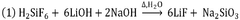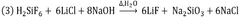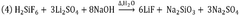How to Measure Lithium Fluoride Purity for Battery Applications
SEP 12, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
LiF Purity Analysis Background and Objectives
Lithium fluoride (LiF) has emerged as a critical component in advanced battery technologies, particularly in solid-state batteries where it serves as an essential constituent of solid electrolytes and protective interface layers. The purity of LiF significantly impacts battery performance metrics including capacity, cycling stability, and overall lifespan. This technical investigation aims to comprehensively examine methodologies for accurately measuring and characterizing LiF purity specifically for battery applications.
The evolution of battery technology has witnessed a transition from traditional liquid electrolytes to solid-state configurations, with LiF playing an increasingly prominent role. Historically, LiF has been utilized in various industrial applications, but its specific requirements for battery-grade purity have only recently become a focal point of research. The technical trajectory indicates a growing emphasis on ultra-high purity materials as battery performance demands intensify.
Current industry standards typically specify 99.9% purity (3N) for battery-grade LiF, yet emerging research suggests that even trace impurities at parts-per-million levels can significantly compromise electrochemical performance. Common contaminants include metallic ions (Na+, K+, Ca2+), moisture, and oxygen-containing compounds that can trigger undesired side reactions within battery systems. These impurities can accelerate capacity fading, increase internal resistance, and compromise the integrity of solid-electrolyte interfaces.
This technical assessment aims to establish comprehensive protocols for LiF purity analysis that address the specific requirements of next-generation battery technologies. Primary objectives include: developing standardized methodologies for quantifying trace impurities in LiF with detection limits below 10 ppm; establishing correlations between specific impurity profiles and battery performance metrics; and creating industry-relevant benchmarks for battery-grade LiF specifications.
The investigation will further explore how various synthesis routes affect the impurity profile of LiF and evaluate the cost-effectiveness of different purification techniques. Additionally, we seek to determine whether current analytical technologies provide sufficient sensitivity and specificity for battery-grade material qualification, or if new methodological approaches are required.
By establishing robust analytical frameworks for LiF purity assessment, this research aims to support quality control processes in battery manufacturing, facilitate material selection decisions, and ultimately contribute to the advancement of high-performance energy storage technologies. The findings will provide valuable insights for materials scientists, battery engineers, and quality assurance professionals working at the intersection of materials chemistry and energy storage applications.
The evolution of battery technology has witnessed a transition from traditional liquid electrolytes to solid-state configurations, with LiF playing an increasingly prominent role. Historically, LiF has been utilized in various industrial applications, but its specific requirements for battery-grade purity have only recently become a focal point of research. The technical trajectory indicates a growing emphasis on ultra-high purity materials as battery performance demands intensify.
Current industry standards typically specify 99.9% purity (3N) for battery-grade LiF, yet emerging research suggests that even trace impurities at parts-per-million levels can significantly compromise electrochemical performance. Common contaminants include metallic ions (Na+, K+, Ca2+), moisture, and oxygen-containing compounds that can trigger undesired side reactions within battery systems. These impurities can accelerate capacity fading, increase internal resistance, and compromise the integrity of solid-electrolyte interfaces.
This technical assessment aims to establish comprehensive protocols for LiF purity analysis that address the specific requirements of next-generation battery technologies. Primary objectives include: developing standardized methodologies for quantifying trace impurities in LiF with detection limits below 10 ppm; establishing correlations between specific impurity profiles and battery performance metrics; and creating industry-relevant benchmarks for battery-grade LiF specifications.
The investigation will further explore how various synthesis routes affect the impurity profile of LiF and evaluate the cost-effectiveness of different purification techniques. Additionally, we seek to determine whether current analytical technologies provide sufficient sensitivity and specificity for battery-grade material qualification, or if new methodological approaches are required.
By establishing robust analytical frameworks for LiF purity assessment, this research aims to support quality control processes in battery manufacturing, facilitate material selection decisions, and ultimately contribute to the advancement of high-performance energy storage technologies. The findings will provide valuable insights for materials scientists, battery engineers, and quality assurance professionals working at the intersection of materials chemistry and energy storage applications.
