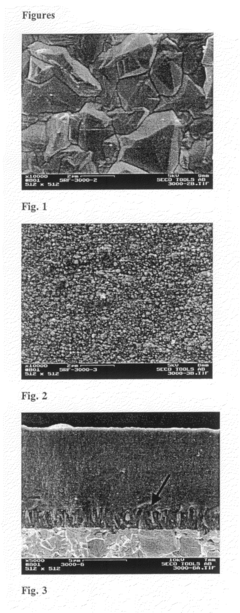
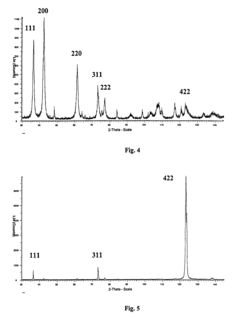
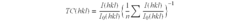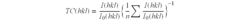Optimize Lithium Fluoride Grain Size for Enhanced Conductivity
SEP 12, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
LiF Grain Size Optimization Background and Objectives
Lithium fluoride (LiF) has emerged as a critical material in advanced energy storage systems, particularly in solid-state batteries where its grain size characteristics directly impact ionic conductivity performance. The optimization of LiF grain size represents a frontier challenge in materials science that has evolved significantly over the past decade. Initially considered merely as a passive component in battery systems, research has progressively revealed LiF's potential as an active contributor to enhanced ionic conductivity when properly engineered at the microstructural level.
The evolution of LiF grain size optimization techniques has followed the broader trajectory of nanomaterials research, transitioning from macro-scale to nano-scale engineering. Early approaches in the 2010s focused primarily on mechanical processing methods to reduce grain size, while more recent developments have incorporated sophisticated chemical synthesis routes and advanced characterization techniques that enable precise control over grain morphology and boundary characteristics.
Current technological trends indicate a convergence toward multi-scale optimization strategies that consider not only the absolute dimensions of LiF grains but also their distribution patterns, interfacial properties, and integration within composite structures. This holistic approach reflects the growing understanding that conductivity enhancement depends on complex interactions between grain boundaries, crystallographic orientation, and defect chemistry.
The primary objective of LiF grain size optimization research is to achieve breakthrough improvements in ionic conductivity that can enable next-generation solid-state batteries with superior energy density, safety, and cycle life compared to conventional lithium-ion technologies. Specifically, researchers aim to develop reproducible methods for synthesizing LiF with grain sizes in the nanometer range (typically 20-100 nm) while maintaining structural stability under operational conditions.
Secondary objectives include understanding the fundamental mechanisms governing ion transport across grain boundaries, developing in-situ characterization techniques for monitoring grain evolution during battery operation, and establishing predictive models that can accelerate the design of optimized LiF microstructures. These objectives align with broader industry goals to commercialize solid-state battery technologies within the next 3-5 years.
The technical challenges associated with LiF grain size optimization are compounded by the material's high sensitivity to environmental conditions, particularly moisture, which necessitates specialized handling protocols and processing environments. Additionally, there exists a critical need to balance conductivity enhancement with mechanical stability, as ultra-fine grain structures may compromise the structural integrity required for long-term battery operation.
As research progresses, the field is increasingly focused on establishing quantitative relationships between grain size parameters and conductivity metrics, moving beyond empirical approaches toward physics-based design principles that can guide systematic optimization efforts.
The evolution of LiF grain size optimization techniques has followed the broader trajectory of nanomaterials research, transitioning from macro-scale to nano-scale engineering. Early approaches in the 2010s focused primarily on mechanical processing methods to reduce grain size, while more recent developments have incorporated sophisticated chemical synthesis routes and advanced characterization techniques that enable precise control over grain morphology and boundary characteristics.
Current technological trends indicate a convergence toward multi-scale optimization strategies that consider not only the absolute dimensions of LiF grains but also their distribution patterns, interfacial properties, and integration within composite structures. This holistic approach reflects the growing understanding that conductivity enhancement depends on complex interactions between grain boundaries, crystallographic orientation, and defect chemistry.
