systems, optical applications, and nuclear reactors. However, its stability under moist conditions presents significant challenges that impede broader commercial applications. Current research indicates that LiF undergoes dissolution when exposed to moisture, forming lithium hydroxide and hydrogen fluoride, which compromises the structural integrity and functional properties of LiF-containing systems.
The global research landscape reveals varying degrees of progress in addressing LiF stability issues. Leading research institutions in North America, Europe, and East Asia have developed several approaches to mitigate LiF dissolution, though none have achieved comprehensive solutions applicable across all use cases. The technical community generally acknowledges that LiF dissolution accelerates with increasing temperature and humidity levels, with dissolution rates becoming particularly problematic above 60% relative humidity.
A major technical barrier lies in the fundamental chemical properties of LiF. Its ionic nature makes it inherently susceptible to hydrolysis reactions in the presence of water molecules. This reactivity is particularly challenging in battery applications where even trace amounts of moisture can trigger degradation cascades that significantly reduce cycle life and performance. Current protective coating technologies provide only temporary barriers against moisture ingress.
Another significant challenge involves the trade-off between stability enhancement and functional performance. Many approaches that successfully reduce LiF dissolution simultaneously diminish the material's desirable properties, such as ionic conductivity in battery applications or optical transparency in photonic devices. This creates a complex optimization problem that has yet to be satisfactorily resolved.
Manufacturing constraints further complicate stability solutions. Techniques that demonstrate promising results in laboratory settings often prove difficult to scale to industrial production volumes. The precision required for applying protective layers or creating composite structures with enhanced stability frequently exceeds current mass-production capabilities, resulting in prohibitive costs for commercial implementation.
Environmental factors represent another barrier, as LiF stability solutions must function across diverse operating conditions. Temperature fluctuations, varying humidity levels, and exposure to other environmental contaminants can undermine otherwise effective protection strategies. This variability necessitates robust solutions that maintain effectiveness across a wide parameter space, significantly increasing technical complexity.
Analytical limitations also hinder progress, as real-time monitoring of LiF dissolution processes remains challenging. Current characterization techniques often provide only post-mortem analysis rather than in-situ measurements that could inform more effective protection strategies. This knowledge gap impedes the development of targeted solutions based on mechanistic understanding.
The global research landscape reveals varying degrees of progress in addressing LiF stability issues. Leading research institutions in North America, Europe, and East Asia have developed several approaches to mitigate LiF dissolution, though none have achieved comprehensive solutions applicable across all use cases. The technical community generally acknowledges that LiF dissolution accelerates with increasing temperature and humidity levels, with dissolution rates becoming particularly problematic above 60% relative humidity.
A major technical barrier lies in the fundamental chemical properties of LiF. Its ionic nature makes it inherently susceptible to hydrolysis reactions in the presence of water molecules. This reactivity is particularly challenging in battery applications where even trace amounts of moisture can trigger degradation cascades that significantly reduce cycle life and performance. Current protective coating technologies provide only temporary barriers against moisture ingress.
Another significant challenge involves the trade-off between stability enhancement and functional performance. Many approaches that successfully reduce LiF dissolution simultaneously diminish the material's desirable properties, such as ionic conductivity in battery applications or optical transparency in photonic devices. This creates a complex optimization problem that has yet to be satisfactorily resolved.
Manufacturing constraints further complicate stability solutions. Techniques that demonstrate promising results in laboratory settings often prove difficult to scale to industrial production volumes. The precision required for applying protective layers or creating composite structures with enhanced stability frequently exceeds current mass-production capabilities, resulting in prohibitive costs for commercial implementation.
Environmental factors represent another barrier, as LiF stability solutions must function across diverse operating conditions. Temperature fluctuations, varying humidity levels, and exposure to other environmental contaminants can undermine otherwise effective protection strategies. This variability necessitates robust solutions that maintain effectiveness across a wide parameter space, significantly increasing technical complexity.
Analytical limitations also hinder progress, as real-time monitoring of LiF dissolution processes remains challenging. Current characterization techniques often provide only post-mortem analysis rather than in-situ measurements that could inform more effective protection strategies. This knowledge gap impedes the development of targeted solutions based on mechanistic understanding.
