Self-Discharge Mechanisms And Mitigation Strategies For Aqueous Zinc Ion Batteries
SEP 12, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Aqueous Zinc Battery Evolution and Research Objectives
Aqueous zinc ion batteries (AZIBs) have emerged as a promising energy storage technology, evolving significantly since their conceptualization in the early 20th century. The fundamental architecture of these batteries consists of a zinc metal anode, an aqueous electrolyte, and a cathode capable of reversibly hosting zinc ions. Their development trajectory has been marked by periods of intense research followed by relative dormancy, with renewed interest in recent decades driven by the global push for sustainable energy solutions.
The evolution of AZIBs can be traced through several distinct phases. Initial explorations in the 1920s-1930s established basic electrochemical principles, followed by commercial applications in primary battery systems. The 1980s-1990s witnessed attempts at rechargeable zinc systems, though these efforts were hampered by persistent issues including dendrite formation and limited cycle life. The current renaissance period, beginning around 2010, has been characterized by significant breakthroughs in materials science and electrolyte chemistry.
Recent advancements have focused on addressing the inherent challenges of zinc electrochemistry in aqueous environments. Researchers have developed novel cathode materials with enhanced zinc ion intercalation properties, explored electrolyte additives to suppress hydrogen evolution, and implemented strategies to mitigate dendrite growth. These innovations have substantially improved the performance metrics of AZIBs, including energy density, cycle life, and rate capability.
Self-discharge represents a critical challenge in the practical deployment of AZIBs. This phenomenon, whereby batteries lose charge during storage without external current flow, significantly impacts their reliability and operational efficiency. The mechanisms underlying self-discharge in AZIBs are multifaceted, involving parasitic reactions at electrode surfaces, shuttle effects of active species, and electrolyte decomposition pathways.
The primary research objectives in this field now center on elucidating these self-discharge mechanisms at a fundamental level and developing effective mitigation strategies. This includes investigating the role of electrode surface chemistry, electrolyte composition, and operating conditions on self-discharge rates. Additionally, research aims to establish standardized testing protocols for quantifying self-discharge behavior and develop predictive models to inform battery management systems.
Long-term research goals extend to achieving practical AZIBs with self-discharge rates comparable to or better than commercial lithium-ion systems, while maintaining the inherent advantages of aqueous zinc chemistry: safety, environmental compatibility, and cost-effectiveness. Success in this domain would significantly enhance the viability of AZIBs for grid-scale energy storage, renewable energy integration, and sustainable portable electronics applications.
The evolution of AZIBs can be traced through several distinct phases. Initial explorations in the 1920s-1930s established basic electrochemical principles, followed by commercial applications in primary battery systems. The 1980s-1990s witnessed attempts at rechargeable zinc systems, though these efforts were hampered by persistent issues including dendrite formation and limited cycle life. The current renaissance period, beginning around 2010, has been characterized by significant breakthroughs in materials science and electrolyte chemistry.
Recent advancements have focused on addressing the inherent challenges of zinc electrochemistry in aqueous environments. Researchers have developed novel cathode materials with enhanced zinc ion intercalation properties, explored electrolyte additives to suppress hydrogen evolution, and implemented strategies to mitigate dendrite growth. These innovations have substantially improved the performance metrics of AZIBs, including energy density, cycle life, and rate capability.
Self-discharge represents a critical challenge in the practical deployment of AZIBs. This phenomenon, whereby batteries lose charge during storage without external current flow, significantly impacts their reliability and operational efficiency. The mechanisms underlying self-discharge in AZIBs are multifaceted, involving parasitic reactions at electrode surfaces, shuttle effects of active species, and electrolyte decomposition pathways.
The primary research objectives in this field now center on elucidating these self-discharge mechanisms at a fundamental level and developing effective mitigation strategies. This includes investigating the role of electrode surface chemistry, electrolyte composition, and operating conditions on self-discharge rates. Additionally, research aims to establish standardized testing protocols for quantifying self-discharge behavior and develop predictive models to inform battery management systems.
Long-term research goals extend to achieving practical AZIBs with self-discharge rates comparable to or better than commercial lithium-ion systems, while maintaining the inherent advantages of aqueous zinc chemistry: safety, environmental compatibility, and cost-effectiveness. Success in this domain would significantly enhance the viability of AZIBs for grid-scale energy storage, renewable energy integration, and sustainable portable electronics applications.