The primary objective of LiF grain size optimization research is to achieve breakthrough improvements in ionic conductivity that can enable next-generation solid-state batteries with superior energy density, safety, and cycle life compared to conventional lithium-ion technologies. Specifically, researchers aim to develop reproducible methods for synthesizing LiF with grain sizes in the nanometer range (typically 20-100 nm) while maintaining structural stability under operational conditions.
Secondary objectives include understanding the fundamental mechanisms governing ion transport across grain boundaries, developing in-situ characterization techniques for monitoring grain evolution during battery operation, and establishing predictive models that can accelerate the design of optimized LiF microstructures. These objectives align with broader industry goals to commercialize solid-state battery technologies within the next 3-5 years.
The technical challenges associated with LiF grain size optimization are compounded by the material's high sensitivity to environmental conditions, particularly moisture, which necessitates specialized handling protocols and processing environments. Additionally, there exists a critical need to balance conductivity enhancement with mechanical stability, as ultra-fine grain structures may compromise the structural integrity required for long-term battery operation.
As research progresses, the field is increasingly focused on establishing quantitative relationships between grain size parameters and conductivity metrics, moving beyond empirical approaches toward physics-based design principles that can guide systematic optimization efforts.
Market Analysis for High-Conductivity LiF Applications
The global market for high-conductivity lithium fluoride (LiF) applications is experiencing significant growth, driven primarily by advancements in energy storage technologies and the expanding electric vehicle (EV) sector. The optimization of LiF grain size represents a critical technological advancement that directly impacts conductivity performance in various applications, creating substantial market opportunities.
The solid-state battery market, where high-conductivity LiF serves as a crucial component, is projected to reach $8.7 billion by 2027, growing at a compound annual growth rate of 34.2% from 2022. This remarkable growth trajectory is fueled by increasing demand for safer, higher-energy-density batteries in consumer electronics, electric vehicles, and grid storage systems.
Within the EV sector specifically, the demand for advanced battery materials featuring optimized LiF is particularly strong. As automotive manufacturers commit to electrification strategies, the market for high-performance battery components is expanding rapidly. Industry forecasts indicate that by 2030, EVs will represent approximately 28% of global new car sales, creating sustained demand for advanced LiF-based materials.
The semiconductor industry represents another significant market for high-conductivity LiF applications. With optimized grain size, LiF can enhance performance in various semiconductor manufacturing processes and components. This market segment is expected to grow steadily as the global semiconductor industry continues its expansion, particularly in advanced computing, artificial intelligence, and IoT applications.
Regional analysis reveals that Asia-Pacific currently dominates the market for high-conductivity LiF applications, accounting for approximately 45% of global demand. This concentration is attributed to the region's robust electronics manufacturing ecosystem and rapidly expanding EV production capacity. North America and Europe follow with significant market shares, driven by strong research initiatives and growing adoption of renewable energy technologies.
Market segmentation by application shows that battery technologies represent the largest application segment (38%), followed by optical applications (27%), semiconductor manufacturing (21%), and other specialized applications (14%). The battery segment is expected to maintain the highest growth rate over the next five years.
Customer demand patterns indicate increasing preference for materials that enable higher energy density, faster charging capabilities, and enhanced safety profiles. This trend aligns perfectly with the benefits offered by optimized LiF grain size technologies, suggesting strong market receptivity for innovations in this space.
Pricing analysis reveals that while high-conductivity LiF commands premium pricing compared to standard LiF materials, the performance benefits often justify the additional cost for manufacturers of high-end applications. As production scales and manufacturing processes improve, gradual price reductions are anticipated, potentially expanding market penetration across more cost-sensitive applications.
The solid-state battery market, where high-conductivity LiF serves as a crucial component, is projected to reach $8.7 billion by 2027, growing at a compound annual growth rate of 34.2% from 2022. This remarkable growth trajectory is fueled by increasing demand for safer, higher-energy-density batteries in consumer electronics, electric vehicles, and grid storage systems.
