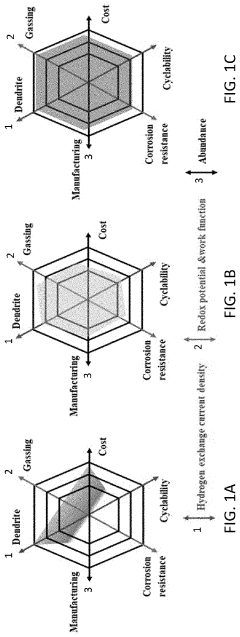
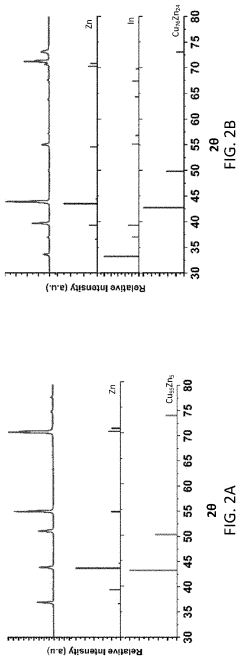
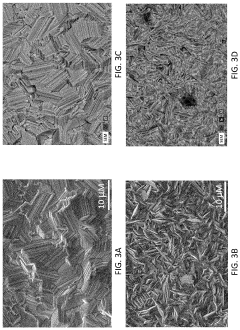
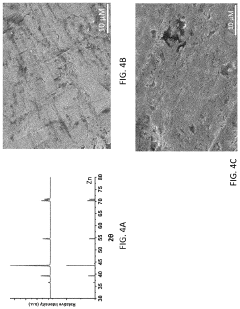
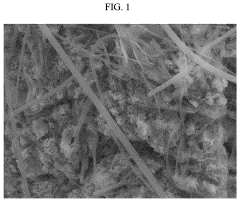
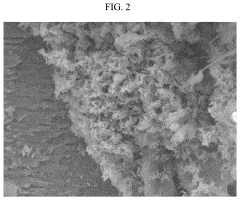
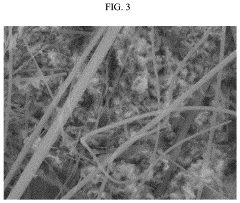
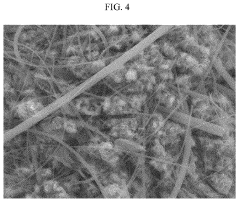
Failure Analysis And Reliability Engineering For Aqueous Zinc Ion Batteries
SEP 12, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Zinc Ion Battery Failure Mechanisms and Research Objectives
Aqueous zinc ion batteries (AZIBs) have emerged as promising candidates for next-generation energy storage systems due to their high safety, low cost, and environmental friendliness. However, their widespread commercial application is hindered by several failure mechanisms that compromise their performance and longevity. Understanding these failure modes is crucial for developing reliable and durable zinc-based energy storage solutions.
The evolution of zinc ion batteries can be traced back to the traditional zinc-carbon and alkaline batteries, with significant advancements occurring in the past decade as researchers sought alternatives to lithium-ion technology. Recent years have witnessed accelerated development in electrode materials, electrolyte formulations, and battery architectures specifically designed for zinc ion storage. This technological progression has been driven by the increasing demand for safe, sustainable, and cost-effective energy storage solutions for grid applications and portable electronics.
A primary challenge in AZIBs is zinc dendrite formation during cycling, which can lead to internal short circuits and capacity fading. This phenomenon is exacerbated by the inherent properties of aqueous electrolytes and the complex electrochemical behavior of zinc metal anodes. Additionally, hydrogen evolution reactions, electrode pulverization, and cathode dissolution represent significant failure mechanisms that require systematic investigation and mitigation strategies.
The corrosion of zinc anodes in aqueous electrolytes presents another critical challenge, as it leads to self-discharge and reduced coulombic efficiency. This process is influenced by electrolyte composition, pH, and operating conditions, necessitating comprehensive studies to establish correlations between these parameters and battery performance. Furthermore, the passivation layer formation on electrode surfaces significantly impacts ion transport kinetics and overall battery efficiency.
