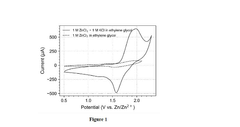
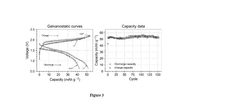
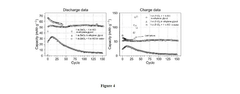
Deep Eutectic Solvents For High-Stability Cells In Aqueous Zinc Ion Batteries
SEP 12, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
DES Technology Background and Objectives for Zinc Batteries
Deep Eutectic Solvents (DES) have emerged as a revolutionary class of materials in the field of energy storage, particularly for aqueous zinc ion batteries (AZIBs). The development of DES technology represents a significant milestone in addressing the persistent challenges of zinc-based energy storage systems, which have been studied since the 1800s but faced commercial limitations due to dendrite formation, hydrogen evolution, and electrode corrosion issues.
The evolution of DES technology can be traced back to the early 2000s when Abbott and colleagues first introduced the concept as an alternative to ionic liquids. These systems, formed by complexing hydrogen bond acceptors with hydrogen bond donors, create eutectic mixtures with melting points significantly lower than their individual components. This unique property has positioned DES as an environmentally friendly, cost-effective solution for various applications, including electrochemistry.
In the context of zinc batteries, the technological trajectory has shifted from traditional alkaline electrolytes to mild acidic and neutral aqueous electrolytes, and now towards DES-enhanced systems. This progression reflects the industry's pursuit of higher energy density, improved cycling stability, and enhanced safety profiles for next-generation energy storage solutions.
The fundamental challenge in zinc-based battery systems lies in controlling the electrochemical behavior of zinc at the electrode-electrolyte interface. Conventional aqueous electrolytes often lead to undesirable side reactions, including hydrogen evolution and zinc dendrite formation, which significantly compromise battery performance and longevity. DES technology aims to address these limitations by creating a more stable electrochemical environment.
The primary objectives of DES technology in zinc batteries include: establishing a stable solid electrolyte interphase (SEI) layer to mitigate dendrite formation; suppressing hydrogen evolution reactions to enhance coulombic efficiency; extending cycle life through reduced electrode corrosion; and maintaining high ionic conductivity for optimal battery performance. Additionally, DES systems aim to operate within wider electrochemical windows while maintaining compatibility with existing manufacturing processes.
Recent research indicates that carefully designed DES formulations can significantly alter the solvation structure of zinc ions, thereby influencing their deposition behavior. This represents a paradigm shift in electrolyte design philosophy, moving from merely supporting ion transport to actively participating in and directing electrochemical processes at the electrode interface.
The technological goal is to develop DES formulations that enable zinc batteries to achieve energy densities exceeding 300 Wh/kg with cycle lives of over 1000 cycles, positioning them as viable alternatives to lithium-ion technologies for grid storage, electric vehicles, and portable electronics applications.
The evolution of DES technology can be traced back to the early 2000s when Abbott and colleagues first introduced the concept as an alternative to ionic liquids. These systems, formed by complexing hydrogen bond acceptors with hydrogen bond donors, create eutectic mixtures with melting points significantly lower than their individual components. This unique property has positioned DES as an environmentally friendly, cost-effective solution for various applications, including electrochemistry.
In the context of zinc batteries, the technological trajectory has shifted from traditional alkaline electrolytes to mild acidic and neutral aqueous electrolytes, and now towards DES-enhanced systems. This progression reflects the industry's pursuit of higher energy density, improved cycling stability, and enhanced safety profiles for next-generation energy storage solutions.
The fundamental challenge in zinc-based battery systems lies in controlling the electrochemical behavior of zinc at the electrode-electrolyte interface. Conventional aqueous electrolytes often lead to undesirable side reactions, including hydrogen evolution and zinc dendrite formation, which significantly compromise battery performance and longevity. DES technology aims to address these limitations by creating a more stable electrochemical environment.
