Conformal Coating in Medical Device Production: Standards Review
SEP 17, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Medical Device Conformal Coating Background and Objectives
Conformal coating has evolved significantly since its initial applications in the aerospace and military sectors in the 1960s and 1970s. The technology was primarily developed to protect electronic components from harsh environmental conditions, including moisture, dust, chemicals, and temperature fluctuations. In the medical device industry, conformal coating adoption began to accelerate in the 1990s as medical electronics became more sophisticated and miniaturized, requiring enhanced protection against environmental factors while maintaining biocompatibility.
The evolution of conformal coating technologies has been marked by transitions from traditional solvent-based acrylics and silicones to more advanced formulations including polyurethanes, epoxies, and parylenes. Each generation has offered improvements in protection capabilities, application methods, and compliance with increasingly stringent medical standards. Recent innovations have focused on developing coatings with antimicrobial properties, enhanced biocompatibility, and reduced environmental impact.
Current market trends indicate a growing emphasis on water-based and UV-curable conformal coatings, which offer reduced volatile organic compound (VOC) emissions and faster processing times. Additionally, the integration of nanotechnology has enabled the development of ultra-thin coatings with superior barrier properties and flexibility, addressing the challenges posed by complex medical device geometries and miniaturization.
The primary objective of conformal coating in medical device production is to ensure long-term reliability and safety of electronic components in medical devices, particularly those used in critical care applications. This includes protection against bodily fluids, sterilization processes, and cleaning agents commonly used in healthcare environments. Secondary objectives include extending device lifespan, reducing maintenance requirements, and enabling functionality in diverse clinical settings.
Regulatory frameworks governing conformal coatings in medical devices have become increasingly comprehensive, with standards such as IEC 60601 for electrical medical equipment, ISO 10993 for biocompatibility, and IPC-CC-830 for coating performance specifications. These standards establish baseline requirements for coating materials, application processes, and testing methodologies to ensure patient safety and device efficacy.
The technological trajectory suggests continued refinement of coating materials and application techniques, with particular focus on automated precision application methods, real-time quality control systems, and coatings specifically engineered for emerging medical technologies such as implantable sensors and wearable monitoring devices. Research efforts are increasingly directed toward developing coatings that not only protect but also enhance device functionality through properties such as improved thermal management and electromagnetic interference shielding.
The evolution of conformal coating technologies has been marked by transitions from traditional solvent-based acrylics and silicones to more advanced formulations including polyurethanes, epoxies, and parylenes. Each generation has offered improvements in protection capabilities, application methods, and compliance with increasingly stringent medical standards. Recent innovations have focused on developing coatings with antimicrobial properties, enhanced biocompatibility, and reduced environmental impact.
Current market trends indicate a growing emphasis on water-based and UV-curable conformal coatings, which offer reduced volatile organic compound (VOC) emissions and faster processing times. Additionally, the integration of nanotechnology has enabled the development of ultra-thin coatings with superior barrier properties and flexibility, addressing the challenges posed by complex medical device geometries and miniaturization.
The primary objective of conformal coating in medical device production is to ensure long-term reliability and safety of electronic components in medical devices, particularly those used in critical care applications. This includes protection against bodily fluids, sterilization processes, and cleaning agents commonly used in healthcare environments. Secondary objectives include extending device lifespan, reducing maintenance requirements, and enabling functionality in diverse clinical settings.
Regulatory frameworks governing conformal coatings in medical devices have become increasingly comprehensive, with standards such as IEC 60601 for electrical medical equipment, ISO 10993 for biocompatibility, and IPC-CC-830 for coating performance specifications. These standards establish baseline requirements for coating materials, application processes, and testing methodologies to ensure patient safety and device efficacy.
The technological trajectory suggests continued refinement of coating materials and application techniques, with particular focus on automated precision application methods, real-time quality control systems, and coatings specifically engineered for emerging medical technologies such as implantable sensors and wearable monitoring devices. Research efforts are increasingly directed toward developing coatings that not only protect but also enhance device functionality through properties such as improved thermal management and electromagnetic interference shielding.
Market Demand Analysis for Coated Medical Devices
The global market for conformal coated medical devices has experienced substantial growth in recent years, driven primarily by increasing demand for implantable devices, wearable health monitors, and advanced diagnostic equipment. Current market valuations place the medical device coating segment at approximately 15 billion USD, with projections indicating a compound annual growth rate of 7-8% through 2028. Conformal coating specifically represents a significant portion of this market, as it addresses critical requirements for device protection against moisture, chemicals, and contaminants.
Healthcare providers are increasingly demanding medical devices with enhanced durability and reliability, particularly for equipment used in critical care settings where device failure could have severe consequences. The growing prevalence of chronic diseases worldwide has accelerated the adoption of long-term implantable devices, which require superior protection against bodily fluids and potential biofouling. This trend is particularly evident in cardiology, neurology, and diabetes management sectors.
Regulatory pressures have also shaped market demand significantly. With the implementation of more stringent standards such as ISO 13485:2016 and the EU Medical Device Regulation (MDR), manufacturers are required to demonstrate improved quality control and device longevity. Conformal coating has emerged as a key solution to meet these enhanced regulatory requirements, driving adoption across the industry.
Regional analysis reveals varying demand patterns, with North America currently leading the market due to its advanced healthcare infrastructure and high adoption rates of cutting-edge medical technologies. However, the Asia-Pacific region is experiencing the fastest growth, attributed to expanding healthcare access, increasing medical tourism, and growing domestic manufacturing capabilities in countries like China, India, and Singapore.
Consumer preferences are shifting toward miniaturized, portable medical devices that can be used in home healthcare settings. This miniaturization trend presents unique challenges for device protection, creating new opportunities for specialized conformal coating solutions that can provide adequate protection without adding significant bulk or weight to compact devices.
The COVID-19 pandemic has accelerated certain market segments, particularly those related to respiratory support equipment and remote patient monitoring devices. These applications often require enhanced protection against frequent disinfection procedures, creating additional demand for chemical-resistant conformal coatings. Post-pandemic healthcare strategies continue to emphasize infection control and device reliability, sustaining this demand trend.
Market research indicates that hospitals and healthcare facilities are increasingly factoring total cost of ownership into purchasing decisions, recognizing that properly coated devices may command premium prices but offer superior longevity and reduced maintenance costs over their operational lifetime.