Market Demand for High-Purity LiF in Battery Industry
The global market for high-purity lithium fluoride (LiF) in battery applications has witnessed substantial growth in recent years, primarily driven by the expanding electric vehicle (EV) sector and increasing adoption of energy storage systems. As lithium-ion batteries continue to dominate the energy storage landscape, the demand for high-quality battery materials, including LiF, has intensified significantly.
Battery manufacturers are increasingly recognizing the critical role of LiF purity in determining battery performance metrics. High-purity LiF contributes to enhanced battery cycle life, improved energy density, and better thermal stability—all crucial factors for next-generation battery technologies. Market research indicates that batteries utilizing high-purity LiF components demonstrate up to 30% longer operational lifespans compared to those with standard-grade materials.
The automotive sector represents the largest consumer segment for high-purity LiF, accounting for approximately two-thirds of the total market demand. This trend correlates directly with the accelerating global transition toward electric mobility, with major automotive manufacturers committing to ambitious electrification targets over the next decade.
Consumer electronics constitutes the second-largest application segment, where the need for longer-lasting, faster-charging batteries drives demand for premium battery components. Grid-scale energy storage systems are emerging as another significant market, especially in regions investing heavily in renewable energy infrastructure.
Geographically, Asia-Pacific dominates the market consumption, with China, Japan, and South Korea collectively representing over 70% of global demand. This regional concentration aligns with the established battery manufacturing ecosystem in these countries. However, North America and Europe are experiencing the fastest growth rates as they develop domestic battery supply chains to reduce dependence on Asian imports.
Price sensitivity varies significantly across application segments. While consumer electronics manufacturers often prioritize cost efficiency, automotive and industrial applications demonstrate greater willingness to pay premium prices for high-purity LiF that delivers demonstrable performance advantages.
Market forecasts project the global high-purity LiF market for battery applications to grow at a compound annual growth rate exceeding 15% through 2030. This growth trajectory is supported by ongoing research into solid-state batteries and other advanced energy storage technologies, where ultra-high-purity LiF plays an even more critical role in electrolyte formulations.
Supply chain considerations are increasingly influencing market dynamics, with battery manufacturers seeking reliable sources of high-purity LiF amid growing concerns about raw material availability and geopolitical factors affecting lithium supply.
Battery manufacturers are increasingly recognizing the critical role of LiF purity in determining battery performance metrics. High-purity LiF contributes to enhanced battery cycle life, improved energy density, and better thermal stability—all crucial factors for next-generation battery technologies. Market research indicates that batteries utilizing high-purity LiF components demonstrate up to 30% longer operational lifespans compared to those with standard-grade materials.
The automotive sector represents the largest consumer segment for high-purity LiF, accounting for approximately two-thirds of the total market demand. This trend correlates directly with the accelerating global transition toward electric mobility, with major automotive manufacturers committing to ambitious electrification targets over the next decade.
Consumer electronics constitutes the second-largest application segment, where the need for longer-lasting, faster-charging batteries drives demand for premium battery components. Grid-scale energy storage systems are emerging as another significant market, especially in regions investing heavily in renewable energy infrastructure.
Geographically, Asia-Pacific dominates the market consumption, with China, Japan, and South Korea collectively representing over 70% of global demand. This regional concentration aligns with the established battery manufacturing ecosystem in these countries. However, North America and Europe are experiencing the fastest growth rates as they develop domestic battery supply chains to reduce dependence on Asian imports.
Price sensitivity varies significantly across application segments. While consumer electronics manufacturers often prioritize cost efficiency, automotive and industrial applications demonstrate greater willingness to pay premium prices for high-purity LiF that delivers demonstrable performance advantages.
Market forecasts project the global high-purity LiF market for battery applications to grow at a compound annual growth rate exceeding 15% through 2030. This growth trajectory is supported by ongoing research into solid-state batteries and other advanced energy storage technologies, where ultra-high-purity LiF plays an even more critical role in electrolyte formulations.
Supply chain considerations are increasingly influencing market dynamics, with battery manufacturers seeking reliable sources of high-purity LiF amid growing concerns about raw material availability and geopolitical factors affecting lithium supply.