The research objectives in this field are multifaceted, aiming to develop fundamental understanding and practical solutions. Key goals include elucidating the mechanisms of zinc dendrite growth and developing effective suppression strategies, designing stable electrolyte systems that minimize side reactions, and creating robust cathode materials resistant to dissolution and structural degradation during long-term cycling.
Advanced characterization techniques, including in-situ and operando methods, are essential for monitoring failure processes in real-time and under realistic operating conditions. Computational modeling and simulation approaches complement experimental investigations by providing atomic-level insights into degradation mechanisms and guiding the rational design of improved battery components.
Ultimately, this research aims to establish design principles and engineering guidelines for high-performance AZIBs with enhanced cycle life, rate capability, and operational stability across diverse environmental conditions and use scenarios.
The evolution of zinc ion batteries can be traced back to the traditional zinc-carbon and alkaline batteries, with significant advancements occurring in the past decade as researchers sought alternatives to lithium-ion technology. Recent years have witnessed accelerated development in electrode materials, electrolyte formulations, and battery architectures specifically designed for zinc ion storage. This technological progression has been driven by the increasing demand for safe, sustainable, and cost-effective energy storage solutions for grid applications and portable electronics.
A primary challenge in AZIBs is zinc dendrite formation during cycling, which can lead to internal short circuits and capacity fading. This phenomenon is exacerbated by the inherent properties of aqueous electrolytes and the complex electrochemical behavior of zinc metal anodes. Additionally, hydrogen evolution reactions, electrode pulverization, and cathode dissolution represent significant failure mechanisms that require systematic investigation and mitigation strategies.
The corrosion of zinc anodes in aqueous electrolytes presents another critical challenge, as it leads to self-discharge and reduced coulombic efficiency. This process is influenced by electrolyte composition, pH, and operating conditions, necessitating comprehensive studies to establish correlations between these parameters and battery performance. Furthermore, the passivation layer formation on electrode surfaces significantly impacts ion transport kinetics and overall battery efficiency.
The research objectives in this field are multifaceted, aiming to develop fundamental understanding and practical solutions. Key goals include elucidating the mechanisms of zinc dendrite growth and developing effective suppression strategies, designing stable electrolyte systems that minimize side reactions, and creating robust cathode materials resistant to dissolution and structural degradation during long-term cycling.
Advanced characterization techniques, including in-situ and operando methods, are essential for monitoring failure processes in real-time and under realistic operating conditions. Computational modeling and simulation approaches complement experimental investigations by providing atomic-level insights into degradation mechanisms and guiding the rational design of improved battery components.
Ultimately, this research aims to establish design principles and engineering guidelines for high-performance AZIBs with enhanced cycle life, rate capability, and operational stability across diverse environmental conditions and use scenarios.
Market Demand Analysis for Aqueous Zinc Ion Battery Technologies
The global energy storage market is witnessing a significant shift towards sustainable and efficient battery technologies, with aqueous zinc ion batteries (AZIBs) emerging as a promising alternative to conventional lithium-ion systems. Market analysis indicates that the global energy storage market is projected to reach $546 billion by 2035, with a compound annual growth rate of approximately 20% between 2023 and 2035. Within this expanding landscape, AZIBs are positioned to capture a growing market share due to their inherent advantages in safety, cost, and environmental impact.
The demand for AZIBs is primarily driven by several key factors. First, the increasing focus on grid-scale energy storage solutions to support renewable energy integration has created a substantial market opportunity. Grid operators and utility companies are actively seeking battery technologies that offer long cycle life, high safety profiles, and cost-effectiveness for large-scale deployment. AZIBs, with their non-flammable aqueous electrolytes and abundant zinc resources, present a compelling value proposition for this segment.
Industrial and commercial sectors represent another significant market for AZIBs, particularly in applications requiring uninterruptible power supplies and backup systems. The telecommunications industry, data centers, and manufacturing facilities are increasingly adopting energy storage solutions that minimize fire risks while maintaining reliable performance. Market research indicates that these sectors collectively account for approximately 30% of the current energy storage demand, with annual growth rates exceeding 15%.