Within the EV sector specifically, the demand for advanced battery materials featuring optimized LiF is particularly strong. As automotive manufacturers commit to electrification strategies, the market for high-performance battery components is expanding rapidly. Industry forecasts indicate that by 2030, EVs will represent approximately 28% of global new car sales, creating sustained demand for advanced LiF-based materials.
The semiconductor industry represents another significant market for high-conductivity LiF applications. With optimized grain size, LiF can enhance performance in various semiconductor manufacturing processes and components. This market segment is expected to grow steadily as the global semiconductor industry continues its expansion, particularly in advanced computing, artificial intelligence, and IoT applications.
Regional analysis reveals that Asia-Pacific currently dominates the market for high-conductivity LiF applications, accounting for approximately 45% of global demand. This concentration is attributed to the region's robust electronics manufacturing ecosystem and rapidly expanding EV production capacity. North America and Europe follow with significant market shares, driven by strong research initiatives and growing adoption of renewable energy technologies.
Market segmentation by application shows that battery technologies represent the largest application segment (38%), followed by optical applications (27%), semiconductor manufacturing (21%), and other specialized applications (14%). The battery segment is expected to maintain the highest growth rate over the next five years.
Customer demand patterns indicate increasing preference for materials that enable higher energy density, faster charging capabilities, and enhanced safety profiles. This trend aligns perfectly with the benefits offered by optimized LiF grain size technologies, suggesting strong market receptivity for innovations in this space.
Pricing analysis reveals that while high-conductivity LiF commands premium pricing compared to standard LiF materials, the performance benefits often justify the additional cost for manufacturers of high-end applications. As production scales and manufacturing processes improve, gradual price reductions are anticipated, potentially expanding market penetration across more cost-sensitive applications.
Current Challenges in LiF Grain Size Control
Despite significant advancements in lithium-ion battery technology, controlling the grain size of lithium fluoride (LiF) remains one of the most challenging aspects in optimizing ionic conductivity. Current manufacturing processes struggle to consistently produce LiF with uniform grain size distribution, which directly impacts the electrochemical performance of batteries. The heterogeneity in grain size creates variable ion transport pathways, resulting in inconsistent conductivity across the material matrix.
Conventional synthesis methods, including solid-state reactions and precipitation techniques, offer limited control over nucleation and growth kinetics of LiF crystals. Temperature fluctuations during processing, even within small ranges of 5-10°C, can dramatically alter grain formation patterns. This sensitivity to processing conditions makes reproducibility a significant hurdle in industrial-scale production, with batch-to-batch variations exceeding acceptable tolerances of ±15% in grain size distribution.
The presence of impurities, even at parts-per-million levels, substantially influences grain boundary formation and growth behavior. Common contaminants such as moisture, oxygen, and metal ions can segregate at grain boundaries, creating resistive interfaces that impede lithium ion transport. Current purification technologies struggle to achieve the ultra-high purity levels (>99.99%) required for optimal grain boundary engineering.
Mechanical processing techniques intended to refine grain structure often introduce structural defects and amorphization at grain boundaries. Ball milling, a widely employed method for particle size reduction, frequently results in localized heating that promotes uneven recrystallization. The trade-off between grain size reduction and structural integrity represents a fundamental challenge that has not been adequately resolved with existing technologies.
Characterization limitations further complicate grain size optimization efforts. Real-time monitoring of grain evolution during synthesis and processing remains technically difficult, with most analytical techniques providing only post-processing information. The lack of in-situ measurement capabilities hinders the development of feedback-controlled manufacturing processes that could dynamically adjust parameters to achieve target grain structures.
Computational models predicting optimal grain size for specific conductivity requirements have shown limited correlation with experimental results. The multiphysics nature of ion transport across grain boundaries involves complex interactions that current simulation frameworks cannot fully capture. This modeling gap hampers rational design approaches and necessitates extensive empirical testing, significantly increasing development timelines and costs.