Current Analytical Challenges in LiF Purity Assessment
The accurate determination of lithium fluoride (LiF) purity presents significant analytical challenges due to its unique chemical properties and the stringent requirements for battery applications. Traditional analytical methods often struggle with the detection of trace impurities in LiF, particularly those below parts-per-million (ppm) levels that can critically impact battery performance. Conventional techniques such as X-ray diffraction (XRD) provide crystallographic information but lack sensitivity for detecting amorphous impurities or those present in minute quantities.
Inductively Coupled Plasma Mass Spectrometry (ICP-MS), while highly sensitive for many elements, faces challenges with fluorine detection due to its high ionization potential. Additionally, sample preparation for LiF analysis is particularly problematic as the material is hygroscopic and can react with moisture during handling, potentially altering the impurity profile before measurement is complete.
Spectroscopic methods like Fourier Transform Infrared Spectroscopy (FTIR) and Raman spectroscopy offer non-destructive analysis but suffer from limited detection limits for certain critical impurities. The presence of transition metal contaminants, particularly iron and copper at sub-ppm levels, can significantly impact battery performance, yet these remain difficult to quantify consistently across different analytical platforms.
Another significant challenge lies in the lack of standardized protocols specifically designed for LiF purity assessment in battery applications. The battery industry has not established consensus on acceptable impurity thresholds, leading to inconsistent quality control practices across manufacturers. This absence of standardization complicates comparative analyses between different LiF sources and batches.
Surface analysis techniques such as X-ray Photoelectron Spectroscopy (XPS) and Secondary Ion Mass Spectrometry (SIMS) can provide valuable information about surface contaminants but are limited in their ability to characterize bulk properties. This creates a disconnect between surface and bulk analysis results, complicating the comprehensive assessment of LiF purity.
The detection of organic impurities presents another analytical hurdle, as these compounds can significantly impact electrolyte stability in batteries. Current methods often require multiple analytical techniques to be employed in parallel, increasing analysis time and cost while introducing potential for inconsistencies between different measurement approaches.
Furthermore, the distinction between harmful and benign impurities remains poorly understood, with limited research correlating specific impurity profiles to battery performance metrics. This knowledge gap hampers the development of targeted analytical methods focused on performance-critical contaminants rather than comprehensive purity assessment.
Inductively Coupled Plasma Mass Spectrometry (ICP-MS), while highly sensitive for many elements, faces challenges with fluorine detection due to its high ionization potential. Additionally, sample preparation for LiF analysis is particularly problematic as the material is hygroscopic and can react with moisture during handling, potentially altering the impurity profile before measurement is complete.
Spectroscopic methods like Fourier Transform Infrared Spectroscopy (FTIR) and Raman spectroscopy offer non-destructive analysis but suffer from limited detection limits for certain critical impurities. The presence of transition metal contaminants, particularly iron and copper at sub-ppm levels, can significantly impact battery performance, yet these remain difficult to quantify consistently across different analytical platforms.
Another significant challenge lies in the lack of standardized protocols specifically designed for LiF purity assessment in battery applications. The battery industry has not established consensus on acceptable impurity thresholds, leading to inconsistent quality control practices across manufacturers. This absence of standardization complicates comparative analyses between different LiF sources and batches.
Surface analysis techniques such as X-ray Photoelectron Spectroscopy (XPS) and Secondary Ion Mass Spectrometry (SIMS) can provide valuable information about surface contaminants but are limited in their ability to characterize bulk properties. This creates a disconnect between surface and bulk analysis results, complicating the comprehensive assessment of LiF purity.
The detection of organic impurities presents another analytical hurdle, as these compounds can significantly impact electrolyte stability in batteries. Current methods often require multiple analytical techniques to be employed in parallel, increasing analysis time and cost while introducing potential for inconsistencies between different measurement approaches.
Furthermore, the distinction between harmful and benign impurities remains poorly understood, with limited research correlating specific impurity profiles to battery performance metrics. This knowledge gap hampers the development of targeted analytical methods focused on performance-critical contaminants rather than comprehensive purity assessment.