Market Analysis for Sustainable Energy Storage Solutions
The global energy storage market is witnessing unprecedented growth, with sustainable solutions becoming increasingly vital for grid stability and renewable energy integration. Aqueous Zinc Ion Batteries (AZIBs) represent a promising segment within this expanding market, offering advantages in safety, cost-effectiveness, and environmental compatibility compared to conventional lithium-ion technologies.
Market projections indicate that the global energy storage market will reach approximately $546 billion by 2035, with a compound annual growth rate of 15-20% between 2023 and 2035. Within this broader context, aqueous battery technologies are expected to capture a growing market share, potentially reaching 25% of stationary storage applications by 2030, driven by their inherent safety advantages and lower production costs.
Consumer demand for sustainable energy storage solutions continues to rise across multiple sectors. Industrial applications represent the largest market segment, with utilities increasingly deploying grid-scale storage to manage peak loads and integrate intermittent renewable energy sources. Commercial and residential sectors are also showing significant growth potential, particularly in regions with high electricity costs or unreliable grid infrastructure.
Regional analysis reveals varying adoption patterns for sustainable storage technologies. Asia-Pacific leads in manufacturing capacity and deployment, with China dominating production of zinc-based battery components. North America and Europe follow with strong research initiatives and policy support for sustainable energy technologies, creating favorable market conditions for advanced aqueous battery systems.
Market drivers for AZIBs include increasing safety concerns with lithium-ion batteries, growing environmental regulations, and the push for domestic supply chains for critical materials. The self-discharge issue addressed in this research represents a significant market barrier that, when overcome, could accelerate adoption across multiple applications.
Price sensitivity analysis indicates that AZIBs could achieve cost parity with lead-acid batteries in the near term while offering superior performance characteristics. With further improvements in cycle life and self-discharge rates, they could compete effectively in markets currently dominated by lithium-ion technologies, particularly in stationary applications where energy density is less critical than safety and cost.
Market forecasts suggest that technologies effectively addressing self-discharge mechanisms could capture premium positioning, with potential price premiums of 15-20% for solutions demonstrating significantly improved shelf life and reliability. This represents a substantial market opportunity for companies that can successfully implement the mitigation strategies outlined in this research.
Market projections indicate that the global energy storage market will reach approximately $546 billion by 2035, with a compound annual growth rate of 15-20% between 2023 and 2035. Within this broader context, aqueous battery technologies are expected to capture a growing market share, potentially reaching 25% of stationary storage applications by 2030, driven by their inherent safety advantages and lower production costs.
Consumer demand for sustainable energy storage solutions continues to rise across multiple sectors. Industrial applications represent the largest market segment, with utilities increasingly deploying grid-scale storage to manage peak loads and integrate intermittent renewable energy sources. Commercial and residential sectors are also showing significant growth potential, particularly in regions with high electricity costs or unreliable grid infrastructure.
Regional analysis reveals varying adoption patterns for sustainable storage technologies. Asia-Pacific leads in manufacturing capacity and deployment, with China dominating production of zinc-based battery components. North America and Europe follow with strong research initiatives and policy support for sustainable energy technologies, creating favorable market conditions for advanced aqueous battery systems.
Market drivers for AZIBs include increasing safety concerns with lithium-ion batteries, growing environmental regulations, and the push for domestic supply chains for critical materials. The self-discharge issue addressed in this research represents a significant market barrier that, when overcome, could accelerate adoption across multiple applications.
Price sensitivity analysis indicates that AZIBs could achieve cost parity with lead-acid batteries in the near term while offering superior performance characteristics. With further improvements in cycle life and self-discharge rates, they could compete effectively in markets currently dominated by lithium-ion technologies, particularly in stationary applications where energy density is less critical than safety and cost.
Market forecasts suggest that technologies effectively addressing self-discharge mechanisms could capture premium positioning, with potential price premiums of 15-20% for solutions demonstrating significantly improved shelf life and reliability. This represents a substantial market opportunity for companies that can successfully implement the mitigation strategies outlined in this research.
Self-Discharge Challenges in Zinc-Based Battery Systems
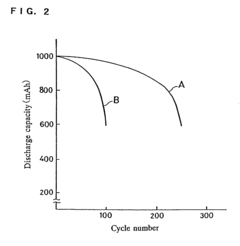
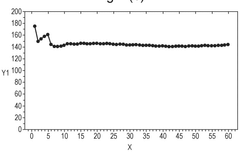
Self-discharge represents a significant challenge in zinc-based battery systems, manifesting as capacity loss during storage without external load connection. This phenomenon substantially impacts the shelf life and reliability of aqueous zinc ion batteries (AZIBs), limiting their practical applications despite their theoretical advantages of high safety, low cost, and environmental friendliness.