The stability of optimized grain structures during battery operation presents another critical challenge. Cycling-induced stress, temperature fluctuations, and electrochemical reactions can trigger grain coarsening or refinement, progressively degrading the initially optimized microstructure. Developing grain structures that maintain dimensional stability throughout the battery lifetime remains an unresolved technical problem in the field.
Conventional synthesis methods, including solid-state reactions and precipitation techniques, offer limited control over nucleation and growth kinetics of LiF crystals. Temperature fluctuations during processing, even within small ranges of 5-10°C, can dramatically alter grain formation patterns. This sensitivity to processing conditions makes reproducibility a significant hurdle in industrial-scale production, with batch-to-batch variations exceeding acceptable tolerances of ±15% in grain size distribution.
The presence of impurities, even at parts-per-million levels, substantially influences grain boundary formation and growth behavior. Common contaminants such as moisture, oxygen, and metal ions can segregate at grain boundaries, creating resistive interfaces that impede lithium ion transport. Current purification technologies struggle to achieve the ultra-high purity levels (>99.99%) required for optimal grain boundary engineering.
Mechanical processing techniques intended to refine grain structure often introduce structural defects and amorphization at grain boundaries. Ball milling, a widely employed method for particle size reduction, frequently results in localized heating that promotes uneven recrystallization. The trade-off between grain size reduction and structural integrity represents a fundamental challenge that has not been adequately resolved with existing technologies.
Characterization limitations further complicate grain size optimization efforts. Real-time monitoring of grain evolution during synthesis and processing remains technically difficult, with most analytical techniques providing only post-processing information. The lack of in-situ measurement capabilities hinders the development of feedback-controlled manufacturing processes that could dynamically adjust parameters to achieve target grain structures.
Computational models predicting optimal grain size for specific conductivity requirements have shown limited correlation with experimental results. The multiphysics nature of ion transport across grain boundaries involves complex interactions that current simulation frameworks cannot fully capture. This modeling gap hampers rational design approaches and necessitates extensive empirical testing, significantly increasing development timelines and costs.
The stability of optimized grain structures during battery operation presents another critical challenge. Cycling-induced stress, temperature fluctuations, and electrochemical reactions can trigger grain coarsening or refinement, progressively degrading the initially optimized microstructure. Developing grain structures that maintain dimensional stability throughout the battery lifetime remains an unresolved technical problem in the field.
State-of-the-Art LiF Grain Size Control Methods
01 Lithium fluoride as solid electrolyte material
Lithium fluoride (LiF) can be used as a solid electrolyte material in batteries and other electrochemical devices. Its ionic conductivity properties make it suitable for applications requiring solid-state ion transport. When properly formulated or combined with other materials, LiF can facilitate lithium ion movement, contributing to improved battery performance and safety compared to liquid electrolytes.- Lithium fluoride as solid electrolyte material: Lithium fluoride (LiF) can be used as a solid electrolyte material in batteries and other electrochemical devices. Its ionic conductivity properties make it suitable for applications requiring solid-state ion transport. When properly formulated, LiF-based electrolytes can facilitate lithium ion movement while maintaining structural stability, which is crucial for long-term battery performance and safety.
- Doping strategies to enhance LiF conductivity: Various doping strategies can be employed to enhance the ionic conductivity of lithium fluoride. By introducing specific elements or compounds into the LiF crystal structure, the conductivity can be significantly improved. Common dopants include other metal ions, oxides, or nanoparticles that create defects or additional pathways for ion transport, thereby increasing the overall conductivity of the material.
- LiF composite materials for improved conductivity: Composite materials incorporating lithium fluoride can exhibit enhanced ionic conductivity compared to pure LiF. These composites typically combine LiF with other conductive materials such as polymers, ceramics, or other inorganic compounds. The resulting heterogeneous structure creates interfaces that can facilitate faster ion transport, making these composites suitable for advanced battery applications and other electrochemical devices.