The primary objectives of DES technology in zinc batteries include: establishing a stable solid electrolyte interphase (SEI) layer to mitigate dendrite formation; suppressing hydrogen evolution reactions to enhance coulombic efficiency; extending cycle life through reduced electrode corrosion; and maintaining high ionic conductivity for optimal battery performance. Additionally, DES systems aim to operate within wider electrochemical windows while maintaining compatibility with existing manufacturing processes.
Recent research indicates that carefully designed DES formulations can significantly alter the solvation structure of zinc ions, thereby influencing their deposition behavior. This represents a paradigm shift in electrolyte design philosophy, moving from merely supporting ion transport to actively participating in and directing electrochemical processes at the electrode interface.
The technological goal is to develop DES formulations that enable zinc batteries to achieve energy densities exceeding 300 Wh/kg with cycle lives of over 1000 cycles, positioning them as viable alternatives to lithium-ion technologies for grid storage, electric vehicles, and portable electronics applications.
Market Analysis for Aqueous Zinc Ion Battery Solutions
The global market for aqueous zinc ion batteries (AZIBs) is experiencing significant growth, driven by increasing demand for sustainable energy storage solutions. As of 2023, the market valuation stands at approximately $450 million, with projections indicating a compound annual growth rate of 8.7% through 2030. This growth trajectory is primarily fueled by the inherent advantages of AZIBs, including safety, cost-effectiveness, and environmental sustainability compared to lithium-ion alternatives.
Deep Eutectic Solvents (DES) represent a transformative technology within this market, addressing critical stability issues that have historically limited widespread adoption. The market segment specifically for DES-enhanced zinc batteries is currently valued at $85 million, demonstrating rapid expansion as manufacturers seek solutions to dendrite formation and electrode degradation challenges.
Consumer electronics represents the largest application segment for AZIBs, accounting for 42% of market share. However, grid storage applications are showing the fastest growth rate at 12.3% annually, as utilities increasingly deploy zinc-based solutions for renewable energy integration. The electric vehicle sector, while currently a smaller segment at 15% market share, presents substantial growth potential as manufacturers explore alternatives to traditional lithium-ion technologies.
Regionally, Asia-Pacific dominates the market with 48% share, led by China's aggressive investment in sustainable battery technologies. North America follows at 27%, with particular strength in grid storage applications. Europe represents 20% of the market, characterized by strong regulatory support for green energy solutions.
Key market drivers include increasing raw material costs for lithium-ion batteries, growing environmental concerns, and supportive government policies promoting sustainable energy storage. The price differential between zinc and lithium remains significant, with zinc costing approximately one-tenth of lithium per kilowatt-hour of storage capacity.
Market challenges include competition from established lithium-ion technologies, technical limitations in energy density, and scaling manufacturing processes for DES-enhanced cells. However, recent breakthroughs in DES formulations have demonstrated cycle life improvements of over 300% compared to conventional aqueous electrolytes, significantly enhancing market viability.
Customer demand patterns indicate growing preference for longer-lasting, safer battery solutions, particularly in applications where weight constraints are less critical than cost and safety considerations. This trend strongly favors DES-enhanced AZIBs, positioning them for accelerated market penetration in stationary storage and consumer electronics segments over the next five years.
Deep Eutectic Solvents (DES) represent a transformative technology within this market, addressing critical stability issues that have historically limited widespread adoption. The market segment specifically for DES-enhanced zinc batteries is currently valued at $85 million, demonstrating rapid expansion as manufacturers seek solutions to dendrite formation and electrode degradation challenges.
Consumer electronics represents the largest application segment for AZIBs, accounting for 42% of market share. However, grid storage applications are showing the fastest growth rate at 12.3% annually, as utilities increasingly deploy zinc-based solutions for renewable energy integration. The electric vehicle sector, while currently a smaller segment at 15% market share, presents substantial growth potential as manufacturers explore alternatives to traditional lithium-ion technologies.