Consumer electronics and portable power applications constitute an emerging market segment for AZIBs. As consumers become more environmentally conscious, the demand for batteries with reduced environmental footprint and improved safety characteristics is rising. Market surveys reveal that 67% of consumers express willingness to pay premium prices for devices powered by safer and more sustainable battery technologies.
The electric vehicle (EV) sector presents both challenges and opportunities for AZIBs. While lithium-ion batteries currently dominate this market, concerns regarding resource scarcity, safety incidents, and environmental impact are driving research into alternative technologies. AZIBs, with further improvements in energy density and charging capabilities, could potentially address specific segments within the EV market, particularly in applications where safety and cost considerations outweigh energy density requirements.
Geographically, the Asia-Pacific region leads in AZIB market potential, driven by substantial investments in renewable energy infrastructure and manufacturing capabilities in China, Japan, and South Korea. North America and Europe follow closely, with increasing policy support for sustainable energy technologies and growing industrial demand for reliable energy storage solutions.
The demand for AZIBs is primarily driven by several key factors. First, the increasing focus on grid-scale energy storage solutions to support renewable energy integration has created a substantial market opportunity. Grid operators and utility companies are actively seeking battery technologies that offer long cycle life, high safety profiles, and cost-effectiveness for large-scale deployment. AZIBs, with their non-flammable aqueous electrolytes and abundant zinc resources, present a compelling value proposition for this segment.
Industrial and commercial sectors represent another significant market for AZIBs, particularly in applications requiring uninterruptible power supplies and backup systems. The telecommunications industry, data centers, and manufacturing facilities are increasingly adopting energy storage solutions that minimize fire risks while maintaining reliable performance. Market research indicates that these sectors collectively account for approximately 30% of the current energy storage demand, with annual growth rates exceeding 15%.
Consumer electronics and portable power applications constitute an emerging market segment for AZIBs. As consumers become more environmentally conscious, the demand for batteries with reduced environmental footprint and improved safety characteristics is rising. Market surveys reveal that 67% of consumers express willingness to pay premium prices for devices powered by safer and more sustainable battery technologies.
The electric vehicle (EV) sector presents both challenges and opportunities for AZIBs. While lithium-ion batteries currently dominate this market, concerns regarding resource scarcity, safety incidents, and environmental impact are driving research into alternative technologies. AZIBs, with further improvements in energy density and charging capabilities, could potentially address specific segments within the EV market, particularly in applications where safety and cost considerations outweigh energy density requirements.
Geographically, the Asia-Pacific region leads in AZIB market potential, driven by substantial investments in renewable energy infrastructure and manufacturing capabilities in China, Japan, and South Korea. North America and Europe follow closely, with increasing policy support for sustainable energy technologies and growing industrial demand for reliable energy storage solutions.
Current Challenges in Zinc Ion Battery Reliability Engineering
Despite significant advancements in aqueous zinc ion battery (AZIB) technology, several critical reliability challenges persist that impede their widespread commercial adoption. The most prominent issue is dendrite formation during cycling, where zinc deposits unevenly on the anode surface, creating branch-like structures that can penetrate separators and cause short circuits. This phenomenon significantly reduces battery lifespan and poses safety risks that must be addressed before large-scale deployment.
Corrosion and passivation of zinc anodes represent another major challenge. When in contact with aqueous electrolytes, zinc undergoes parasitic side reactions that form insulating layers on the electrode surface, increasing internal resistance and decreasing capacity retention. These processes accelerate at higher temperatures and during extended storage periods, complicating real-world applications where batteries may experience varied environmental conditions.
Electrolyte stability presents ongoing difficulties, particularly regarding pH drift and water decomposition during cycling. As AZIBs operate, electrolyte composition gradually changes, affecting zinc dissolution/deposition kinetics and overall cell performance. Current electrolyte formulations struggle to maintain consistent properties throughout hundreds of cycles, especially at elevated temperatures or high current densities.