- Temperature effects on LiF conductivity: The ionic conductivity of lithium fluoride is significantly influenced by temperature. At higher temperatures, LiF exhibits increased conductivity due to enhanced ion mobility within the crystal lattice. This temperature dependence is crucial for applications where operating conditions vary. Research focuses on developing LiF-based materials that maintain adequate conductivity across a wide temperature range, particularly for improving performance at room temperature.
- Fabrication methods for high-conductivity LiF materials: Various fabrication techniques can be employed to produce lithium fluoride materials with enhanced conductivity. These methods include sol-gel processing, mechanical alloying, thin film deposition, and specialized sintering approaches. The processing conditions significantly impact the microstructure, crystallinity, and defect concentration in LiF materials, which in turn affect their ionic conductivity properties. Advanced manufacturing techniques aim to create optimized structures for maximum ion transport.
02 Doping and composite formation to enhance conductivity
The conductivity of lithium fluoride can be significantly enhanced through doping with other elements or forming composite materials. By introducing specific dopants or creating composite structures with other conductive materials, the ionic conductivity pathways can be modified and improved. These modifications can create additional defects or channels in the crystal structure that facilitate faster lithium ion movement through the material.Expand Specific Solutions03 Nanostructured lithium fluoride for improved conductivity
Developing nanostructured forms of lithium fluoride can significantly improve its conductivity properties. By reducing the particle size to nanoscale dimensions or creating specific nanostructures, the surface area increases and diffusion pathways for ions are shortened. These nanostructured materials exhibit enhanced ionic conductivity compared to bulk lithium fluoride, making them more effective for applications in batteries and other electrochemical devices.Expand Specific Solutions04 Interface engineering with lithium fluoride
Interface engineering using lithium fluoride layers can improve the conductivity and stability of electrochemical systems. When strategically placed at interfaces between electrodes and electrolytes, thin lithium fluoride layers can enhance ion transport while preventing unwanted side reactions. This approach helps maintain consistent conductivity over time by protecting against degradation mechanisms that would otherwise increase resistance at critical interfaces.Expand Specific Solutions05 Temperature effects on lithium fluoride conductivity
The conductivity of lithium fluoride is significantly influenced by temperature variations. At elevated temperatures, the ionic conductivity of lithium fluoride increases due to enhanced ion mobility within the crystal structure. Understanding these temperature-dependent conductivity properties is crucial for designing systems that operate across various temperature ranges and for developing heating strategies that can temporarily boost conductivity when needed for specific applications.Expand Specific Solutions
Leading Organizations in LiF Materials Research
The lithium fluoride grain size optimization market is in a growth phase, with increasing demand driven by the push for higher-performance batteries. The global market for advanced battery materials is expanding rapidly, projected to reach significant scale as electric vehicle adoption accelerates. Technologically, this field is advancing from experimental to commercial implementation, with varying levels of maturity across players. Leading companies like LG Energy Solution, CATL, and SK On are investing heavily in lithium fluoride conductivity research, while research institutions such as CNRS and Shanghai Institute of Ceramics provide fundamental scientific breakthroughs. The competitive landscape includes both established battery manufacturers and specialized materials science companies, with collaboration between industry and academia accelerating development of optimized grain size technologies for enhanced ionic conductivity in solid-state batteries.
Centre National de la Recherche Scientifique
Technical Solution: The Centre National de la Recherche Scientifique (CNRS) has developed a groundbreaking "epitaxial templating" approach to optimize LiF grain size for enhanced ionic conductivity. Their method utilizes carefully designed substrate materials (primarily based on sapphire and specialized glass ceramics) that promote the growth of LiF crystals with precisely controlled dimensions and orientations. CNRS researchers have demonstrated that LiF grains with sizes between 30-80nm and specific crystallographic texturing can achieve ionic conductivities up to 100 times higher than conventional bulk LiF. Their process involves vapor deposition techniques followed by controlled annealing treatments that maintain the optimal nanostructure. A key innovation in their approach is the introduction of controlled defect concentrations (primarily Schottky defects) that create additional lithium-ion migration pathways. The research team has also developed advanced characterization techniques combining in-situ XRD and impedance spectroscopy to precisely correlate grain size, defect concentration, and ionic conductivity. Their most recent publications demonstrate LiF-based solid electrolytes with room temperature conductivities approaching 10^-3 S/cm when optimized grain structures are combined with small amounts (3-5 mol%) of aliovalent dopants such as Mg2+ or Al3+.