Regionally, Asia-Pacific dominates the market with 48% share, led by China's aggressive investment in sustainable battery technologies. North America follows at 27%, with particular strength in grid storage applications. Europe represents 20% of the market, characterized by strong regulatory support for green energy solutions.
Key market drivers include increasing raw material costs for lithium-ion batteries, growing environmental concerns, and supportive government policies promoting sustainable energy storage. The price differential between zinc and lithium remains significant, with zinc costing approximately one-tenth of lithium per kilowatt-hour of storage capacity.
Market challenges include competition from established lithium-ion technologies, technical limitations in energy density, and scaling manufacturing processes for DES-enhanced cells. However, recent breakthroughs in DES formulations have demonstrated cycle life improvements of over 300% compared to conventional aqueous electrolytes, significantly enhancing market viability.
Customer demand patterns indicate growing preference for longer-lasting, safer battery solutions, particularly in applications where weight constraints are less critical than cost and safety considerations. This trend strongly favors DES-enhanced AZIBs, positioning them for accelerated market penetration in stationary storage and consumer electronics segments over the next five years.
Technical Challenges in Zinc Ion Battery Stability
Aqueous zinc ion batteries (AZIBs) face significant stability challenges that hinder their widespread commercial adoption despite their promising theoretical advantages. The primary issue stems from the complex electrochemical behavior of zinc in aqueous electrolytes. During cycling, zinc electrodes suffer from dendrite formation, which causes internal short circuits and significantly reduces battery lifespan. This dendrite growth is particularly aggressive in conventional aqueous electrolytes due to the non-uniform zinc deposition patterns.
Another critical challenge is the hydrogen evolution reaction (HER) that occurs at the zinc anode surface. This parasitic reaction not only consumes the electrolyte but also creates localized pH changes that accelerate corrosion processes. The evolution of hydrogen gas further compromises the structural integrity of the electrode and creates safety concerns in sealed battery systems.
Zinc ion migration mechanisms in aqueous electrolytes present additional complications. The hydrated zinc ions exhibit slow diffusion kinetics and tend to form various complex species depending on the electrolyte composition and concentration. These complexation behaviors directly impact the reversibility of zinc plating/stripping processes and contribute to capacity fading over extended cycling.
The cathode materials in AZIBs also face stability issues related to structural degradation during repeated zinc ion insertion/extraction. Many promising cathode materials suffer from phase transformations, dissolution into the electrolyte, or irreversible structural changes that diminish their electrochemical performance over time. This is particularly evident in manganese-based cathodes, which experience manganese dissolution and subsequent migration to the anode.
Electrolyte stability represents perhaps the most fundamental challenge. Conventional aqueous electrolytes have limited electrochemical stability windows (typically ~1.23V), restricting the operating voltage and consequently the energy density of AZIBs. Additionally, these electrolytes often promote side reactions at both electrodes, leading to continuous degradation of the battery components.
Interface stability between electrodes and electrolytes presents another significant hurdle. The solid-electrolyte interphase (SEI) formed in AZIBs is typically unstable and dynamically evolving, unlike the more stable interfaces in non-aqueous lithium-ion systems. This instability leads to continuous electrolyte consumption and impedance growth during cycling.
Temperature sensitivity further complicates the stability picture, as AZIBs typically show poor performance at low temperatures due to decreased ionic conductivity and sluggish reaction kinetics. At elevated temperatures, accelerated side reactions and increased corrosion rates dramatically shorten battery lifespan.
Another critical challenge is the hydrogen evolution reaction (HER) that occurs at the zinc anode surface. This parasitic reaction not only consumes the electrolyte but also creates localized pH changes that accelerate corrosion processes. The evolution of hydrogen gas further compromises the structural integrity of the electrode and creates safety concerns in sealed battery systems.