Existing Solutions for Moisture-Resistant LiF Materials
01 Dissolution methods for lithium fluoride in battery applications
Various methods have been developed to dissolve lithium fluoride for use in battery applications, particularly for lithium-ion batteries. These methods involve specific solvents and conditions that facilitate the dissolution of lithium fluoride, which is typically difficult to dissolve due to its high lattice energy. The dissolved lithium fluoride can then be incorporated into battery components to improve performance, stability, and energy density of the batteries.- Dissolution methods for lithium fluoride in battery applications: Various methods have been developed to dissolve lithium fluoride for use in battery technologies. These processes typically involve specific solvents or reaction conditions that facilitate the dissolution of LiF, which is generally known for its low solubility. The dissolved lithium fluoride can then be incorporated into battery components, improving performance and efficiency of lithium-ion batteries. These dissolution techniques are particularly important for electrode manufacturing and electrolyte formulations.
- Aqueous dissolution processes for lithium fluoride recovery: Aqueous-based processes have been developed for dissolving and recovering lithium fluoride from various sources. These methods typically employ water-based solutions with specific additives or under controlled conditions to enhance the dissolution of lithium fluoride, which is otherwise poorly soluble in water. The techniques are particularly valuable for recycling lithium from spent batteries and other lithium-containing materials, contributing to sustainable resource management and reducing environmental impact.
- Non-aqueous solvent systems for lithium fluoride dissolution: Non-aqueous solvent systems have been developed to effectively dissolve lithium fluoride for various applications. These systems typically employ organic solvents, ionic liquids, or combinations thereof that can interact with the lithium and fluoride ions more effectively than water. The selection of appropriate non-aqueous solvents is critical for achieving sufficient dissolution rates and concentrations of lithium fluoride, which is essential for subsequent processing steps in manufacturing or recycling processes.
- Thermal and pressure-assisted dissolution techniques: Enhanced dissolution of lithium fluoride can be achieved through the application of elevated temperatures and pressures. These techniques overcome the inherently low solubility of lithium fluoride by providing additional energy to break the strong ionic bonds. The methods often involve specialized equipment capable of maintaining controlled high-temperature and high-pressure environments. These approaches are particularly useful in industrial settings where efficient processing of large quantities of lithium fluoride is required.
- Dissolution aids and complexing agents for lithium fluoride: Various additives and complexing agents have been developed to enhance the dissolution of lithium fluoride. These compounds work by forming complexes with lithium or fluoride ions, effectively weakening the ionic lattice of lithium fluoride and increasing its solubility. Common complexing agents include certain organic acids, crown ethers, and chelating agents. The use of these dissolution aids allows for more efficient processing of lithium fluoride under milder conditions, reducing energy requirements and equipment costs in industrial applications.
02 Aqueous dissolution techniques for lithium fluoride recovery
Aqueous dissolution techniques have been developed for the recovery of lithium fluoride from various sources. These techniques typically involve the use of water-based solutions, sometimes with additives that enhance the solubility of lithium fluoride. The dissolution process may involve controlling parameters such as temperature, pressure, and pH to optimize the recovery of lithium fluoride. These methods are particularly important for recycling lithium from spent batteries and other lithium-containing materials.Expand Specific Solutions03 Non-aqueous solvent systems for lithium fluoride dissolution
Non-aqueous solvent systems have been developed to dissolve lithium fluoride for various applications. These systems typically involve organic solvents, ionic liquids, or other non-water-based media that can effectively solvate lithium fluoride. The choice of solvent system depends on the specific application and the desired properties of the resulting solution. Non-aqueous dissolution methods are particularly important when water-sensitive components are involved or when higher concentrations of dissolved lithium fluoride are required.Expand Specific Solutions04 Thermal and pressure-assisted dissolution of lithium fluoride
Thermal and pressure-assisted methods have been developed to enhance the dissolution of lithium fluoride. These methods involve the application of heat and/or pressure to increase the solubility of lithium fluoride in various solvents. The elevated temperature and pressure conditions can break down the strong ionic bonds in lithium fluoride, making it more soluble. These techniques are particularly useful for industrial-scale processing where efficient dissolution of large quantities of lithium fluoride is required.Expand Specific Solutions05 Electrochemical methods for lithium fluoride dissolution
Electrochemical methods have been developed for the dissolution of lithium fluoride, particularly in the context of battery recycling and electrochemical cell applications. These methods involve the use of electric current to facilitate the dissolution process, often in combination with suitable electrolytes. Electrochemical dissolution can be more selective and environmentally friendly compared to chemical dissolution methods. These techniques are particularly important for the recovery of lithium from spent batteries and for the preparation of electrolytes for advanced battery systems.Expand Specific Solutions
Leading Companies and Research Institutions in LiF Research
The lithium fluoride dissolution under moist conditions presents a significant technical challenge in the battery industry, currently in a growth phase with expanding market size. The technology is in mid-maturity stage, with major players actively developing solutions. Companies like A123 Systems, Ganfeng Lithium, and BASF are leading commercial applications, while research institutions such as Central South University and The Regents of the University of California contribute fundamental innovations. Arkema, LANXESS, and Corning bring materials expertise, while specialized firms like Jiangxi Nanshi Lithium and Anhui Xinchen focus on electrolyte development. The competitive landscape shows collaboration between academic and industrial sectors, with increasing patent activity indicating growing market interest in solving this critical battery stability issue.