Healthcare providers are increasingly demanding medical devices with enhanced durability and reliability, particularly for equipment used in critical care settings where device failure could have severe consequences. The growing prevalence of chronic diseases worldwide has accelerated the adoption of long-term implantable devices, which require superior protection against bodily fluids and potential biofouling. This trend is particularly evident in cardiology, neurology, and diabetes management sectors.
Regulatory pressures have also shaped market demand significantly. With the implementation of more stringent standards such as ISO 13485:2016 and the EU Medical Device Regulation (MDR), manufacturers are required to demonstrate improved quality control and device longevity. Conformal coating has emerged as a key solution to meet these enhanced regulatory requirements, driving adoption across the industry.
Regional analysis reveals varying demand patterns, with North America currently leading the market due to its advanced healthcare infrastructure and high adoption rates of cutting-edge medical technologies. However, the Asia-Pacific region is experiencing the fastest growth, attributed to expanding healthcare access, increasing medical tourism, and growing domestic manufacturing capabilities in countries like China, India, and Singapore.
Consumer preferences are shifting toward miniaturized, portable medical devices that can be used in home healthcare settings. This miniaturization trend presents unique challenges for device protection, creating new opportunities for specialized conformal coating solutions that can provide adequate protection without adding significant bulk or weight to compact devices.
The COVID-19 pandemic has accelerated certain market segments, particularly those related to respiratory support equipment and remote patient monitoring devices. These applications often require enhanced protection against frequent disinfection procedures, creating additional demand for chemical-resistant conformal coatings. Post-pandemic healthcare strategies continue to emphasize infection control and device reliability, sustaining this demand trend.
Market research indicates that hospitals and healthcare facilities are increasingly factoring total cost of ownership into purchasing decisions, recognizing that properly coated devices may command premium prices but offer superior longevity and reduced maintenance costs over their operational lifetime.
Current Standards and Technical Challenges
The global medical device industry is currently governed by a complex framework of standards for conformal coating applications. IPC-CC-830 and IPC-A-610 serve as foundational standards, establishing baseline requirements for coating materials, application processes, and quality control. More specifically tailored to medical applications, ISO 10993 series addresses biocompatibility concerns, ensuring coatings do not cause adverse biological reactions when used in medical contexts. The ISO 13485 standard provides comprehensive quality management system requirements for medical device manufacturing, including conformal coating processes.
Regulatory bodies worldwide have established region-specific standards that manufacturers must navigate. The FDA in the United States enforces 21 CFR Part 820 (Quality System Regulation), which includes specific provisions for coating processes in medical devices. In Europe, the Medical Device Regulation (MDR) and the IEC 60601 series impose stringent requirements for electrical medical equipment safety, where conformal coatings play a critical protective role.
Despite this robust regulatory framework, significant technical challenges persist in conformal coating applications for medical devices. Achieving consistent coating thickness across complex geometries remains problematic, particularly for miniaturized devices with intricate surface features. The industry struggles with balancing adequate protection while avoiding excessive material application that could affect device functionality or create stress points.
Material compatibility presents another major challenge, as coating materials must maintain long-term adhesion to diverse substrate materials while not interfering with device performance. This is particularly critical for implantable devices where coating degradation could lead to device failure or patient harm. Additionally, environmental concerns and regulatory pressure are driving the industry away from traditional solvent-based coatings toward water-based and UV-curable alternatives, which often present different application challenges.
Testing and validation methodologies represent a significant technical hurdle. Current standards require extensive testing for environmental resistance, electrical insulation properties, and long-term reliability, but standardized methodologies sometimes lag behind the rapid evolution of medical device technology. This creates uncertainty in validation processes, particularly for innovative devices with novel form factors or operating environments.
Manufacturing scalability remains problematic as coating processes that work effectively in laboratory settings often encounter difficulties when scaled to production volumes. Automated application systems must maintain precision across large production runs while accommodating the increasing complexity of modern medical devices. This challenge is compounded by the industry's trend toward personalized medicine, which demands flexible manufacturing processes capable of accommodating customized devices.
Regulatory bodies worldwide have established region-specific standards that manufacturers must navigate. The FDA in the United States enforces 21 CFR Part 820 (Quality System Regulation), which includes specific provisions for coating processes in medical devices. In Europe, the Medical Device Regulation (MDR) and the IEC 60601 series impose stringent requirements for electrical medical equipment safety, where conformal coatings play a critical protective role.
Despite this robust regulatory framework, significant technical challenges persist in conformal coating applications for medical devices. Achieving consistent coating thickness across complex geometries remains problematic, particularly for miniaturized devices with intricate surface features. The industry struggles with balancing adequate protection while avoiding excessive material application that could affect device functionality or create stress points.
Material compatibility presents another major challenge, as coating materials must maintain long-term adhesion to diverse substrate materials while not interfering with device performance. This is particularly critical for implantable devices where coating degradation could lead to device failure or patient harm. Additionally, environmental concerns and regulatory pressure are driving the industry away from traditional solvent-based coatings toward water-based and UV-curable alternatives, which often present different application challenges.
Testing and validation methodologies represent a significant technical hurdle. Current standards require extensive testing for environmental resistance, electrical insulation properties, and long-term reliability, but standardized methodologies sometimes lag behind the rapid evolution of medical device technology. This creates uncertainty in validation processes, particularly for innovative devices with novel form factors or operating environments.
Manufacturing scalability remains problematic as coating processes that work effectively in laboratory settings often encounter difficulties when scaled to production volumes. Automated application systems must maintain precision across large production runs while accommodating the increasing complexity of modern medical devices. This challenge is compounded by the industry's trend toward personalized medicine, which demands flexible manufacturing processes capable of accommodating customized devices.
Current Conformal Coating Solutions
01 Types of conformal coating materials
Various materials are used for conformal coatings, including acrylics, silicones, polyurethanes, epoxies, and parylene. Each material offers different properties in terms of moisture resistance, chemical resistance, temperature stability, and dielectric strength. The selection of coating material depends on the specific application requirements, environmental conditions, and the electronic components being protected.- Types and compositions of conformal coatings: Various types of conformal coatings are used for electronic protection, including acrylic, silicone, polyurethane, and epoxy-based formulations. These coatings differ in their chemical compositions, providing specific properties such as moisture resistance, chemical protection, and thermal stability. The selection of coating material depends on the application requirements, environmental conditions, and the level of protection needed for the electronic components.