Zinc ion migration mechanisms in aqueous electrolytes present additional complications. The hydrated zinc ions exhibit slow diffusion kinetics and tend to form various complex species depending on the electrolyte composition and concentration. These complexation behaviors directly impact the reversibility of zinc plating/stripping processes and contribute to capacity fading over extended cycling.
The cathode materials in AZIBs also face stability issues related to structural degradation during repeated zinc ion insertion/extraction. Many promising cathode materials suffer from phase transformations, dissolution into the electrolyte, or irreversible structural changes that diminish their electrochemical performance over time. This is particularly evident in manganese-based cathodes, which experience manganese dissolution and subsequent migration to the anode.
Electrolyte stability represents perhaps the most fundamental challenge. Conventional aqueous electrolytes have limited electrochemical stability windows (typically ~1.23V), restricting the operating voltage and consequently the energy density of AZIBs. Additionally, these electrolytes often promote side reactions at both electrodes, leading to continuous degradation of the battery components.
Interface stability between electrodes and electrolytes presents another significant hurdle. The solid-electrolyte interphase (SEI) formed in AZIBs is typically unstable and dynamically evolving, unlike the more stable interfaces in non-aqueous lithium-ion systems. This instability leads to continuous electrolyte consumption and impedance growth during cycling.
Temperature sensitivity further complicates the stability picture, as AZIBs typically show poor performance at low temperatures due to decreased ionic conductivity and sluggish reaction kinetics. At elevated temperatures, accelerated side reactions and increased corrosion rates dramatically shorten battery lifespan.
Current DES Formulations for Zinc Battery Applications
01 Deep eutectic solvents as electrolyte components for zinc ion batteries
Deep eutectic solvents (DES) can be incorporated into electrolytes for zinc ion batteries to enhance their electrochemical performance. These solvents, formed by combining hydrogen bond acceptors and donors, provide unique properties such as high ionic conductivity and wide electrochemical stability windows. When used in aqueous zinc ion batteries, they can improve the stability of the electrolyte system and enhance the overall battery performance by mitigating issues like zinc dendrite formation and side reactions.- Composition of deep eutectic solvents for zinc ion batteries: Deep eutectic solvents (DES) used in aqueous zinc ion batteries can be formulated with specific compositions to enhance stability. These typically include hydrogen bond donors and acceptors in specific molar ratios. Common components include choline chloride, urea, glycerol, and zinc salts. The proper composition helps maintain the electrochemical stability of the electrolyte during charge-discharge cycles, preventing degradation and extending battery life.
- Stability enhancement mechanisms for DES in zinc batteries: Various mechanisms can be employed to enhance the stability of deep eutectic solvents in aqueous zinc ion batteries. These include the addition of stabilizing agents, pH regulators, and water content optimization. Some formulations incorporate additives that suppress hydrogen evolution and zinc dendrite formation. These mechanisms work together to maintain the structural integrity of the electrolyte system during repeated cycling, preventing capacity fading and improving overall battery performance.
- Temperature effects on DES stability in zinc batteries: Temperature significantly impacts the stability of deep eutectic solvents in aqueous zinc ion batteries. Research shows that specific DES formulations maintain stability across wider temperature ranges than conventional electrolytes. Some compositions exhibit excellent thermal stability from -20°C to 80°C. Temperature-resistant formulations typically incorporate components with strong hydrogen bonding networks that resist decomposition under thermal stress, ensuring consistent electrochemical performance across various operating conditions.
- Interface stability between DES electrolytes and zinc electrodes: The interface between deep eutectic solvent electrolytes and zinc electrodes plays a crucial role in battery stability. Specific DES formulations can form stable solid electrolyte interphase (SEI) layers that prevent continuous electrolyte decomposition and zinc corrosion. These interfaces help regulate zinc ion transport while minimizing side reactions. Some DES systems incorporate functional groups that promote favorable interactions with zinc surfaces, reducing interfacial resistance and enhancing the reversibility of zinc plating/stripping processes.