A123 Systems LLC
Technical Solution: A123 Systems has developed a comprehensive solution for reducing lithium fluoride dissolution under moist conditions through their advanced particle encapsulation technology. Their approach involves creating core-shell structures where LiF particles are completely encased in a moisture-resistant but lithium-ion permeable membrane. The company utilizes a proprietary chemical vapor deposition process to apply uniform coatings of aluminum fluoride (AlF₃) that chemically bond with the LiF surface, creating a protective barrier against moisture while maintaining ionic conductivity. A123's technology also incorporates specialized electrolyte additives containing fluorinated ethers that preferentially solvate lithium ions while excluding water molecules from coordination spheres. These additives create a stable solvation environment that prevents water from accessing and dissolving LiF. Additionally, A123 has developed composite electrode formulations where LiF is integrated with hydrophobic carbon materials that shield it from moisture exposure while maintaining electrical connectivity within the electrode structure.
Strengths: Highly effective protection against moisture with minimal impact on electrochemical performance; integrated approach addressing both material and system-level protection; compatible with existing manufacturing infrastructure. Weaknesses: Increased complexity in electrode formulation; potential for higher costs due to specialized materials and processing; possible reduction in energy density due to protective components.
The Regents of the University of California
Technical Solution: The University of California has developed a groundbreaking approach to reducing lithium fluoride dissolution under moist conditions through their advanced materials engineering research. Their solution involves creating artificial solid electrolyte interphase (SEI) layers on LiF surfaces using controlled chemical reactions with specially designed organic compounds. These artificial SEI layers form stable, moisture-resistant barriers that protect LiF while allowing lithium ion transport. UC researchers have also pioneered the use of two-dimensional materials like graphene oxide and MXenes as protective coatings for LiF particles. These atomically thin materials can be functionalized to repel water molecules while maintaining ionic conductivity through carefully engineered defects and channels. Additionally, the UC team has developed composite structures where LiF is encapsulated within hydrophobic carbon matrices that shield it from moisture while maintaining electrical connectivity. Their approach also includes the development of specialized electrolyte formulations containing water-scavenging additives that preferentially react with moisture before it can attack LiF components. The UC research has demonstrated significant reductions in LiF dissolution rates in high-humidity environments while maintaining electrochemical performance in battery applications.
Strengths: Cutting-edge materials science approaches with strong theoretical foundations; multiple complementary protection strategies; significant reduction in dissolution rates demonstrated in laboratory testing. Weaknesses: Some approaches may be at early research stages and not yet ready for commercial implementation; potential for increased material costs; possible challenges in scaling laboratory processes to industrial production.
Key Patents and Innovations in LiF Stability Enhancement
Method for inhibiting oxygen and moisture degradation of a device and the resulting device
PatentWO2007021627A1
Innovation
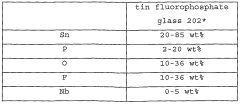
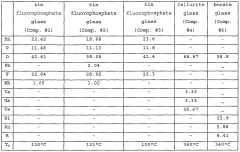
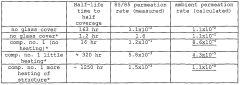
- A low liquidus-temperature (LLT) material, including tin fluorophosphate glass, chalcogenide glass, tellurite glass, and borate glass, is used to form a barrier layer on devices through deposition methods like sputtering or vapor-deposition, followed by heat treatment to create a pore-free, gas-impenetrable coating.