Cathode material degradation manifests through several mechanisms, including structural collapse during repeated ion insertion/extraction, dissolution of active materials into the electrolyte, and surface film formation that blocks ion transport pathways. These processes contribute to capacity fading and voltage decay, particularly in manganese-based cathodes where Mn dissolution remains problematic.
Self-discharge rates in AZIBs exceed those of commercial lithium-ion batteries, limiting their suitability for applications requiring long-term energy storage. The thermodynamic instability of charged states in many zinc battery chemistries results in gradual capacity loss even during idle periods, necessitating improved electrode and electrolyte designs to mitigate this effect.
Scale-up and manufacturing consistency pose significant engineering challenges. Laboratory-scale cells often demonstrate promising performance metrics that prove difficult to maintain in larger formats due to heat distribution issues, electrolyte gradient formation, and mechanical stresses. Establishing reliable quality control parameters and accelerated testing protocols specific to zinc battery chemistry remains underdeveloped compared to lithium-ion battery manufacturing.
Standardized testing protocols for AZIBs are still evolving, making cross-comparison between different research reports challenging. The reliability engineering community lacks consensus on appropriate stress tests, failure criteria, and lifetime prediction models specifically calibrated for zinc-based systems, hampering systematic improvement efforts.
Corrosion and passivation of zinc anodes represent another major challenge. When in contact with aqueous electrolytes, zinc undergoes parasitic side reactions that form insulating layers on the electrode surface, increasing internal resistance and decreasing capacity retention. These processes accelerate at higher temperatures and during extended storage periods, complicating real-world applications where batteries may experience varied environmental conditions.
Electrolyte stability presents ongoing difficulties, particularly regarding pH drift and water decomposition during cycling. As AZIBs operate, electrolyte composition gradually changes, affecting zinc dissolution/deposition kinetics and overall cell performance. Current electrolyte formulations struggle to maintain consistent properties throughout hundreds of cycles, especially at elevated temperatures or high current densities.
Cathode material degradation manifests through several mechanisms, including structural collapse during repeated ion insertion/extraction, dissolution of active materials into the electrolyte, and surface film formation that blocks ion transport pathways. These processes contribute to capacity fading and voltage decay, particularly in manganese-based cathodes where Mn dissolution remains problematic.
Self-discharge rates in AZIBs exceed those of commercial lithium-ion batteries, limiting their suitability for applications requiring long-term energy storage. The thermodynamic instability of charged states in many zinc battery chemistries results in gradual capacity loss even during idle periods, necessitating improved electrode and electrolyte designs to mitigate this effect.
Scale-up and manufacturing consistency pose significant engineering challenges. Laboratory-scale cells often demonstrate promising performance metrics that prove difficult to maintain in larger formats due to heat distribution issues, electrolyte gradient formation, and mechanical stresses. Establishing reliable quality control parameters and accelerated testing protocols specific to zinc battery chemistry remains underdeveloped compared to lithium-ion battery manufacturing.
Standardized testing protocols for AZIBs are still evolving, making cross-comparison between different research reports challenging. The reliability engineering community lacks consensus on appropriate stress tests, failure criteria, and lifetime prediction models specifically calibrated for zinc-based systems, hampering systematic improvement efforts.
Current Failure Analysis Methodologies for Aqueous Zinc Batteries
01 Electrolyte composition and stability for aqueous zinc ion batteries
The electrolyte composition plays a crucial role in the stability and reliability of aqueous zinc ion batteries. Various additives and formulations can be used to mitigate issues such as zinc dendrite formation, hydrogen evolution, and electrode corrosion. Optimized electrolyte compositions can effectively extend the cycle life and improve the overall reliability of the battery system by controlling the zinc ion transport and deposition behavior.- Electrolyte degradation and dendrite formation mechanisms: Aqueous zinc ion batteries often fail due to electrolyte degradation and zinc dendrite formation during cycling. The formation of dendrites can lead to internal short circuits and battery failure. Research focuses on understanding the mechanisms behind dendrite growth and developing methods to suppress it, such as electrolyte additives and surface modifications that promote uniform zinc deposition. Advanced characterization techniques are used to analyze the degradation processes and failure modes in real-time.