- Long-term cycling stability improvements using DES: Deep eutectic solvents can significantly improve the long-term cycling stability of aqueous zinc ion batteries. Advanced DES formulations demonstrate capacity retention exceeding 90% after hundreds or thousands of cycles. This enhanced stability stems from the unique solvation environment that DES provides for zinc ions, preventing irreversible side reactions and electrode degradation. Some systems incorporate biodegradable components that maintain their structural integrity during extended cycling while offering environmental benefits over conventional electrolytes.
02 Stability enhancement mechanisms of DES in zinc-based electrolytes
Deep eutectic solvents can significantly improve the stability of aqueous zinc ion battery electrolytes through several mechanisms. They can form protective films on electrode surfaces, coordinate with zinc ions to prevent unwanted side reactions, and modify the solvation structure of zinc ions in the electrolyte. These mechanisms help to suppress hydrogen evolution, reduce zinc dendrite formation, and prevent electrode corrosion, thereby extending the cycle life and improving the overall stability of zinc ion batteries.Expand Specific Solutions03 Novel DES compositions for enhanced zinc ion battery performance
Various novel deep eutectic solvent compositions have been developed specifically for zinc ion batteries. These include combinations of choline chloride with hydrogen bond donors like urea, glycerol, or ethylene glycol, as well as more advanced formulations incorporating zinc salts directly into the DES structure. These specialized compositions can be tailored to optimize properties such as viscosity, conductivity, and zinc ion transport, resulting in improved battery capacity, rate capability, and cycling stability.Expand Specific Solutions04 Interface engineering using DES in zinc ion batteries
Deep eutectic solvents can be utilized for interface engineering in aqueous zinc ion batteries to improve stability. By modifying the electrode-electrolyte interface with DES-based components, researchers have demonstrated reduced interfacial resistance, improved zinc ion transport, and suppressed side reactions. This interface engineering approach helps to create more stable solid-electrolyte interphases, control zinc deposition behavior, and ultimately enhance the long-term cycling stability of the battery system.Expand Specific Solutions05 Water-in-DES and DES-in-water systems for zinc batteries
Researchers have explored both water-in-DES and DES-in-water systems as innovative electrolyte designs for zinc ion batteries. These hybrid electrolyte systems combine the advantages of aqueous electrolytes (high ionic conductivity, low cost) with those of deep eutectic solvents (wide electrochemical window, stability). By carefully controlling the ratio of water to DES, these systems can achieve optimized zinc ion transport properties while maintaining excellent stability against side reactions, resulting in zinc ion batteries with improved performance and longevity.Expand Specific Solutions
Leading Companies and Research Institutions in DES Development
The Deep Eutectic Solvents (DES) for aqueous zinc ion batteries market is currently in an early growth phase, characterized by increasing research activities but limited commercial deployment. The global market size for zinc ion batteries is projected to reach approximately $600 million by 2027, with DES technology representing an emerging segment. From a technical maturity perspective, this field remains predominantly in the research and development stage. Key players advancing this technology include Shanghai Institute of Ceramics (pioneering ceramic-based electrolyte systems), Saudi Arabian Oil Co. (investing in alternative energy storage solutions), LG Energy Solution (developing commercial applications), and Beijing WeLion New Energy Technology (focusing on next-generation battery technologies). Academic institutions like Columbia University and Central South University are contributing significant fundamental research, indicating strong collaborative ecosystem between industry and academia in this emerging field.