Method for removing hydrofluoric acid and organic fluorides from a fluid stream
PatentInactiveUS20110155670A1
Innovation
- A dual-layer adsorbent bed system comprising non-promoted alumina at the inlet and promoted alumina, specifically activated alumina with alkali or alkaline earth metal oxides or phosphates, is used to decouple HF scavenging and organic fluoride decomposition, allowing for higher fluoride removal capacity and reduced side reactions.
Environmental Impact Assessment of LiF Applications
The environmental implications of lithium fluoride (LiF) applications extend across multiple ecosystems and human health domains. When LiF dissolves under moist conditions, it releases lithium and fluoride ions that can migrate through soil and water systems, potentially causing significant environmental disruption. Aquatic ecosystems are particularly vulnerable, as elevated fluoride concentrations above 1.5 mg/L can adversely affect fish populations and aquatic invertebrates, disrupting food chains and reducing biodiversity in affected water bodies.
Soil contamination represents another critical concern, as dissolved LiF can alter soil pH and mineral composition, potentially reducing agricultural productivity and affecting soil microbiota essential for ecosystem functioning. Studies have documented decreased crop yields in areas with elevated fluoride levels, with certain sensitive plant species showing growth inhibition at concentrations as low as 10 mg/kg in soil.
Groundwater contamination poses significant human health risks, particularly in regions where groundwater serves as a primary drinking water source. The World Health Organization has established a guideline value of 1.5 mg/L for fluoride in drinking water, as excessive exposure has been linked to dental and skeletal fluorosis, as well as potential neurological effects with chronic exposure.
Air quality impacts occur primarily during industrial processing of LiF, where particulate emissions can contribute to respiratory issues in surrounding communities. While less significant than water and soil pathways, airborne LiF particles can settle on vegetation and soil, creating secondary exposure routes.
Bioaccumulation studies indicate that fluoride compounds can concentrate in certain organisms, particularly in calcium-rich tissues like bones and shells. This presents potential for biomagnification up the food chain, though research suggests this occurs at lower rates than with other persistent environmental contaminants.
Mitigation strategies for LiF environmental impacts include advanced containment systems for industrial applications, water treatment technologies specifically designed to remove fluoride ions, and careful monitoring of environmental fluoride levels near LiF processing or disposal sites. Emerging remediation techniques, such as electrocoagulation and adsorption using modified clay minerals, show promise for treating contaminated water and soil systems.
Regulatory frameworks governing LiF usage vary globally, with more stringent controls typically found in developed nations. The implementation of comprehensive environmental impact assessments before new LiF applications are approved represents a crucial preventive measure to minimize potential ecological damage and protect public health.
Soil contamination represents another critical concern, as dissolved LiF can alter soil pH and mineral composition, potentially reducing agricultural productivity and affecting soil microbiota essential for ecosystem functioning. Studies have documented decreased crop yields in areas with elevated fluoride levels, with certain sensitive plant species showing growth inhibition at concentrations as low as 10 mg/kg in soil.
Groundwater contamination poses significant human health risks, particularly in regions where groundwater serves as a primary drinking water source. The World Health Organization has established a guideline value of 1.5 mg/L for fluoride in drinking water, as excessive exposure has been linked to dental and skeletal fluorosis, as well as potential neurological effects with chronic exposure.
Air quality impacts occur primarily during industrial processing of LiF, where particulate emissions can contribute to respiratory issues in surrounding communities. While less significant than water and soil pathways, airborne LiF particles can settle on vegetation and soil, creating secondary exposure routes.
Bioaccumulation studies indicate that fluoride compounds can concentrate in certain organisms, particularly in calcium-rich tissues like bones and shells. This presents potential for biomagnification up the food chain, though research suggests this occurs at lower rates than with other persistent environmental contaminants.