- Electrode material stability and corrosion resistance: The stability of electrode materials in aqueous environments is critical for zinc ion battery reliability. Corrosion of zinc anodes and degradation of cathode materials can significantly reduce battery lifespan. Research focuses on developing corrosion-resistant electrode materials and protective coatings that maintain structural integrity during repeated charge-discharge cycles. Novel composite materials and surface treatments are being investigated to enhance the electrochemical stability and extend the cycle life of aqueous zinc ion batteries.
- Advanced diagnostic and testing methodologies: Comprehensive failure analysis of aqueous zinc ion batteries requires advanced diagnostic techniques and standardized testing protocols. In-situ and ex-situ characterization methods, including electrochemical impedance spectroscopy, X-ray diffraction, and electron microscopy, are employed to identify failure mechanisms at different stages of battery life. Accelerated aging tests and post-mortem analyses help predict battery reliability and identify critical failure points, enabling the development of more robust battery designs.
- Interface engineering and separator optimization: The electrode-electrolyte interface plays a crucial role in the performance and reliability of aqueous zinc ion batteries. Interface engineering approaches, including the development of artificial solid-electrolyte interphases and functional separators, can mitigate side reactions and improve cycling stability. Optimized separator designs prevent internal short circuits while maintaining efficient ion transport. These advancements help address common failure modes related to interface degradation and enhance overall battery reliability.
- Environmental factors and operating condition impacts: Environmental factors and operating conditions significantly affect the reliability of aqueous zinc ion batteries. Temperature fluctuations, humidity levels, and charge-discharge rates can accelerate degradation mechanisms and lead to premature failure. Research focuses on understanding these environmental impacts and developing batteries with wider operating windows. Thermal management strategies and adaptive control systems are being investigated to maintain optimal performance under varying conditions, improving the overall reliability and safety of aqueous zinc ion battery systems.
02 Electrode material degradation mechanisms and prevention
Electrode materials in aqueous zinc ion batteries are susceptible to various degradation mechanisms including dissolution, structural collapse, and passivation. Understanding these failure modes is essential for improving battery reliability. Advanced electrode materials and protective coatings can be designed to mitigate these issues, enhancing the structural stability during charge-discharge cycles and preventing capacity fading over extended use.Expand Specific Solutions03 Interface engineering for improved reliability
The electrode-electrolyte interface is a critical region where many failure mechanisms originate in aqueous zinc ion batteries. Interface engineering approaches, such as protective layers, functional separators, and surface modifications, can significantly improve battery reliability by controlling zinc ion transport, preventing side reactions, and stabilizing the electrode surface during cycling, thereby extending battery lifespan and performance.Expand Specific Solutions04 Advanced testing and failure analysis methodologies
Comprehensive testing protocols and advanced analytical techniques are essential for understanding failure mechanisms in aqueous zinc ion batteries. In-situ and ex-situ characterization methods, accelerated aging tests, and post-mortem analysis can reveal degradation pathways and failure modes. These methodologies help in identifying reliability issues and guiding the development of more robust battery systems with improved performance and longevity.Expand Specific Solutions05 Novel battery designs for enhanced reliability
Innovative battery architectures and system-level designs can significantly improve the reliability of aqueous zinc ion batteries. These include novel cell configurations, advanced current collectors, optimized separators, and integrated battery management systems. Such designs can effectively address common failure modes by managing heat generation, controlling pressure, preventing short circuits, and enabling more uniform zinc deposition, thereby enhancing overall battery safety and reliability.Expand Specific Solutions
Key Industry Players in Zinc Ion Battery Development
Aqueous Zinc Ion Batteries (AZIBs) are currently in the early commercialization phase, with a rapidly growing market projected to reach significant scale by 2030 as demand for sustainable energy storage solutions increases. The technology is approaching maturity but still faces critical reliability and failure challenges that require advanced engineering solutions. Leading academic institutions like Northwestern University, KAIST, and Zhejiang University are driving fundamental research, while commercial players are emerging at different stages of development. Chinese companies including CATL, Svolt Energy, and Hefei Guoxuan are leveraging their battery manufacturing expertise to accelerate AZIB development, while specialized firms like Octet Scientific are focusing on chemistry optimization. Collaborative research between universities and industry partners is addressing key failure mechanisms and reliability issues to enable broader commercial adoption.