The primary mechanisms driving self-discharge in zinc-based systems are multifaceted. Foremost among these is the hydrogen evolution reaction (HER), where zinc metal reacts with water in the electrolyte, generating hydrogen gas and causing irreversible capacity loss. This reaction is thermodynamically favorable in aqueous environments, making it particularly problematic for long-term storage.
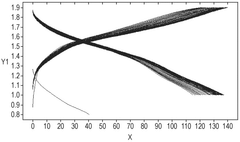
Zinc dendrite formation constitutes another critical self-discharge pathway. During charging cycles, zinc ions can deposit unevenly on the anode surface, creating dendrites that may eventually penetrate the separator and cause internal short circuits. These dendrites also increase the reactive surface area, accelerating side reactions and exacerbating self-discharge rates.
Electrolyte decomposition further contributes to capacity fade. The relatively high voltage window of many zinc-based systems can trigger electrolyte degradation at the electrode-electrolyte interface, forming resistive layers that impede ion transport and increase internal resistance over time.
Corrosion of the zinc anode presents an additional challenge unique to zinc-based systems. The amphoteric nature of zinc makes it susceptible to corrosion in both acidic and alkaline environments, leading to continuous material loss even during idle periods. This process is accelerated by impurities in the electrolyte or electrode materials.
Temperature fluctuations significantly influence self-discharge rates in zinc-based batteries. Higher temperatures accelerate all chemical reactions involved in self-discharge mechanisms, with studies indicating that self-discharge rates can double with every 10°C increase in temperature.
The presence of oxygen dissolved in the electrolyte can also drive parasitic reactions at the electrode surfaces, particularly at the zinc anode where oxygen reduction can occur simultaneously with zinc oxidation, creating a micro-galvanic cell effect that accelerates self-discharge.
Impurities in battery components, including trace metals in the electrolyte or contaminants introduced during manufacturing, can catalyze side reactions and significantly increase self-discharge rates. Even parts-per-million levels of certain metal impurities can dramatically accelerate hydrogen evolution and other degradation pathways.
Understanding these self-discharge mechanisms is essential for developing effective mitigation strategies and advancing zinc-based battery technologies toward commercial viability in energy storage applications requiring extended shelf life and operational reliability.
The primary mechanisms driving self-discharge in zinc-based systems are multifaceted. Foremost among these is the hydrogen evolution reaction (HER), where zinc metal reacts with water in the electrolyte, generating hydrogen gas and causing irreversible capacity loss. This reaction is thermodynamically favorable in aqueous environments, making it particularly problematic for long-term storage.
Zinc dendrite formation constitutes another critical self-discharge pathway. During charging cycles, zinc ions can deposit unevenly on the anode surface, creating dendrites that may eventually penetrate the separator and cause internal short circuits. These dendrites also increase the reactive surface area, accelerating side reactions and exacerbating self-discharge rates.
Electrolyte decomposition further contributes to capacity fade. The relatively high voltage window of many zinc-based systems can trigger electrolyte degradation at the electrode-electrolyte interface, forming resistive layers that impede ion transport and increase internal resistance over time.
Corrosion of the zinc anode presents an additional challenge unique to zinc-based systems. The amphoteric nature of zinc makes it susceptible to corrosion in both acidic and alkaline environments, leading to continuous material loss even during idle periods. This process is accelerated by impurities in the electrolyte or electrode materials.
Temperature fluctuations significantly influence self-discharge rates in zinc-based batteries. Higher temperatures accelerate all chemical reactions involved in self-discharge mechanisms, with studies indicating that self-discharge rates can double with every 10°C increase in temperature.
The presence of oxygen dissolved in the electrolyte can also drive parasitic reactions at the electrode surfaces, particularly at the zinc anode where oxygen reduction can occur simultaneously with zinc oxidation, creating a micro-galvanic cell effect that accelerates self-discharge.
Impurities in battery components, including trace metals in the electrolyte or contaminants introduced during manufacturing, can catalyze side reactions and significantly increase self-discharge rates. Even parts-per-million levels of certain metal impurities can dramatically accelerate hydrogen evolution and other degradation pathways.