Established Methods for LiF Purity Determination
01 Purification methods for lithium fluoride
Various methods are employed to purify lithium fluoride to achieve high purity levels. These include recrystallization, precipitation, and filtration techniques. Advanced purification processes can remove impurities such as metal ions, hydroxides, and other contaminants that affect the quality of lithium fluoride. These methods are crucial for applications requiring ultra-high purity lithium fluoride.- Purification methods for lithium fluoride: Various methods are employed to purify lithium fluoride to achieve high purity levels. These include recrystallization techniques, chemical precipitation, and solvent extraction processes. Advanced purification methods can remove impurities such as metal ions, hydroxides, and other contaminants that affect the quality of lithium fluoride. These purification processes are critical for applications requiring ultra-high purity materials.
- High-purity lithium fluoride for optical applications: High-purity lithium fluoride is essential for optical applications due to its excellent transmission properties in the ultraviolet to infrared spectrum. The material requires extremely low levels of impurities to prevent scattering and absorption effects. Purification techniques focus on removing transition metal ions and other contaminants that can cause color centers or reduce transparency. These high-purity materials are used in specialized windows, lenses, and prisms for scientific instruments.
- Lithium fluoride purity standards for battery applications: Battery-grade lithium fluoride requires specific purity standards to ensure optimal performance in lithium-ion and other advanced battery systems. Impurities can significantly impact electrochemical performance, cycle life, and safety. Purification processes focus on removing moisture, metal contaminants, and other ionic species that could interfere with battery chemistry. Standardized testing methods are employed to verify purity levels suitable for battery applications.
- Equipment and systems for lithium fluoride purity analysis: Specialized equipment and analytical systems have been developed to measure and verify the purity of lithium fluoride. These include spectroscopic methods, chromatography techniques, and elemental analysis tools that can detect impurities at parts-per-million or even parts-per-billion levels. Advanced monitoring systems allow for real-time purity assessment during production processes, ensuring consistent quality control of high-purity lithium fluoride.
- Industrial production of high-purity lithium fluoride: Industrial-scale production of high-purity lithium fluoride involves specialized manufacturing processes designed to minimize contamination. These processes include controlled reaction environments, advanced filtration systems, and multi-stage purification techniques. Quality control measures throughout the production chain ensure consistent purity levels. The manufacturing methods balance efficiency with purity requirements to meet the demands of various high-tech applications.
02 High-purity lithium fluoride for optical applications
High-purity lithium fluoride is essential for optical applications such as lenses, windows, and prisms. The material must have minimal impurities to ensure optimal transparency and performance in the ultraviolet to infrared spectrum. Purification techniques specifically designed for optical-grade lithium fluoride focus on eliminating trace elements that can cause absorption bands or scattering centers, affecting optical transmission properties.Expand Specific Solutions03 Lithium fluoride purity standards for battery applications
In battery applications, especially for lithium-ion batteries, the purity of lithium fluoride significantly impacts performance and safety. Standards for battery-grade lithium fluoride typically require purity levels above 99.9%, with strict limits on moisture content, metal impurities, and particle size distribution. Purification processes for battery applications focus on removing contaminants that could interfere with electrochemical reactions or reduce battery life.Expand Specific Solutions04 Analysis and testing methods for lithium fluoride purity
Various analytical techniques are used to determine the purity of lithium fluoride, including inductively coupled plasma mass spectrometry (ICP-MS), X-ray fluorescence (XRF), and atomic absorption spectroscopy. These methods can detect impurities at parts-per-million or even parts-per-billion levels. Quality control procedures involve multiple testing stages to ensure consistent purity levels and identify specific contaminants that might affect the performance in target applications.Expand Specific Solutions05 Equipment and systems for lithium fluoride purification
Specialized equipment and systems have been developed for the production and purification of high-purity lithium fluoride. These include custom reactors, filtration systems, and crystallization equipment designed to minimize contamination during processing. Advanced purification systems often incorporate multiple stages of purification, controlled atmosphere processing, and automated monitoring to maintain consistent quality and purity levels throughout the production process.Expand Specific Solutions
Key Industry Players in Battery-Grade LiF Production
The lithium fluoride purity measurement market for battery applications is in a growth phase, driven by increasing demand for high-performance lithium-ion batteries. The market is expanding rapidly with the global push toward electric vehicles and energy storage solutions, as evidenced by investments from major players like LG Energy Solution, Ganfeng Lithium, and Renault. Technical maturity varies across measurement methodologies, with companies like Soulbrain, Alkeemia, and CHUNBO leading in advanced analytical techniques for high-purity LiF detection. Research institutions such as Central South University and industrial manufacturers like AGC and Resonac are collaborating to develop standardized purity assessment protocols. The competitive landscape features specialized chemical suppliers (Morita New Energy Materials) working alongside battery manufacturers (Toyota, LG Chem) to establish quality control benchmarks for this critical battery material.