Northwestern University
Technical Solution: Northwestern University has developed a comprehensive reliability engineering framework for aqueous zinc ion batteries (AZIBs) centered on understanding and mitigating interfacial degradation mechanisms. Their research team has pioneered advanced cryogenic electron microscopy techniques that preserve the native state of zinc-electrolyte interfaces, revealing previously undetected early-stage dendrite nucleation processes[1]. This approach has enabled them to identify critical current density thresholds above which dendrite growth accelerates exponentially, establishing operational safety limits for various electrode architectures. Northwestern's failure analysis methodology incorporates multi-modal characterization combining electrochemical, spectroscopic, and microscopic techniques to create comprehensive degradation maps that correlate specific failure modes with operating conditions. Their work has demonstrated that controlled electrolyte additives containing specific nitrogen-containing heterocycles can extend cycle life by 300-400% by forming stable solid-electrolyte interphase layers that suppress both hydrogen evolution and zinc corrosion[3]. Additionally, they've developed machine learning algorithms that can predict battery failure 50-100 cycles before conventional detection methods by analyzing subtle electrochemical signature changes during early cycling stages[5].
Strengths: Northwestern's cryogenic characterization techniques provide unprecedented insights into degradation mechanisms at the atomic scale. Their machine learning approach enables early failure prediction, potentially allowing for adaptive control systems that extend battery life. Weaknesses: The specialized equipment and expertise required for their analysis methods may limit widespread industrial adoption, and their solutions may add complexity and cost to battery manufacturing processes.
Contemporary Amperex Technology Co., Ltd.
Technical Solution: Contemporary Amperex Technology Co., Ltd. (CATL) has developed advanced failure analysis methodologies for aqueous zinc ion batteries (AZIBs) focusing on electrode degradation mechanisms. Their approach combines in-situ characterization techniques with post-mortem analysis to identify zinc dendrite formation and manganese dissolution issues. CATL employs electrochemical impedance spectroscopy (EIS) to monitor internal resistance changes during cycling, correlating these measurements with battery performance degradation. Their proprietary electrolyte additives have demonstrated significant improvement in cycling stability by forming protective films on zinc anodes, reducing dendrite formation by approximately 60% compared to conventional formulations[1]. CATL has also implemented accelerated aging protocols specifically designed for AZIBs that can predict long-term reliability within shortened testing periods, enabling faster product development cycles while maintaining accuracy in lifetime predictions[3].
Strengths: CATL's comprehensive failure analysis approach combines multiple characterization techniques, providing deeper insights into degradation mechanisms. Their electrolyte additives effectively address zinc dendrite formation, a critical failure mode in AZIBs. Weaknesses: Their solutions may increase manufacturing complexity and cost, potentially limiting commercial viability for mass production of AZIBs compared to other battery technologies.
Critical Technologies for Zinc Ion Battery Reliability Enhancement
Indium zinc-based alloy anodes forming porous structure for aqueous zinc batteries
PatentPendingUS20230282816A1
Innovation
- The development of InxMyZnz alloy anodes, where M is Al, Ag, Bi, Pb, Sn, or Cd, with specific compositions and structures that mitigate dendrite formation through indium and zinc domain distribution, porosity, and oxidation, enhancing cycling performance and corrosion resistance.
Zinc rechargeable battery
PatentPendingUS20240055587A1
Innovation
- Incorporating a compound with a donor number of 32 or more as an additive in the electrolyte to suppress side reactions and modify the zinc negative electrode surface, preventing dendrite growth and enhancing electrodeposition uniformity.
Environmental Impact Assessment of Aqueous Zinc Battery Technologies
The environmental impact of aqueous zinc battery technologies represents a critical dimension in evaluating their sustainability and long-term viability. Unlike lithium-ion batteries that rely on rare earth metals and organic electrolytes, aqueous zinc batteries utilize abundant zinc resources and water-based electrolytes, potentially offering significant environmental advantages.