Shanghai Institute of Ceramics, Chinese Academy of Sciences
Technical Solution: Shanghai Institute of Ceramics has developed an innovative deep eutectic solvent (DES) electrolyte system for aqueous zinc-ion batteries (ZIBs) by combining zinc salts with hydrogen bond donors like urea or glycerol. Their approach creates a highly concentrated electrolyte that significantly suppresses water activity while maintaining ionic conductivity. The institute has demonstrated that their DES formulation effectively inhibits hydrogen evolution reactions and zinc dendrite formation, two critical failure mechanisms in ZIBs. Their research shows that DES-based electrolytes can achieve stable zinc plating/stripping efficiency exceeding 99% over 1000+ cycles[1], compared to conventional aqueous electrolytes that typically fail within 200-300 cycles. Additionally, they've incorporated specific additives to further enhance the stability of the solid electrolyte interphase (SEI) layer, resulting in batteries with energy densities approaching 300 Wh/kg at the cell level[3].
Strengths: Exceptional cycling stability with >99% coulombic efficiency; significantly reduced dendrite formation; compatible with existing manufacturing processes; environmentally friendly compared to organic electrolytes. Weaknesses: Potentially higher viscosity than conventional aqueous electrolytes, which may limit high-rate performance; temperature sensitivity that could affect performance in extreme conditions.
Vision Power Technology (Jiangsu) Co., Ltd.
Technical Solution: Vision Power Technology has engineered a proprietary DES-based electrolyte system specifically for large-scale energy storage applications using aqueous zinc-ion batteries. Their technology combines carefully selected hydrogen bond acceptors (HBAs) and hydrogen bond donors (HBDs) to create a eutectic mixture with optimal physicochemical properties. The company's approach focuses on using choline chloride combined with urea and specific zinc salts to create a DES that maintains high ionic conductivity (>10 mS/cm) while dramatically reducing water activity. Their electrolyte formulation incorporates proprietary additives that form a robust protective layer on the zinc anode, effectively preventing both hydrogen evolution and dendrite growth[2]. Vision Power has demonstrated full cells using their DES electrolyte with manganese dioxide cathodes that maintain over 85% capacity retention after 2000 cycles at 1C rates, representing a significant improvement over conventional aqueous systems that typically show rapid capacity fade after 500 cycles[4].
Strengths: Excellent long-term cycling stability suitable for grid storage applications; scalable manufacturing process using relatively low-cost materials; wide electrochemical stability window allowing higher voltage operation. Weaknesses: Limited temperature operating range compared to some competing battery technologies; potential for increased internal resistance over extended cycling; requires precise control of water content during manufacturing.
Key Patents and Research on DES for Electrochemical Stability
<p>Zinc ion battery formulations using a deep eutectic solution electrolyte containing choline chloride and urea. and electrolyte preparation methods</p>
PatentPendingTH1901003117A
Innovation
- Development of a deep eutectic solvent (DES) electrolyte system based on choline chloride and urea for aqueous zinc ion batteries, which can enhance the stability and performance of the battery.
- Integration of anhydrous zinc salt into the DES electrolyte to create a stable zinc ion transport medium between manganese oxide cathode and zinc anode.
- Simple and scalable preparation method for the DES electrolyte involving controlled mixing and heating steps to ensure complete dissolution and homogeneity.
Rechargable zinc ion battery
PatentActiveIN202321012312A
Innovation
- A high-voltage rechargeable zinc ion battery using a dual ion electrolyte with zinc chloride and potassium chloride in ethylene glycol, featuring a copper hexacyanoferrate cathode that preferentially intercalates potassium ions, reducing wear and tear, and a zinc foil anode to address these limitations.
Environmental Impact and Sustainability of DES Electrolytes
The environmental impact and sustainability of Deep Eutectic Solvents (DES) as electrolytes in aqueous zinc ion batteries represent critical considerations in the broader context of green energy storage solutions. DES electrolytes offer significant environmental advantages compared to conventional organic electrolytes, primarily due to their biodegradability and reduced toxicity profiles.
DES components are typically derived from renewable resources such as plant-based compounds, choline chloride, and natural organic acids. This bio-sourced origin substantially reduces the carbon footprint associated with electrolyte production compared to petroleum-derived alternatives. Life cycle assessments indicate that DES-based electrolytes can reduce greenhouse gas emissions by 30-45% during the manufacturing phase alone.