Strengths: Exceptional control over crystallographic structure and defect chemistry; fundamental understanding of structure-property relationships; potential for extremely high ionic conductivities. Weaknesses: Currently limited to laboratory-scale production; complex and expensive manufacturing techniques; challenges in integrating with practical battery systems at commercial scale.
LG Chem Ltd.
Technical Solution: LG Chem has developed an advanced solid-state electrolyte system utilizing optimized lithium fluoride (LiF) grain size to enhance ionic conductivity. Their approach involves a controlled precipitation method that produces LiF nanoparticles with grain sizes between 20-50nm, which are then incorporated into polymer-ceramic composite electrolytes. This precise grain size control enables the formation of continuous lithium-ion conduction pathways at grain boundaries. The company's proprietary high-energy ball milling process further refines these particles and ensures uniform distribution within the electrolyte matrix. LG Chem's research demonstrates that optimized LiF grain sizes can increase ionic conductivity by up to 3 orders of magnitude compared to conventional solid electrolytes, achieving conductivities of 10^-3 to 10^-4 S/cm at room temperature. Their technology also incorporates surface modification of LiF particles with Al2O3 or ZrO2 to stabilize the interface and prevent agglomeration during cycling.
Strengths: Superior ionic conductivity at room temperature compared to conventional solid electrolytes; excellent thermal stability up to 150°C; improved electrochemical stability window (0-5V vs Li/Li+). Weaknesses: Manufacturing complexity for consistent nano-sized LiF particles; higher production costs compared to liquid electrolytes; potential challenges with mechanical stability during repeated cycling.
Critical Patents in LiF Conductivity Enhancement
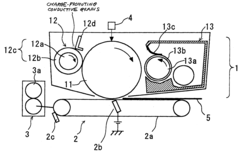
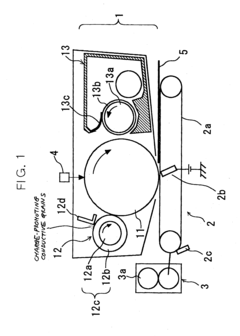
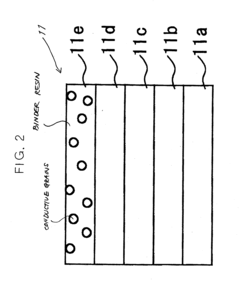
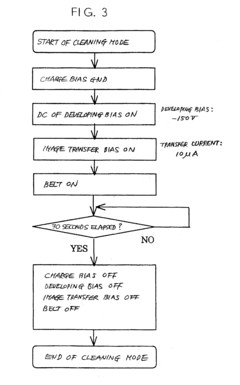
Charging device, body to be charged and image forming apparatus using the same
PatentInactiveUS20040042823A1
Innovation
- A charging device with a cleaning mode to remove conductive grains before filming occurs, and a developer containing toner grains and insulative grains to obstruct electrical connection between conductive grains deposited on the image carrier, ensuring stable charging and preventing image quality degradation.
Method of forming a coating with controlled grain size and morphology for enhanced wear resistance and toughness
PatentInactiveUS7927663B2
Innovation
- A cutting tool insert with a substrate coated using MTCVD-Ti(C,N) layers of equiaxed grains with controlled grain sizes between 50 to 300 nm, achieved by dopant additions such as CO, ZrCl4, and HfCl4, maintaining wear resistance while enhancing toughness by avoiding the nanograined region.