Life cycle assessment (LCA) studies indicate that zinc-based battery systems generally exhibit lower carbon footprints during manufacturing compared to lithium-ion counterparts. The extraction and processing of zinc requires approximately 30-40% less energy than equivalent lithium processing, translating to reduced greenhouse gas emissions. Additionally, the water-based electrolyte systems eliminate the need for toxic organic solvents commonly used in conventional batteries, substantially reducing volatile organic compound (VOC) emissions.
Water consumption presents both advantages and challenges for aqueous zinc battery technologies. While these systems require water resources for electrolyte preparation, they typically consume 50-70% less water throughout their lifecycle than lithium-ion batteries when considering mining operations and manufacturing processes collectively. However, potential zinc leaching from improper disposal remains a concern for aquatic ecosystems.
End-of-life management offers promising environmental benefits. The recyclability rate of zinc from spent batteries exceeds 90% with current technologies, significantly higher than recovery rates for lithium and cobalt. This circular economy potential substantially reduces the need for primary resource extraction and associated environmental impacts. Furthermore, the non-flammable nature of aqueous electrolytes eliminates fire hazards during recycling processes.
Land use impacts from zinc mining operations are generally less intensive than those associated with lithium extraction, particularly when compared to lithium brine operations that can disrupt sensitive desert ecosystems. However, zinc mining still presents challenges related to habitat disruption and potential acid mine drainage if not properly managed.
Toxicity profiles of aqueous zinc batteries show mixed results. While avoiding the highly toxic and flammable components of conventional batteries, zinc itself can be ecotoxic at elevated concentrations. Recent studies indicate that modern zinc battery designs with improved sealing and containment systems have reduced leakage risks by approximately 85% compared to earlier generations, significantly mitigating potential environmental exposure.
Future environmental improvements will likely focus on developing zinc recovery processes with lower energy requirements, implementing water recycling systems in manufacturing, and designing batteries with biodegradable separators to further enhance end-of-life sustainability.
Life cycle assessment (LCA) studies indicate that zinc-based battery systems generally exhibit lower carbon footprints during manufacturing compared to lithium-ion counterparts. The extraction and processing of zinc requires approximately 30-40% less energy than equivalent lithium processing, translating to reduced greenhouse gas emissions. Additionally, the water-based electrolyte systems eliminate the need for toxic organic solvents commonly used in conventional batteries, substantially reducing volatile organic compound (VOC) emissions.
Water consumption presents both advantages and challenges for aqueous zinc battery technologies. While these systems require water resources for electrolyte preparation, they typically consume 50-70% less water throughout their lifecycle than lithium-ion batteries when considering mining operations and manufacturing processes collectively. However, potential zinc leaching from improper disposal remains a concern for aquatic ecosystems.
End-of-life management offers promising environmental benefits. The recyclability rate of zinc from spent batteries exceeds 90% with current technologies, significantly higher than recovery rates for lithium and cobalt. This circular economy potential substantially reduces the need for primary resource extraction and associated environmental impacts. Furthermore, the non-flammable nature of aqueous electrolytes eliminates fire hazards during recycling processes.
Land use impacts from zinc mining operations are generally less intensive than those associated with lithium extraction, particularly when compared to lithium brine operations that can disrupt sensitive desert ecosystems. However, zinc mining still presents challenges related to habitat disruption and potential acid mine drainage if not properly managed.
Toxicity profiles of aqueous zinc batteries show mixed results. While avoiding the highly toxic and flammable components of conventional batteries, zinc itself can be ecotoxic at elevated concentrations. Recent studies indicate that modern zinc battery designs with improved sealing and containment systems have reduced leakage risks by approximately 85% compared to earlier generations, significantly mitigating potential environmental exposure.
Future environmental improvements will likely focus on developing zinc recovery processes with lower energy requirements, implementing water recycling systems in manufacturing, and designing batteries with biodegradable separators to further enhance end-of-life sustainability.