- Application methods for conformal coatings: Conformal coatings can be applied using various techniques including spray coating, dip coating, brush application, and automated selective coating systems. Each method offers different advantages in terms of coverage, thickness control, and production efficiency. Advanced application technologies enable precise deposition of coating materials on specific areas of electronic assemblies while masking sensitive components that should remain uncoated.
- Protection of electronic components and PCBs: Conformal coatings provide critical protection for printed circuit boards (PCBs) and electronic components against environmental factors such as moisture, dust, chemicals, and temperature fluctuations. These protective layers help prevent corrosion, electrical leakage, and short circuits, thereby extending the operational life of electronic devices. The coatings are particularly important in harsh environments or applications where reliability is crucial.
- Curing and processing technologies: Various curing methods are employed for conformal coatings, including UV curing, thermal curing, moisture curing, and room temperature vulcanization. These processes transform the liquid coating into a solid protective film with the desired properties. Advanced curing technologies focus on reducing processing time, energy consumption, and environmental impact while ensuring complete polymerization and optimal adhesion to substrates.
- Specialized conformal coatings for specific applications: Specialized conformal coatings are developed for specific applications such as automotive electronics, aerospace components, medical devices, and renewable energy systems. These tailored formulations may incorporate additional properties like flame retardancy, high-temperature resistance, optical clarity, or electromagnetic interference (EMI) shielding. Some advanced coatings also feature self-healing capabilities or enhanced adhesion to difficult substrates.
02 Application methods for conformal coatings
Conformal coatings can be applied using various techniques including spraying, dipping, brushing, selective coating, and vapor deposition. Each method has advantages depending on production volume, component geometry, and precision requirements. Automated application systems can improve consistency and coverage while reducing material waste. Masking techniques are often employed to protect areas that should remain uncoated.Expand Specific Solutions03 Protection of electronic components
Conformal coatings provide critical protection for electronic components against environmental factors such as moisture, dust, chemicals, and temperature fluctuations. These coatings form a protective barrier that prevents corrosion, electrical shorts, and mechanical damage, thereby extending the operational life of electronic assemblies. They are particularly important in harsh environments or applications where reliability is crucial.Expand Specific Solutions04 Specialized coatings for specific applications
Specialized conformal coatings have been developed for specific applications such as automotive electronics, aerospace components, medical devices, and renewable energy systems. These formulations may include additives for enhanced UV resistance, flame retardancy, thermal conductivity, or flexibility. Some coatings are designed to withstand extreme conditions like high vibration, radiation exposure, or wide temperature ranges.Expand Specific Solutions05 Curing and removal processes
Conformal coatings require specific curing processes to achieve optimal performance, including UV curing, thermal curing, moisture curing, or room temperature vulcanization. The curing method affects production speed, energy consumption, and coating properties. Some applications also require consideration of reworkability, where coatings may need to be selectively removed for component repair or replacement without damaging the underlying circuitry.Expand Specific Solutions
Key Industry Players and Manufacturers
The conformal coating market in medical device production is currently in a growth phase, with increasing demand driven by stringent regulatory standards and the need for enhanced device protection. The global market size is expanding rapidly, projected to reach significant value as medical technology advances. Technologically, conformal coating applications are maturing with innovations in biocompatible materials and application methods. Leading players like Medtronic, Boston Scientific, and Terumo are investing heavily in advanced coating technologies for their cardiovascular and neurovascular devices, while specialized coating providers such as Nordson Corp. and 3M Innovative Properties offer comprehensive solutions. Companies like Wacker Chemie and Covestro are developing next-generation coating materials specifically engineered for medical applications, focusing on compliance with international standards including ISO 10993 and IEC 60601.
Nordson Corp.
Technical Solution: Nordson has pioneered automated conformal coating systems specifically optimized for medical device production that comply with IPC-CC-830, UL 746E, and MIL-I-46058C standards. Their technology integrates precision dispensing with advanced vision systems for real-time quality control, ensuring consistent application across complex medical device geometries. Nordson's selective coating technology allows for targeted application to specific areas while masking sensitive components, reducing material waste by up to 30% compared to traditional methods. Their systems incorporate multi-axis motion control with accuracy to ±25 microns, enabling coating of miniaturized medical components with complex topographies. Nordson has developed specialized formulations including silicone, acrylic, and parylene coatings that maintain electrical insulation properties while meeting biocompatibility requirements under ISO 10993. Their coating systems feature controlled environment chambers that regulate temperature, humidity, and particulate levels to ensure optimal curing conditions and minimize defects in the finished coating layer.
Strengths: Market-leading automated dispensing technology with exceptional precision; comprehensive process control systems that ensure traceability and compliance; extensive global service network supporting medical device manufacturers. Weaknesses: Higher initial capital investment compared to manual application methods; requires specialized technical expertise for programming and maintenance; system changeovers between different coating materials can be time-consuming.
Medtronic, Inc.
Technical Solution: Medtronic has developed proprietary conformal coating technologies specifically for implantable medical devices that must function reliably in the harsh environment of the human body. Their approach incorporates multilayer coating systems with specialized biocompatible polymers that provide both electrical insulation and protection against bodily fluids. Medtronic's conformal coating process includes plasma surface preparation to enhance adhesion, followed by precision-controlled application methods that ensure uniform coverage even on complex geometries with tolerances as tight as ±10 microns. Their coatings undergo rigorous testing including accelerated aging studies simulating 10+ years of implantation, salt fog exposure, and thermal cycling between body and ambient temperatures. Medtronic has pioneered the use of parylene coatings for neurostimulation devices, achieving thicknesses as low as 5 microns while maintaining complete coverage and excellent dielectric properties. Their coating systems comply with ISO 13485 manufacturing standards and have been validated through extensive clinical studies demonstrating long-term stability in vivo. Medtronic's conformal coating technology includes specialized formulations that resist enzymatic degradation and maintain performance through multiple sterilization cycles.
Strengths: Extensive experience with implantable medical devices requiring the highest reliability standards; vertically integrated testing capabilities that simulate real-world conditions; proven clinical performance data spanning decades. Weaknesses: Highly specialized coatings may have limited application beyond specific medical device categories; longer validation timelines due to stringent regulatory requirements for implantable devices; higher production costs compared to non-implantable device coatings.