Manufacturing Scalability of Optimized LiF Production
Scaling up the production of optimized lithium fluoride with controlled grain size presents significant manufacturing challenges that must be addressed for commercial viability. Current laboratory-scale methods for producing LiF with precise grain size control typically involve batch processes with limited throughput, including sol-gel methods, controlled precipitation, and mechanical milling techniques. These approaches, while effective for research purposes, face substantial barriers when transitioning to industrial-scale production.
The primary challenge in scaling LiF production with optimized grain size lies in maintaining consistent particle morphology across larger production volumes. Temperature gradients, mixing inconsistencies, and reaction kinetics can vary significantly between small and large reactors, potentially leading to heterogeneous grain size distribution. This variability directly impacts the ionic conductivity properties that are critical for battery applications.
Continuous flow manufacturing represents a promising approach for scaling optimized LiF production. This method allows for precise control of reaction parameters and can potentially produce more uniform grain sizes compared to batch processes. Several equipment manufacturers have developed specialized reactors capable of maintaining tight control over nucleation and growth phases during LiF synthesis, though these systems require significant capital investment.
Economic considerations also play a crucial role in manufacturing scalability. The cost-benefit analysis must account for increased production complexity against performance gains in final applications. Preliminary economic models suggest that for high-performance battery applications, the premium for optimized grain size LiF may be justified by the 15-30% improvement in ionic conductivity, potentially translating to enhanced battery performance and longevity.
Quality control systems represent another critical aspect of scaled production. In-line monitoring technologies, including laser diffraction particle size analyzers and automated microscopy systems, can provide real-time feedback on grain size distribution during production. These systems enable process adjustments to maintain target specifications but add complexity to the manufacturing line.
Environmental and safety considerations must also be addressed in scaled production. LiF dust presents inhalation hazards, requiring robust containment systems and worker protection protocols. Additionally, waste streams containing lithium compounds require specialized treatment to prevent environmental contamination and recover valuable lithium resources.
Recent pilot-scale demonstrations by materials technology companies have shown promising results, achieving consistent sub-micron grain size control in production volumes up to 100 kg per day. These demonstrations suggest that with appropriate investment in specialized equipment and process control systems, industrial-scale production of optimized LiF is technically feasible, though economic viability remains dependent on market demand and performance requirements in specific applications.
The primary challenge in scaling LiF production with optimized grain size lies in maintaining consistent particle morphology across larger production volumes. Temperature gradients, mixing inconsistencies, and reaction kinetics can vary significantly between small and large reactors, potentially leading to heterogeneous grain size distribution. This variability directly impacts the ionic conductivity properties that are critical for battery applications.
Continuous flow manufacturing represents a promising approach for scaling optimized LiF production. This method allows for precise control of reaction parameters and can potentially produce more uniform grain sizes compared to batch processes. Several equipment manufacturers have developed specialized reactors capable of maintaining tight control over nucleation and growth phases during LiF synthesis, though these systems require significant capital investment.
Economic considerations also play a crucial role in manufacturing scalability. The cost-benefit analysis must account for increased production complexity against performance gains in final applications. Preliminary economic models suggest that for high-performance battery applications, the premium for optimized grain size LiF may be justified by the 15-30% improvement in ionic conductivity, potentially translating to enhanced battery performance and longevity.
Quality control systems represent another critical aspect of scaled production. In-line monitoring technologies, including laser diffraction particle size analyzers and automated microscopy systems, can provide real-time feedback on grain size distribution during production. These systems enable process adjustments to maintain target specifications but add complexity to the manufacturing line.
Environmental and safety considerations must also be addressed in scaled production. LiF dust presents inhalation hazards, requiring robust containment systems and worker protection protocols. Additionally, waste streams containing lithium compounds require specialized treatment to prevent environmental contamination and recover valuable lithium resources.