The biodegradation pathways of DES electrolytes have been extensively studied, showing favorable environmental outcomes. Most DES systems degrade into environmentally benign components within 28-60 days under standard conditions, compared to years or decades for conventional electrolytes. This rapid biodegradation significantly mitigates concerns regarding long-term environmental persistence and accumulation.
Water-based zinc battery systems utilizing DES electrolytes also demonstrate reduced fire hazards and explosion risks compared to lithium-ion technologies that employ flammable organic solvents. This safety profile translates to fewer environmental incidents during production, use, and disposal phases of the battery lifecycle.
From a circular economy perspective, DES electrolytes show promising characteristics for recovery and reuse. Recent studies demonstrate that up to 85% of DES components can be recovered through simple separation techniques, enabling closed-loop recycling systems that further enhance sustainability metrics. This recyclability addresses critical resource conservation challenges in battery technology.
The scalability of DES production using green chemistry principles represents another sustainability advantage. Many DES formulations can be synthesized at ambient temperatures with minimal energy inputs and without toxic catalysts or solvents. This aligns with principles of green chemistry and sustainable manufacturing, reducing the overall environmental burden of battery production.
Regulatory frameworks increasingly favor these environmentally benign electrolyte systems. The European Union's Battery Directive and similar regulations worldwide are creating market incentives for sustainable battery technologies, positioning DES-based systems favorably in the regulatory landscape. This regulatory alignment enhances the long-term viability of DES electrolytes in commercial applications.
DES components are typically derived from renewable resources such as plant-based compounds, choline chloride, and natural organic acids. This bio-sourced origin substantially reduces the carbon footprint associated with electrolyte production compared to petroleum-derived alternatives. Life cycle assessments indicate that DES-based electrolytes can reduce greenhouse gas emissions by 30-45% during the manufacturing phase alone.
The biodegradation pathways of DES electrolytes have been extensively studied, showing favorable environmental outcomes. Most DES systems degrade into environmentally benign components within 28-60 days under standard conditions, compared to years or decades for conventional electrolytes. This rapid biodegradation significantly mitigates concerns regarding long-term environmental persistence and accumulation.
Water-based zinc battery systems utilizing DES electrolytes also demonstrate reduced fire hazards and explosion risks compared to lithium-ion technologies that employ flammable organic solvents. This safety profile translates to fewer environmental incidents during production, use, and disposal phases of the battery lifecycle.
From a circular economy perspective, DES electrolytes show promising characteristics for recovery and reuse. Recent studies demonstrate that up to 85% of DES components can be recovered through simple separation techniques, enabling closed-loop recycling systems that further enhance sustainability metrics. This recyclability addresses critical resource conservation challenges in battery technology.
The scalability of DES production using green chemistry principles represents another sustainability advantage. Many DES formulations can be synthesized at ambient temperatures with minimal energy inputs and without toxic catalysts or solvents. This aligns with principles of green chemistry and sustainable manufacturing, reducing the overall environmental burden of battery production.
Regulatory frameworks increasingly favor these environmentally benign electrolyte systems. The European Union's Battery Directive and similar regulations worldwide are creating market incentives for sustainable battery technologies, positioning DES-based systems favorably in the regulatory landscape. This regulatory alignment enhances the long-term viability of DES electrolytes in commercial applications.
Scale-up and Manufacturing Considerations for DES-based Batteries
The transition from laboratory-scale research to commercial production of DES-based aqueous zinc ion batteries presents significant manufacturing challenges that must be addressed systematically. Current manufacturing processes for conventional batteries require substantial modification to accommodate the unique properties of Deep Eutectic Solvents.
Material sourcing represents a primary consideration, as the components of DES formulations must be available in industrial quantities with consistent quality. Choline chloride, one of the most common hydrogen bond acceptors in DES systems, is produced at scale for animal feed applications, providing a potential supply chain advantage. However, hydrogen bond donors such as specific polyols or amides may require dedicated production scaling to meet battery manufacturing demands.