Critical Standards and Compliance Requirements
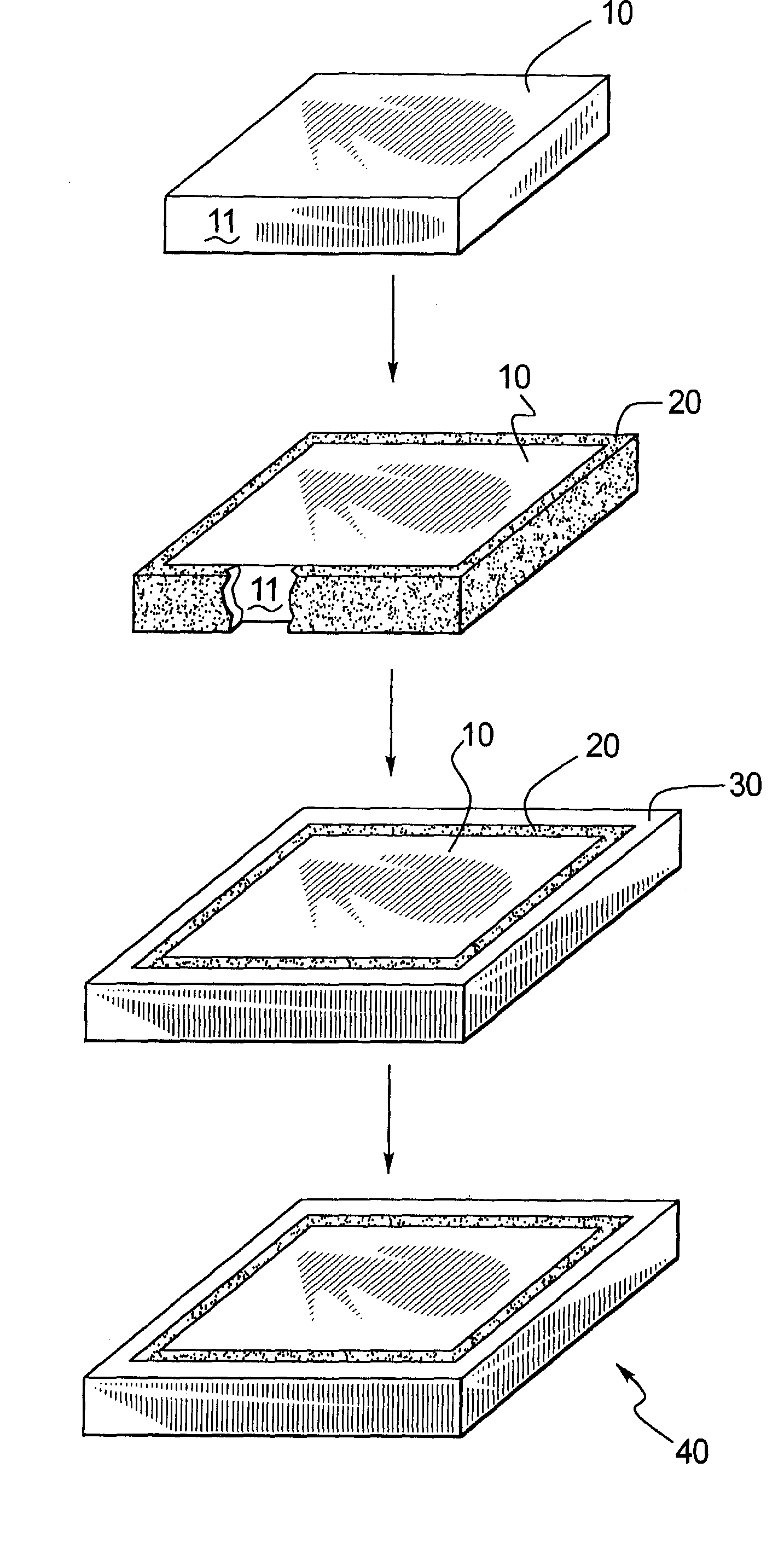
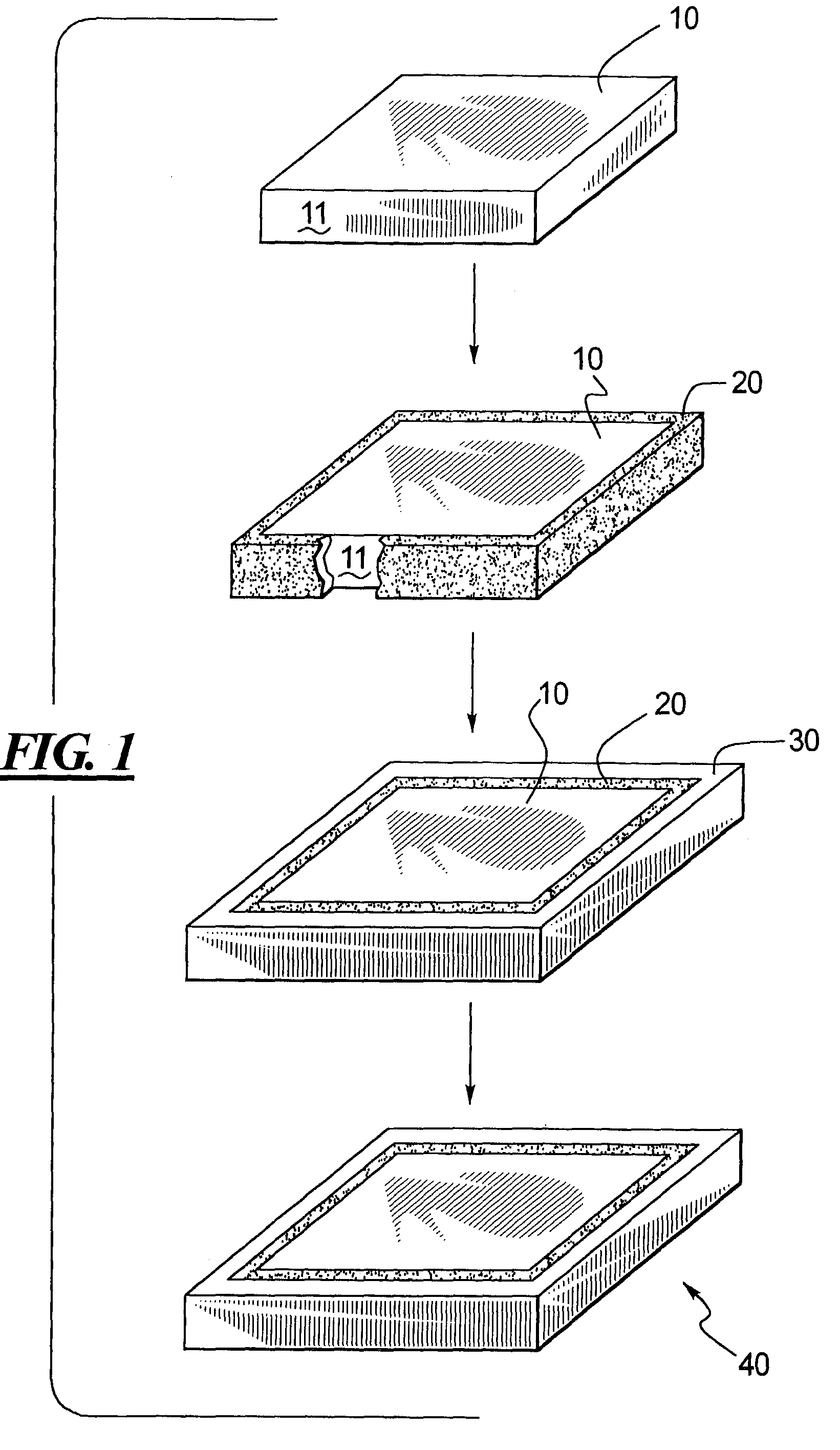
Method of manufacture of smart microfluidic medical device with universal coating
PatentInactiveUS7279112B2
Innovation
- A 'smart' medical device with a universal diamond or diamond-like coating that enables optical, electrochemical, and thermal signal transmission, incorporating optical sensors and microprocessors for controlled drug delivery, corrosion resistance, and simplified electrode fabrication, allowing for real-time monitoring and adjustment of drug concentrations and mixtures.
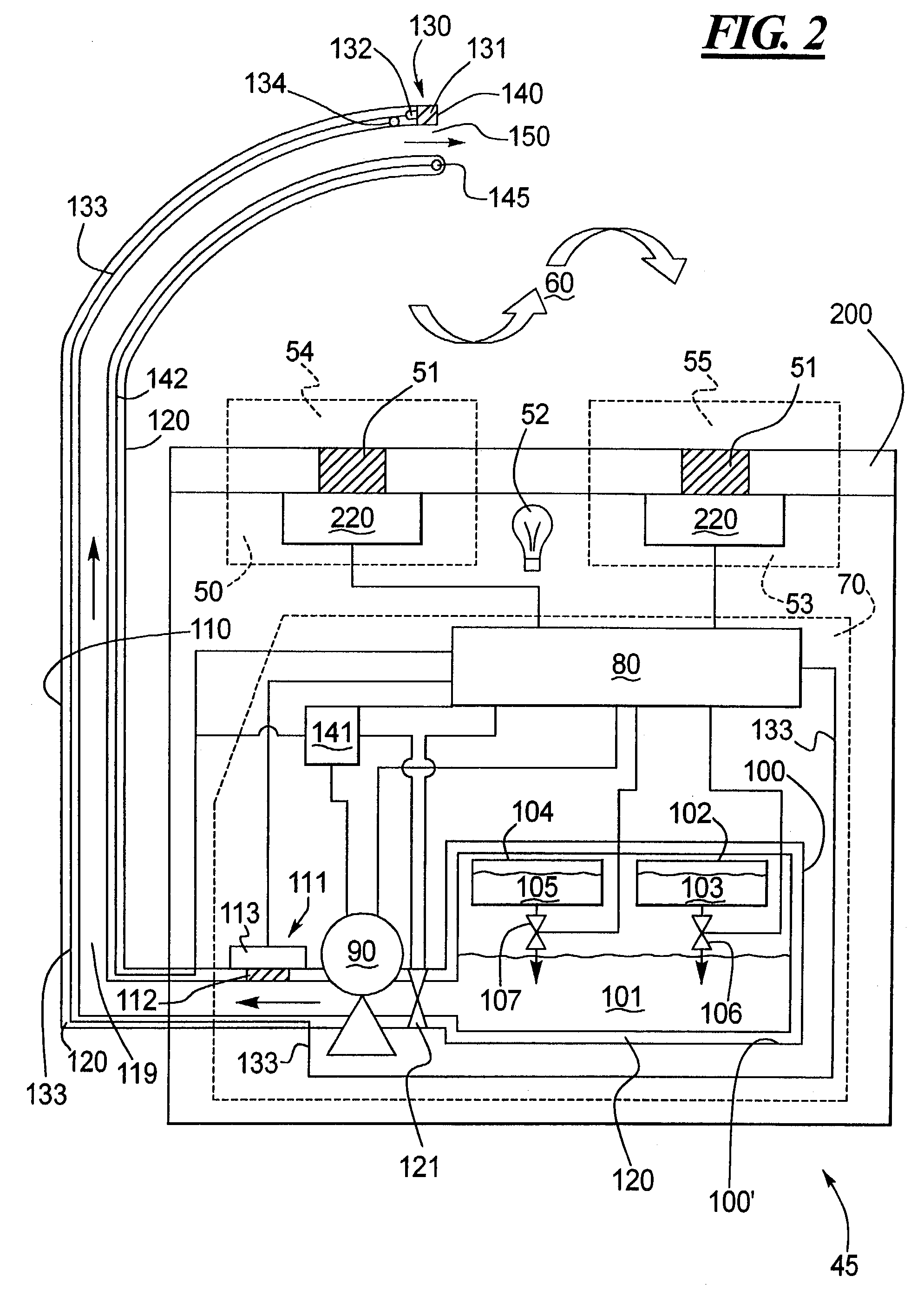
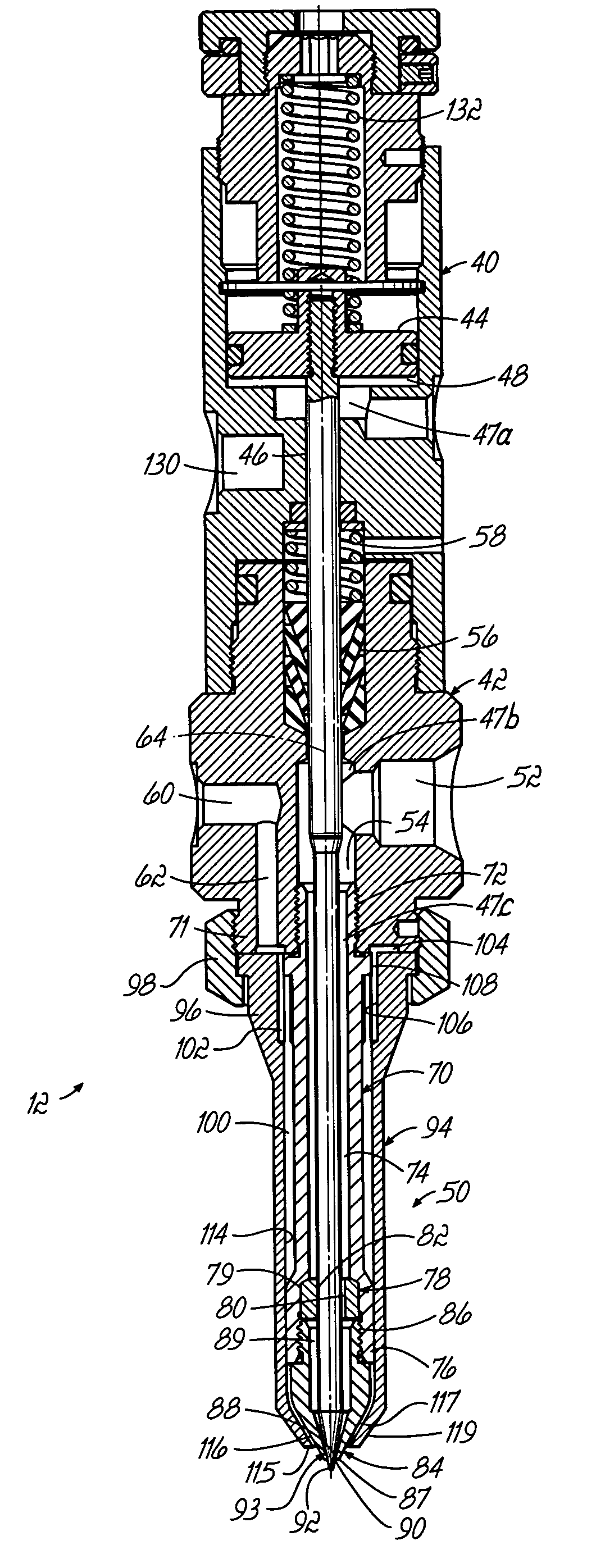
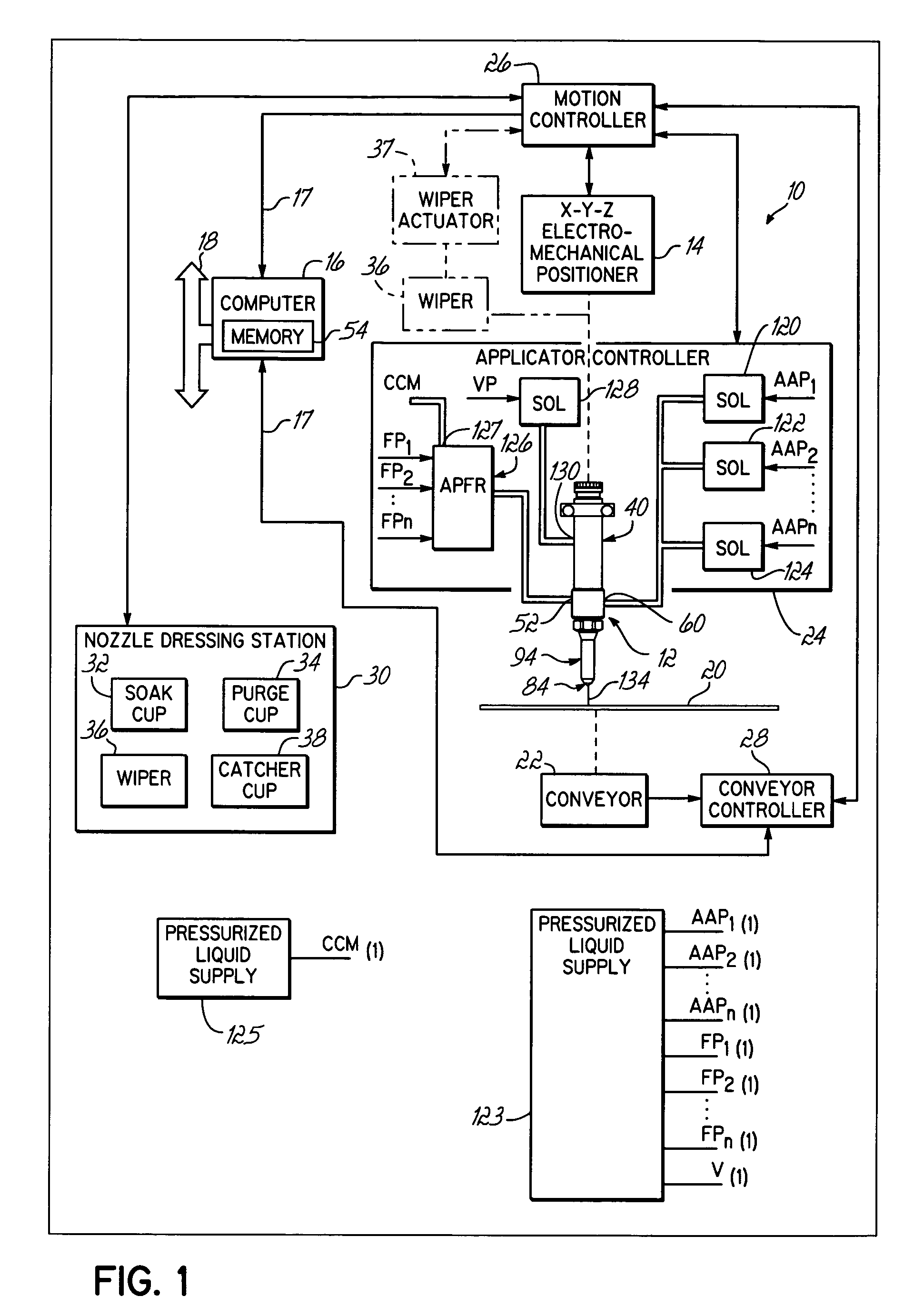
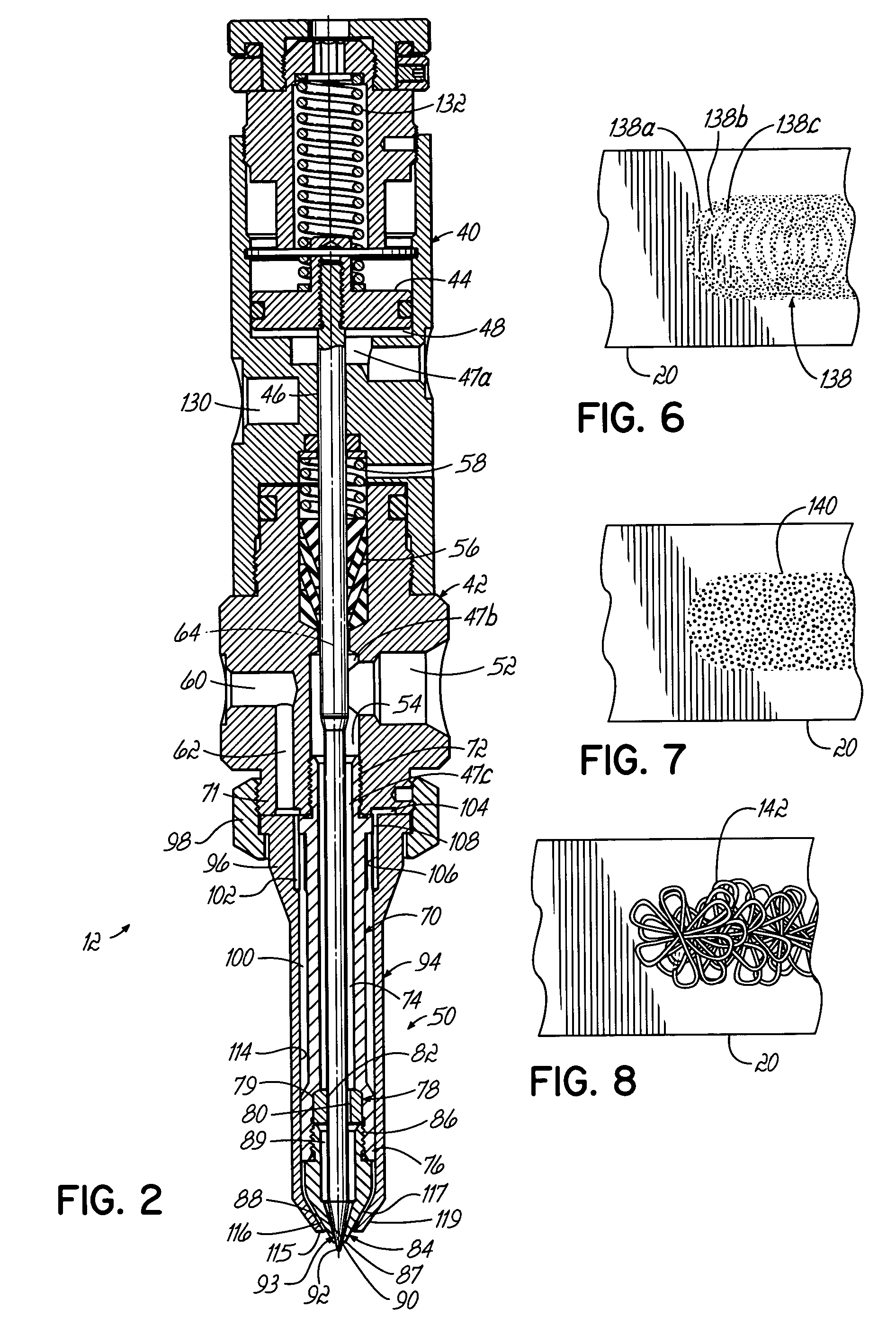
Conformal coating applicator and method
PatentInactiveUS7028867B2
Innovation