Hefei Guoxuan High-Tech Power Energy Co., Ltd.
Technical Solution: Hefei Guoxuan has developed an integrated analytical approach for LiF purity assessment focused on battery applications. Their method centers on a combination of X-ray fluorescence (XRF) spectroscopy for rapid screening and ICP-MS for detailed impurity profiling. The company has created a specialized sample preparation protocol that minimizes environmental contamination during analysis. Their approach includes a unique dissolution method that ensures complete sample homogeneity while preventing the formation of insoluble fluoride complexes that can interfere with accurate measurement. Guoxuan has established a database of impurity profiles correlated with battery performance metrics, allowing for predictive quality assessment. Their system incorporates machine learning algorithms that analyze spectral data to identify subtle patterns associated with specific manufacturing defects or contamination sources[4]. The company has also developed portable XRF systems for in-line quality control during LiF production, enabling real-time purity monitoring without disrupting manufacturing processes. Their research has demonstrated that certain trace impurities, particularly transition metals, have disproportionate effects on battery performance even at sub-ppm levels.
Strengths: Combination of rapid screening (XRF) and detailed analysis (ICP-MS); machine learning integration for pattern recognition; portable systems for in-line monitoring; established correlation database between impurity profiles and battery performance. Weaknesses: XRF has limited sensitivity for light elements; requires careful calibration and matrix matching; potential for spectral interferences in complex samples; significant initial investment in analytical infrastructure.
LG Energy Solution Ltd.
Technical Solution: LG Energy Solution has developed a comprehensive lithium fluoride purity measurement system specifically for battery applications. Their approach combines multiple analytical techniques including X-ray Diffraction (XRD), Inductively Coupled Plasma Mass Spectrometry (ICP-MS), and Ion Chromatography (IC). The company employs a proprietary multi-stage verification process that first identifies crystalline structure through XRD to confirm LiF presence, then utilizes ICP-MS for detecting metallic impurities down to ppb levels. Their method includes specialized sample preparation techniques to minimize contamination during handling. LG Energy Solution has also developed an automated quality control system that continuously monitors LiF purity throughout the production process, with real-time data analytics to identify potential quality issues before they affect battery performance[1]. Their research has established correlations between specific impurity profiles and battery degradation patterns, allowing for more targeted quality control measures.
Strengths: Highly sensitive detection capabilities down to ppb levels; integrated quality control system with real-time monitoring; established correlation between impurity profiles and battery performance. Weaknesses: Complex multi-technique approach requires significant technical expertise and expensive equipment; sample preparation protocols can be time-consuming and labor-intensive.
Critical Technologies for Trace Impurity Detection in LiF
Process for the production of battery grade lithium fluoride
PatentWO2024261004A1
Innovation
- A process utilizing fluorosilicic acid (H2SiF6) as an alternative fluorine source, which is a by-product from HF manufacturing and fertilizer production, to produce highly pure LiF by reacting it with a lithium source in a controlled pH environment, avoiding the need for HF and enabling recycling, thus ensuring high purity and safety while allowing for global plant development.
Process for the production of battery grade lithium fluoride
PatentActiveEP4480919A1
Innovation
- A process using fluorosilicic acid (H2SiF6) as an alternative source of fluorine, which is a by-product from HF manufacturing and fertilizers, to produce high-purity LiF without HF, involving a reaction with a lithium source and a base at controlled pH and temperature, allowing for recycling and reducing environmental impact.