Understanding these self-discharge mechanisms is essential for developing effective mitigation strategies and advancing zinc-based battery technologies toward commercial viability in energy storage applications requiring extended shelf life and operational reliability.
Leading Manufacturers and Research Institutions in Zinc Battery Field
The aqueous zinc ion battery market is in an early growth phase, characterized by increasing research focus on addressing self-discharge mechanisms. The global market size remains relatively small but is expanding rapidly due to zinc batteries' cost advantages and safety benefits compared to lithium-ion technologies. From a technical maturity perspective, the field is still evolving, with key players at different development stages. Companies like NGK Insulators and Toyota have established research capabilities, while Chinese firms including CATL (Ningde Amperex) and Guoxuan are investing heavily in zinc battery R&D. Academic institutions such as Tsinghua University and University of Maryland are contributing fundamental research on electrolyte stability and electrode materials. The industry faces challenges in commercialization despite promising lab-scale demonstrations, with self-discharge mitigation remaining a critical hurdle for widespread adoption.
Tsinghua University
Technical Solution: Tsinghua University researchers have developed an innovative approach to address self-discharge in aqueous zinc ion batteries through a comprehensive materials engineering strategy. Their solution involves a novel cathode structure using layered manganese oxide with precisely controlled interlayer spacing, modified with nitrogen-doped carbon to enhance electronic conductivity and structural stability. This design significantly reduces manganese dissolution, a primary cause of self-discharge. The research team has also engineered a zinc anode with a calcium-doped surface layer that forms a protective barrier against side reactions while maintaining excellent zinc ion diffusion kinetics. Their electrolyte formulation incorporates specific organic additives that create a stable solid electrolyte interphase on electrode surfaces, suppressing hydrogen evolution and other parasitic reactions. Additionally, they've developed a composite polymer gel electrolyte that reduces water activity while maintaining high ionic conductivity, effectively minimizing self-discharge mechanisms related to water decomposition. The university's approach also includes a specialized battery management algorithm that identifies optimal charging protocols to minimize conditions that accelerate self-discharge.
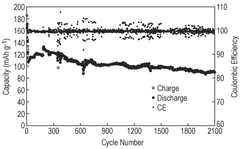
Strengths: Exceptional theoretical foundation with comprehensive understanding of zinc ion battery chemistry; innovative materials design with multiple synergistic protection mechanisms; demonstrated long-term stability in laboratory testing with self-discharge rates below 1% per month. Weaknesses: Currently at laboratory scale with challenges in scaling to commercial production; some specialized materials may increase manufacturing complexity and costs.
Hefei Guoxuan High-Tech Power Energy Co., Ltd.
Technical Solution: Hefei Guoxuan has pioneered a multi-faceted approach to mitigate self-discharge in aqueous zinc ion batteries. Their technology centers on a novel zinc anode design with nano-structured zinc particles that are surface-modified with carbon-based materials to suppress hydrogen evolution. The company has developed a proprietary electrolyte formulation containing specific organic additives that form a stable solid electrolyte interphase (SEI) on the zinc surface, effectively reducing parasitic reactions. Their cathode materials feature manganese oxide structures with vanadium doping to enhance structural stability and prevent manganese dissolution during cycling. Guoxuan's batteries also incorporate a composite separator with selective ion transport properties that blocks dendrite growth while maintaining high ionic conductivity. The company has implemented an advanced encapsulation technology that minimizes water loss and contamination from atmospheric CO2, which can accelerate self-discharge mechanisms through pH changes in the electrolyte.
Strengths: Exceptional energy density (>100 Wh/kg) while maintaining low self-discharge rates; cost-effective manufacturing process compatible with existing production lines; environmentally friendly materials with reduced toxicity. Weaknesses: Performance degradation at extreme temperatures (below 0°C or above 50°C); requires precise manufacturing controls to ensure consistent quality of the protective surface layers.