Standardization and Testing Protocols for Zinc Ion Battery Reliability
The standardization of testing protocols for zinc ion battery reliability represents a critical frontier in advancing aqueous zinc ion battery (AZIB) technology toward commercial viability. Currently, the field suffers from significant inconsistencies in testing methodologies, making cross-study comparisons challenging and hindering technological progress. Establishing unified testing standards would enable more accurate benchmarking of battery performance and failure mechanisms across different research groups and manufacturers.
Key parameters requiring standardization include cycle life testing conditions, depth of discharge specifications, temperature ranges for performance evaluation, and accelerated aging protocols. The absence of industry-wide standards has led to widely varying reported performance metrics, with some studies focusing exclusively on ideal laboratory conditions that poorly represent real-world applications. This disconnect between laboratory testing and practical deployment scenarios creates significant barriers to commercial adoption.
Testing protocols must address the unique failure modes of zinc ion batteries, particularly zinc dendrite formation, electrode corrosion, and electrolyte degradation. Standard methodologies should incorporate specific tests for monitoring zinc plating/stripping efficiency, quantifying side reactions, and evaluating separator integrity under various operational conditions. Post-mortem analysis techniques also require standardization to ensure consistent evaluation of failure mechanisms.
International organizations including the International Electrotechnical Commission (IEC) and IEEE have begun preliminary work on zinc-specific battery standards, but these efforts remain in early stages compared to the well-established protocols for lithium-ion technologies. Industry consortia involving battery manufacturers, academic institutions, and regulatory bodies are forming to accelerate this standardization process, with several working groups focusing on developing testing frameworks specifically tailored to aqueous zinc systems.
Reliability testing must incorporate both calendar aging and cycle aging under various environmental conditions. Standard protocols should define specific temperature ranges, humidity levels, and mechanical stress parameters that accurately reflect intended application environments. Additionally, safety testing standards must address the unique characteristics of aqueous electrolytes, including protocols for evaluating hydrogen evolution, electrolyte leakage, and corrosion-related failures.
Data reporting formats also require standardization to facilitate meaningful comparison between different battery systems. This includes establishing uniform methods for calculating and reporting capacity retention, coulombic efficiency, energy density, and power capability. Standardized statistical methods for reliability data analysis would further enhance the value of testing results, enabling more accurate lifetime predictions and failure rate estimations across the industry.
Key parameters requiring standardization include cycle life testing conditions, depth of discharge specifications, temperature ranges for performance evaluation, and accelerated aging protocols. The absence of industry-wide standards has led to widely varying reported performance metrics, with some studies focusing exclusively on ideal laboratory conditions that poorly represent real-world applications. This disconnect between laboratory testing and practical deployment scenarios creates significant barriers to commercial adoption.
Testing protocols must address the unique failure modes of zinc ion batteries, particularly zinc dendrite formation, electrode corrosion, and electrolyte degradation. Standard methodologies should incorporate specific tests for monitoring zinc plating/stripping efficiency, quantifying side reactions, and evaluating separator integrity under various operational conditions. Post-mortem analysis techniques also require standardization to ensure consistent evaluation of failure mechanisms.
International organizations including the International Electrotechnical Commission (IEC) and IEEE have begun preliminary work on zinc-specific battery standards, but these efforts remain in early stages compared to the well-established protocols for lithium-ion technologies. Industry consortia involving battery manufacturers, academic institutions, and regulatory bodies are forming to accelerate this standardization process, with several working groups focusing on developing testing frameworks specifically tailored to aqueous zinc systems.
Reliability testing must incorporate both calendar aging and cycle aging under various environmental conditions. Standard protocols should define specific temperature ranges, humidity levels, and mechanical stress parameters that accurately reflect intended application environments. Additionally, safety testing standards must address the unique characteristics of aqueous electrolytes, including protocols for evaluating hydrogen evolution, electrolyte leakage, and corrosion-related failures.
Data reporting formats also require standardization to facilitate meaningful comparison between different battery systems. This includes establishing uniform methods for calculating and reporting capacity retention, coulombic efficiency, energy density, and power capability. Standardized statistical methods for reliability data analysis would further enhance the value of testing results, enabling more accurate lifetime predictions and failure rate estimations across the industry.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!