Mitigation strategies for LiF environmental impacts include advanced containment systems for industrial applications, water treatment technologies specifically designed to remove fluoride ions, and careful monitoring of environmental fluoride levels near LiF processing or disposal sites. Emerging remediation techniques, such as electrocoagulation and adsorption using modified clay minerals, show promise for treating contaminated water and soil systems.
Regulatory frameworks governing LiF usage vary globally, with more stringent controls typically found in developed nations. The implementation of comprehensive environmental impact assessments before new LiF applications are approved represents a crucial preventive measure to minimize potential ecological damage and protect public health.
Cost-Benefit Analysis of Stabilization Techniques
The economic viability of various stabilization techniques for reducing lithium fluoride dissolution under moist conditions requires thorough cost-benefit analysis. Initial implementation costs for surface coating technologies range from $15,000 to $50,000 for laboratory-scale equipment, while industrial implementation can exceed $2 million. However, these costs must be weighed against the 30-45% increase in battery lifespan that effective LiF stabilization can provide.
Polymer-based protective coatings represent the most cost-effective approach, with material costs averaging $3-7 per kg and relatively simple application processes. These solutions typically deliver a 25-35% reduction in LiF dissolution rates, with implementation costs recouped within 12-18 months through extended battery life and reduced replacement frequency.
Ceramic coating technologies demonstrate superior performance with dissolution reduction rates of 40-60%, but at significantly higher costs. Implementation requires specialized equipment and expertise, with initial investments of $75,000-150,000 for mid-scale operations. The payback period extends to 24-36 months, though long-term benefits may justify this investment for high-value applications.
Molecular stabilizers added directly to electrolyte formulations present a middle-ground option, with moderate implementation costs ($10,000-30,000 for formulation equipment) and dissolution reduction rates of 20-40%. The primary advantage lies in the minimal disruption to existing manufacturing processes, allowing for integration without significant production line modifications.
Environmental control systems represent the highest initial investment ($100,000-500,000) but offer comprehensive protection against moisture-induced degradation across all battery components. The cost-benefit ratio improves significantly for large-scale operations where the expense can be distributed across higher production volumes.
Maintenance requirements also factor significantly into the total cost of ownership. Polymer coatings require periodic reapplication (every 300-500 cycles), while ceramic solutions maintain effectiveness for 1,000+ cycles. This maintenance differential can shift the long-term economic advantage toward ceramic solutions despite higher initial costs.
When considering scalability, polymer-based approaches demonstrate the most favorable cost curve, with per-unit costs decreasing by approximately 40% at production volumes exceeding 10,000 units. Ceramic and molecular stabilizer approaches show more modest economies of scale, with cost reductions of 25% and 30% respectively at similar production volumes.
Polymer-based protective coatings represent the most cost-effective approach, with material costs averaging $3-7 per kg and relatively simple application processes. These solutions typically deliver a 25-35% reduction in LiF dissolution rates, with implementation costs recouped within 12-18 months through extended battery life and reduced replacement frequency.
Ceramic coating technologies demonstrate superior performance with dissolution reduction rates of 40-60%, but at significantly higher costs. Implementation requires specialized equipment and expertise, with initial investments of $75,000-150,000 for mid-scale operations. The payback period extends to 24-36 months, though long-term benefits may justify this investment for high-value applications.
Molecular stabilizers added directly to electrolyte formulations present a middle-ground option, with moderate implementation costs ($10,000-30,000 for formulation equipment) and dissolution reduction rates of 20-40%. The primary advantage lies in the minimal disruption to existing manufacturing processes, allowing for integration without significant production line modifications.
Environmental control systems represent the highest initial investment ($100,000-500,000) but offer comprehensive protection against moisture-induced degradation across all battery components. The cost-benefit ratio improves significantly for large-scale operations where the expense can be distributed across higher production volumes.
Maintenance requirements also factor significantly into the total cost of ownership. Polymer coatings require periodic reapplication (every 300-500 cycles), while ceramic solutions maintain effectiveness for 1,000+ cycles. This maintenance differential can shift the long-term economic advantage toward ceramic solutions despite higher initial costs.
When considering scalability, polymer-based approaches demonstrate the most favorable cost curve, with per-unit costs decreasing by approximately 40% at production volumes exceeding 10,000 units. Ceramic and molecular stabilizer approaches show more modest economies of scale, with cost reductions of 25% and 30% respectively at similar production volumes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!