Environmental Impact of LiF Production Processes
The production of Lithium Fluoride (LiF) for battery applications involves several processes that can have significant environmental implications. Traditional LiF manufacturing methods typically rely on the reaction between lithium carbonate and hydrofluoric acid, a process that generates carbon dioxide emissions and requires careful handling of highly corrosive materials. These production pathways contribute to greenhouse gas emissions and pose potential risks of environmental contamination if not properly managed.
Water consumption represents another critical environmental concern in LiF production. The purification processes often require substantial amounts of water, particularly in regions where lithium mining operations are concentrated, such as the lithium triangle in South America. This water usage can lead to depletion of local water resources and disruption of fragile ecosystems, especially in arid regions where water scarcity is already a pressing issue.
Waste management challenges also emerge throughout the LiF production lifecycle. The generation of fluoride-containing wastewater requires specialized treatment facilities to prevent contamination of groundwater and surface water bodies. Additionally, solid waste byproducts may contain heavy metals and other contaminants that necessitate proper disposal protocols to minimize environmental harm.
Energy intensity represents another significant environmental factor in LiF production. The high-temperature processes required for synthesis and purification demand substantial energy inputs, often derived from fossil fuel sources in many manufacturing regions. This energy consumption pattern contributes to the overall carbon footprint of battery materials production.
Recent advancements in green chemistry approaches have begun addressing these environmental challenges. Closed-loop production systems that recycle process water and recover chemical reagents are being implemented by leading manufacturers. Additionally, alternative synthesis routes using less hazardous precursors are under development, potentially reducing the environmental impact of LiF production.
Life cycle assessment (LCA) studies indicate that the environmental footprint of LiF varies significantly depending on production methods and energy sources. Manufacturers utilizing renewable energy for production processes can achieve substantially lower carbon emissions compared to those relying on coal-powered electricity. This variability highlights the importance of considering production methods when evaluating the environmental sustainability of LiF for battery applications.
Regulatory frameworks governing LiF production are evolving globally, with increasing emphasis on environmental protection measures. Companies pursuing more sustainable production methods may gain competitive advantages as battery manufacturers increasingly prioritize environmentally responsible supply chains in response to consumer and regulatory pressures.
Water consumption represents another critical environmental concern in LiF production. The purification processes often require substantial amounts of water, particularly in regions where lithium mining operations are concentrated, such as the lithium triangle in South America. This water usage can lead to depletion of local water resources and disruption of fragile ecosystems, especially in arid regions where water scarcity is already a pressing issue.
Waste management challenges also emerge throughout the LiF production lifecycle. The generation of fluoride-containing wastewater requires specialized treatment facilities to prevent contamination of groundwater and surface water bodies. Additionally, solid waste byproducts may contain heavy metals and other contaminants that necessitate proper disposal protocols to minimize environmental harm.
Energy intensity represents another significant environmental factor in LiF production. The high-temperature processes required for synthesis and purification demand substantial energy inputs, often derived from fossil fuel sources in many manufacturing regions. This energy consumption pattern contributes to the overall carbon footprint of battery materials production.
Recent advancements in green chemistry approaches have begun addressing these environmental challenges. Closed-loop production systems that recycle process water and recover chemical reagents are being implemented by leading manufacturers. Additionally, alternative synthesis routes using less hazardous precursors are under development, potentially reducing the environmental impact of LiF production.
Life cycle assessment (LCA) studies indicate that the environmental footprint of LiF varies significantly depending on production methods and energy sources. Manufacturers utilizing renewable energy for production processes can achieve substantially lower carbon emissions compared to those relying on coal-powered electricity. This variability highlights the importance of considering production methods when evaluating the environmental sustainability of LiF for battery applications.
Regulatory frameworks governing LiF production are evolving globally, with increasing emphasis on environmental protection measures. Companies pursuing more sustainable production methods may gain competitive advantages as battery manufacturers increasingly prioritize environmentally responsible supply chains in response to consumer and regulatory pressures.