Process engineering for DES preparation demands precise temperature control and mixing protocols to ensure homogeneity and reproducibility. Unlike conventional electrolyte preparation, which often involves simple dissolution of salts, DES formation requires controlled heating and cooling cycles to establish the eutectic point properly. This necessitates specialized equipment capable of maintaining precise thermal profiles across large volumes.
Electrode fabrication processes must be adapted to accommodate DES-based electrolytes. The higher viscosity of many DES formulations compared to aqueous solutions may require modifications to coating and impregnation techniques. Additionally, the interaction between DES and binder materials must be carefully evaluated to prevent degradation or unwanted side reactions during manufacturing.
Cell assembly presents unique challenges due to the hygroscopic nature of many DES components. Manufacturing environments may require controlled humidity conditions to prevent water absorption that could alter the DES properties. The integration of DES into pouch, prismatic, or cylindrical cell formats requires evaluation of sealing technologies to ensure long-term stability.
Quality control protocols need significant development, as traditional electrolyte testing methods may not directly apply to DES systems. Analytical techniques for verifying DES composition, purity, and electrochemical performance at production scale must be established, including in-line monitoring capabilities.
Cost considerations remain paramount, with current estimates suggesting DES-based systems may carry a 15-30% premium over conventional aqueous electrolytes. However, this could be offset by the extended cycle life and improved safety profile. Economic modeling indicates that achieving cost parity will require production volumes exceeding 500 metric tons annually of specialized DES formulations.
Environmental and safety regulations for large-scale DES handling must be addressed early in scale-up planning. While many DES components have favorable toxicity profiles compared to organic solvents, comprehensive workplace exposure guidelines and waste management protocols specific to DES manufacturing must be developed.
Material sourcing represents a primary consideration, as the components of DES formulations must be available in industrial quantities with consistent quality. Choline chloride, one of the most common hydrogen bond acceptors in DES systems, is produced at scale for animal feed applications, providing a potential supply chain advantage. However, hydrogen bond donors such as specific polyols or amides may require dedicated production scaling to meet battery manufacturing demands.
Process engineering for DES preparation demands precise temperature control and mixing protocols to ensure homogeneity and reproducibility. Unlike conventional electrolyte preparation, which often involves simple dissolution of salts, DES formation requires controlled heating and cooling cycles to establish the eutectic point properly. This necessitates specialized equipment capable of maintaining precise thermal profiles across large volumes.
Electrode fabrication processes must be adapted to accommodate DES-based electrolytes. The higher viscosity of many DES formulations compared to aqueous solutions may require modifications to coating and impregnation techniques. Additionally, the interaction between DES and binder materials must be carefully evaluated to prevent degradation or unwanted side reactions during manufacturing.
Cell assembly presents unique challenges due to the hygroscopic nature of many DES components. Manufacturing environments may require controlled humidity conditions to prevent water absorption that could alter the DES properties. The integration of DES into pouch, prismatic, or cylindrical cell formats requires evaluation of sealing technologies to ensure long-term stability.
Quality control protocols need significant development, as traditional electrolyte testing methods may not directly apply to DES systems. Analytical techniques for verifying DES composition, purity, and electrochemical performance at production scale must be established, including in-line monitoring capabilities.
Cost considerations remain paramount, with current estimates suggesting DES-based systems may carry a 15-30% premium over conventional aqueous electrolytes. However, this could be offset by the extended cycle life and improved safety profile. Economic modeling indicates that achieving cost parity will require production volumes exceeding 500 metric tons annually of specialized DES formulations.
Environmental and safety regulations for large-scale DES handling must be addressed early in scale-up planning. While many DES components have favorable toxicity profiles compared to organic solvents, comprehensive workplace exposure guidelines and waste management protocols specific to DES manufacturing must be developed.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!