- A conformal coating applicator with a nozzle design that minimizes downstream material accumulation, allows for automated cleaning, and uses programmable controls to select various liquid and air pressures for different dispensing patterns, featuring a zero-cavity design and exposed nozzle for easy cleaning, and a fluid extension with adjustable splines for consistent airflow.
Regulatory Framework and Approval Pathways
The regulatory landscape for conformal coating in medical device production is governed by a complex framework of international standards and regional regulations. At the global level, ISO 13485 serves as the cornerstone standard for medical device quality management systems, requiring manufacturers to implement appropriate controls for production processes including conformal coating. This standard emphasizes risk management and validation of processes that cannot be fully verified by subsequent inspection.
In the United States, the FDA regulates medical devices through the Federal Food, Drug, and Cosmetic Act and its amendments. Conformal coating processes must comply with 21 CFR Part 820 (Quality System Regulation), which mandates validation of manufacturing processes and establishment of process controls. The FDA categorizes medical devices into three classes based on risk, with each class subject to different regulatory requirements affecting the conformal coating validation process.
The European Union's regulatory framework has undergone significant transformation with the implementation of the Medical Device Regulation (MDR 2017/745) and In Vitro Diagnostic Regulation (IVDR 2017/746), replacing the previous Medical Device Directive. These regulations impose more stringent requirements for technical documentation, post-market surveillance, and clinical evaluation, directly impacting conformal coating processes and materials selection.
For conformal coating specifically, IPC-CC-830C provides detailed specifications for electrical insulating compounds, while IPC-HDBK-830 offers guidance on design, material selection, and quality control. These standards are often referenced in regulatory submissions to demonstrate compliance with broader medical device regulations.
The approval pathway for a medical device with conformal coating varies by jurisdiction and risk classification. In the US, Class I devices generally require only registration, while Class II devices typically follow the 510(k) pathway requiring demonstration of substantial equivalence to a legally marketed device. Class III devices undergo the more rigorous Premarket Approval (PMA) process, requiring clinical evidence of safety and effectiveness.