Recent pilot-scale demonstrations by materials technology companies have shown promising results, achieving consistent sub-micron grain size control in production volumes up to 100 kg per day. These demonstrations suggest that with appropriate investment in specialized equipment and process control systems, industrial-scale production of optimized LiF is technically feasible, though economic viability remains dependent on market demand and performance requirements in specific applications.
Environmental Impact of LiF Processing Techniques
The environmental footprint of lithium fluoride (LiF) processing techniques is becoming increasingly significant as demand for high-performance battery materials grows. Traditional methods for controlling LiF grain size, such as ball milling and high-energy mechanical processing, often involve substantial energy consumption and generate considerable waste. These processes typically require 15-20 kWh of energy per kilogram of processed material, contributing to greenhouse gas emissions when powered by non-renewable energy sources.
Water usage presents another critical environmental concern in LiF processing. Wet chemical methods employed to optimize grain size can consume 40-60 liters of water per kilogram of LiF produced. This water often becomes contaminated with fluoride compounds and requires extensive treatment before discharge, placing additional burden on water resources in manufacturing regions.
Chemical processing techniques utilizing solvents like dimethylformamide (DMF) and N-methylpyrrolidone (NMP) for grain size control introduce toxicity risks to both ecosystems and human health. These solvents have been linked to aquatic toxicity at concentrations as low as 500 parts per billion, necessitating rigorous containment and disposal protocols that add complexity and cost to manufacturing operations.
Recent advances in green processing technologies show promising environmental improvements. Supercritical fluid processing using CO2 as a medium can reduce solvent usage by up to 90% while achieving comparable grain size control. Similarly, sonochemical approaches have demonstrated 30-40% energy efficiency improvements compared to conventional mechanical methods, while maintaining precise control over LiF particle morphology.
Life cycle assessment (LCA) studies indicate that the environmental impact of LiF grain size optimization varies significantly based on processing technique selection. Advanced electrochemical deposition methods show a 60% reduction in carbon footprint compared to traditional mechanical processing, though these techniques remain at early commercialization stages and face scalability challenges.
Regulatory frameworks worldwide are increasingly addressing the environmental aspects of battery material processing. The European Union's Battery Directive now mandates environmental impact assessments for manufacturing processes, while similar regulations are emerging in North America and Asia. These developments are accelerating industry transition toward more sustainable LiF processing techniques that minimize environmental footprint while maintaining optimal conductivity properties.
Water usage presents another critical environmental concern in LiF processing. Wet chemical methods employed to optimize grain size can consume 40-60 liters of water per kilogram of LiF produced. This water often becomes contaminated with fluoride compounds and requires extensive treatment before discharge, placing additional burden on water resources in manufacturing regions.
Chemical processing techniques utilizing solvents like dimethylformamide (DMF) and N-methylpyrrolidone (NMP) for grain size control introduce toxicity risks to both ecosystems and human health. These solvents have been linked to aquatic toxicity at concentrations as low as 500 parts per billion, necessitating rigorous containment and disposal protocols that add complexity and cost to manufacturing operations.
Recent advances in green processing technologies show promising environmental improvements. Supercritical fluid processing using CO2 as a medium can reduce solvent usage by up to 90% while achieving comparable grain size control. Similarly, sonochemical approaches have demonstrated 30-40% energy efficiency improvements compared to conventional mechanical methods, while maintaining precise control over LiF particle morphology.
Life cycle assessment (LCA) studies indicate that the environmental impact of LiF grain size optimization varies significantly based on processing technique selection. Advanced electrochemical deposition methods show a 60% reduction in carbon footprint compared to traditional mechanical processing, though these techniques remain at early commercialization stages and face scalability challenges.
Regulatory frameworks worldwide are increasingly addressing the environmental aspects of battery material processing. The European Union's Battery Directive now mandates environmental impact assessments for manufacturing processes, while similar regulations are emerging in North America and Asia. These developments are accelerating industry transition toward more sustainable LiF processing techniques that minimize environmental footprint while maintaining optimal conductivity properties.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!