Standardization and Certification Requirements for Battery-Grade LiF
The standardization and certification landscape for battery-grade lithium fluoride (LiF) remains fragmented globally, with varying requirements across different regions and applications. Currently, the most widely recognized standards come from organizations such as ASTM International, the International Organization for Standardization (ISO), and the International Electrotechnical Commission (IEC), which have established baseline purity requirements for battery materials.
For battery applications specifically, LiF must typically meet a minimum purity of 99.9%, with strict limitations on metallic impurities such as sodium, potassium, and heavy metals that can significantly impact battery performance and safety. The certification process generally requires comprehensive analytical testing using validated methods such as ICP-MS, XRD, and titration techniques to verify compliance with these specifications.
Manufacturers seeking certification must navigate a complex ecosystem of requirements. In North America, UL (Underwriters Laboratories) certification is often necessary, while in Europe, compliance with REACH regulations and CE marking presents additional hurdles. Asian markets, particularly Japan and South Korea, have established their own rigorous certification frameworks through organizations like JIS (Japanese Industrial Standards) and KS (Korean Standards).
The certification process typically involves multiple stages: initial material qualification, production process validation, and ongoing quality assurance testing. Third-party testing laboratories accredited by organizations such as A2LA (American Association for Laboratory Accreditation) or ILAC (International Laboratory Accreditation Cooperation) are generally required to verify compliance, adding credibility to certification claims.
Recent developments in battery technology have prompted calls for more specialized standards specifically addressing LiF used in solid-state electrolytes and cathode coatings. Industry consortia such as the Battery Materials Standardization Working Group are currently developing more targeted specifications that address particle size distribution, surface area, and moisture content—parameters particularly critical for next-generation battery applications.
Emerging certification requirements are increasingly focusing on sustainability aspects as well. The Initiative for Responsible Mining Assurance (IRMA) and similar programs are beginning to incorporate environmental and ethical sourcing criteria into material certification frameworks, reflecting growing consumer and regulatory pressure for sustainable battery supply chains.
For manufacturers and researchers, navigating this complex standardization landscape requires dedicated resources and expertise. Many organizations are establishing specialized compliance teams to manage the certification process, which can take 6-18 months depending on the target markets and specific applications.
For battery applications specifically, LiF must typically meet a minimum purity of 99.9%, with strict limitations on metallic impurities such as sodium, potassium, and heavy metals that can significantly impact battery performance and safety. The certification process generally requires comprehensive analytical testing using validated methods such as ICP-MS, XRD, and titration techniques to verify compliance with these specifications.
Manufacturers seeking certification must navigate a complex ecosystem of requirements. In North America, UL (Underwriters Laboratories) certification is often necessary, while in Europe, compliance with REACH regulations and CE marking presents additional hurdles. Asian markets, particularly Japan and South Korea, have established their own rigorous certification frameworks through organizations like JIS (Japanese Industrial Standards) and KS (Korean Standards).
The certification process typically involves multiple stages: initial material qualification, production process validation, and ongoing quality assurance testing. Third-party testing laboratories accredited by organizations such as A2LA (American Association for Laboratory Accreditation) or ILAC (International Laboratory Accreditation Cooperation) are generally required to verify compliance, adding credibility to certification claims.
Recent developments in battery technology have prompted calls for more specialized standards specifically addressing LiF used in solid-state electrolytes and cathode coatings. Industry consortia such as the Battery Materials Standardization Working Group are currently developing more targeted specifications that address particle size distribution, surface area, and moisture content—parameters particularly critical for next-generation battery applications.
Emerging certification requirements are increasingly focusing on sustainability aspects as well. The Initiative for Responsible Mining Assurance (IRMA) and similar programs are beginning to incorporate environmental and ethical sourcing criteria into material certification frameworks, reflecting growing consumer and regulatory pressure for sustainable battery supply chains.
For manufacturers and researchers, navigating this complex standardization landscape requires dedicated resources and expertise. Many organizations are establishing specialized compliance teams to manage the certification process, which can take 6-18 months depending on the target markets and specific applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!