In the EU, manufacturers must obtain CE marking through conformity assessment procedures appropriate to the device classification. This process involves technical documentation review and quality management system assessment by Notified Bodies for higher-risk devices. The documentation must include validation data for the conformal coating process, demonstrating that it consistently produces coatings meeting predetermined specifications.
Japan's Pharmaceuticals and Medical Devices Agency (PMDA) and China's National Medical Products Administration (NMPA) have established their own regulatory frameworks, adding complexity for global manufacturers seeking multi-market approvals for devices with conformal coatings.
In the United States, the FDA regulates medical devices through the Federal Food, Drug, and Cosmetic Act and its amendments. Conformal coating processes must comply with 21 CFR Part 820 (Quality System Regulation), which mandates validation of manufacturing processes and establishment of process controls. The FDA categorizes medical devices into three classes based on risk, with each class subject to different regulatory requirements affecting the conformal coating validation process.
The European Union's regulatory framework has undergone significant transformation with the implementation of the Medical Device Regulation (MDR 2017/745) and In Vitro Diagnostic Regulation (IVDR 2017/746), replacing the previous Medical Device Directive. These regulations impose more stringent requirements for technical documentation, post-market surveillance, and clinical evaluation, directly impacting conformal coating processes and materials selection.
For conformal coating specifically, IPC-CC-830C provides detailed specifications for electrical insulating compounds, while IPC-HDBK-830 offers guidance on design, material selection, and quality control. These standards are often referenced in regulatory submissions to demonstrate compliance with broader medical device regulations.
The approval pathway for a medical device with conformal coating varies by jurisdiction and risk classification. In the US, Class I devices generally require only registration, while Class II devices typically follow the 510(k) pathway requiring demonstration of substantial equivalence to a legally marketed device. Class III devices undergo the more rigorous Premarket Approval (PMA) process, requiring clinical evidence of safety and effectiveness.
In the EU, manufacturers must obtain CE marking through conformity assessment procedures appropriate to the device classification. This process involves technical documentation review and quality management system assessment by Notified Bodies for higher-risk devices. The documentation must include validation data for the conformal coating process, demonstrating that it consistently produces coatings meeting predetermined specifications.
Japan's Pharmaceuticals and Medical Devices Agency (PMDA) and China's National Medical Products Administration (NMPA) have established their own regulatory frameworks, adding complexity for global manufacturers seeking multi-market approvals for devices with conformal coatings.
Biocompatibility and Safety Considerations
Biocompatibility and safety considerations represent critical aspects of conformal coating applications in medical device production. The interaction between coating materials and human tissues must be thoroughly evaluated to ensure patient safety and regulatory compliance. ISO 10993 series standards provide the framework for biological evaluation of medical devices, with specific attention to the potential cytotoxicity, sensitization, and irritation properties of conformal coatings.
When selecting conformal coating materials for medical applications, manufacturers must prioritize biocompatibility testing according to the device's intended use and duration of contact with the body. Parylene coatings have demonstrated excellent biocompatibility profiles, making them particularly suitable for implantable devices. Silicone and acrylic coatings also show favorable biological responses but require careful formulation to eliminate potentially harmful additives.
Leachable compounds present a significant safety concern in medical device coatings. Under physiological conditions, certain coating components may migrate from the device surface into surrounding tissues or bloodstream. Comprehensive extraction studies following ISO 10993-18 guidelines are essential to identify and quantify these substances. Manufacturers must establish acceptable limits for leachables based on toxicological risk assessments and ensure their coatings remain below these thresholds throughout the device lifecycle.
The sterilization compatibility of conformal coatings represents another crucial safety consideration. Medical devices typically undergo sterilization processes such as ethylene oxide treatment, gamma irradiation, or autoclave procedures. These methods can potentially degrade coating materials, compromising their protective functions or generating toxic byproducts. Coating selection must account for the intended sterilization method, with appropriate validation studies confirming material stability post-sterilization.
Long-term aging effects must also be evaluated when assessing coating safety. Environmental factors such as temperature fluctuations, humidity, and oxidative stress can accelerate coating degradation over time. Accelerated aging studies following ASTM F1980 protocols help predict coating performance throughout the device's expected service life. Particular attention should be given to potential changes in surface properties, barrier integrity, and biocompatibility characteristics during aging.
Regulatory bodies increasingly emphasize the importance of chemical characterization for conformal coatings. The FDA's guidance on the use of ISO 10993-18 recommends thorough material characterization to identify chemicals of concern before conducting biological tests. This approach, known as the chemical-first paradigm, helps manufacturers develop safer coating formulations by eliminating potentially harmful substances early in the development process.
When selecting conformal coating materials for medical applications, manufacturers must prioritize biocompatibility testing according to the device's intended use and duration of contact with the body. Parylene coatings have demonstrated excellent biocompatibility profiles, making them particularly suitable for implantable devices. Silicone and acrylic coatings also show favorable biological responses but require careful formulation to eliminate potentially harmful additives.
Leachable compounds present a significant safety concern in medical device coatings. Under physiological conditions, certain coating components may migrate from the device surface into surrounding tissues or bloodstream. Comprehensive extraction studies following ISO 10993-18 guidelines are essential to identify and quantify these substances. Manufacturers must establish acceptable limits for leachables based on toxicological risk assessments and ensure their coatings remain below these thresholds throughout the device lifecycle.
The sterilization compatibility of conformal coatings represents another crucial safety consideration. Medical devices typically undergo sterilization processes such as ethylene oxide treatment, gamma irradiation, or autoclave procedures. These methods can potentially degrade coating materials, compromising their protective functions or generating toxic byproducts. Coating selection must account for the intended sterilization method, with appropriate validation studies confirming material stability post-sterilization.
Long-term aging effects must also be evaluated when assessing coating safety. Environmental factors such as temperature fluctuations, humidity, and oxidative stress can accelerate coating degradation over time. Accelerated aging studies following ASTM F1980 protocols help predict coating performance throughout the device's expected service life. Particular attention should be given to potential changes in surface properties, barrier integrity, and biocompatibility characteristics during aging.
Regulatory bodies increasingly emphasize the importance of chemical characterization for conformal coatings. The FDA's guidance on the use of ISO 10993-18 recommends thorough material characterization to identify chemicals of concern before conducting biological tests. This approach, known as the chemical-first paradigm, helps manufacturers develop safer coating formulations by eliminating potentially harmful substances early in the development process.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!