Key Patents and Innovations in Zinc Ion Battery Stability
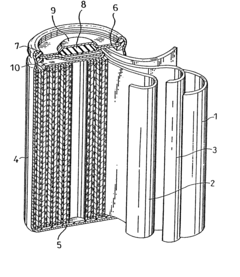
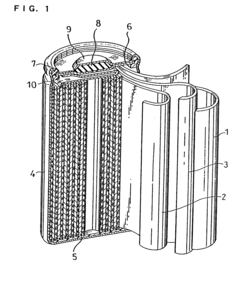
Alkaline zinc secondary cell and method for preparation thereof
PatentInactiveUS7153607B2
Innovation
- A separator layer comprising a gel electrolyte made from a water absorbent polymer, such as cross-linked acrylate or methacrylate units, combined with an alkaline aqueous solution, and optionally a water repellent like fluorinated carbon, integrated with a core material like polyolefin or cellulose, which maintains ion conductivity and prevents zinc corrosion.
battery
PatentWO2025114546A1
Innovation
- The development of an aqueous zinc-ion battery with a thick manganese oxide-carbon composite cathode and a zinc powder-carbon composite anode, along with a thin solid zinc ion separator to suppress dendrite growth. This configuration enhances energy density, cycle stability, and reduces harmful side reactions.
Materials Science Advancements for Zinc Battery Electrodes
Recent advancements in materials science have significantly enhanced the performance of zinc battery electrodes, addressing key challenges in aqueous zinc ion batteries (AZIBs). The evolution of electrode materials has focused on mitigating self-discharge issues through innovative structural designs and compositional modifications.
Manganese dioxide (MnO2) has emerged as a promising cathode material due to its abundant availability and high theoretical capacity. Researchers have developed various polymorphs of MnO2 (α, β, γ, δ, and λ) with different crystallographic structures that demonstrate varying degrees of resistance to self-discharge. The incorporation of defect engineering in these structures has proven effective in stabilizing zinc ions within the lattice, thereby reducing capacity fade during idle periods.
Vanadium-based materials, particularly vanadium oxides and vanadium phosphates, have shown remarkable improvements through surface modification techniques. The introduction of conductive carbon coatings and polymer layers creates physical barriers that prevent parasitic reactions at the electrode-electrolyte interface, significantly reducing self-discharge rates. These modifications maintain the structural integrity of the electrodes while enhancing their electrochemical stability.
Prussian blue analogs (PBAs) represent another breakthrough in electrode materials, offering open framework structures with large interstitial spaces for efficient zinc ion storage. Recent developments in PBA synthesis have focused on controlling crystallinity and defect concentration, resulting in materials with enhanced resistance to self-discharge mechanisms. The incorporation of transition metal substitutions within the PBA framework has further improved their stability during prolonged storage periods.
For zinc anodes, alloying strategies with elements such as bismuth, indium, and calcium have demonstrated significant improvements in preventing dendritic growth and surface passivation. These alloyed anodes exhibit reduced hydrogen evolution reactions, which are major contributors to self-discharge in AZIBs. Additionally, three-dimensional porous zinc architectures have been developed to accommodate volume changes during cycling while maintaining high surface area for efficient ion transport.
Composite electrode materials combining organic and inorganic components have shown synergistic effects in mitigating self-discharge. Polymer-reinforced zinc electrodes demonstrate enhanced mechanical stability and reduced surface reactivity, while carbon-based composites provide improved conductivity and reduced internal resistance, both factors that contribute to lower self-discharge rates in practical applications.
Manganese dioxide (MnO2) has emerged as a promising cathode material due to its abundant availability and high theoretical capacity. Researchers have developed various polymorphs of MnO2 (α, β, γ, δ, and λ) with different crystallographic structures that demonstrate varying degrees of resistance to self-discharge. The incorporation of defect engineering in these structures has proven effective in stabilizing zinc ions within the lattice, thereby reducing capacity fade during idle periods.
Vanadium-based materials, particularly vanadium oxides and vanadium phosphates, have shown remarkable improvements through surface modification techniques. The introduction of conductive carbon coatings and polymer layers creates physical barriers that prevent parasitic reactions at the electrode-electrolyte interface, significantly reducing self-discharge rates. These modifications maintain the structural integrity of the electrodes while enhancing their electrochemical stability.
Prussian blue analogs (PBAs) represent another breakthrough in electrode materials, offering open framework structures with large interstitial spaces for efficient zinc ion storage. Recent developments in PBA synthesis have focused on controlling crystallinity and defect concentration, resulting in materials with enhanced resistance to self-discharge mechanisms. The incorporation of transition metal substitutions within the PBA framework has further improved their stability during prolonged storage periods.
For zinc anodes, alloying strategies with elements such as bismuth, indium, and calcium have demonstrated significant improvements in preventing dendritic growth and surface passivation. These alloyed anodes exhibit reduced hydrogen evolution reactions, which are major contributors to self-discharge in AZIBs. Additionally, three-dimensional porous zinc architectures have been developed to accommodate volume changes during cycling while maintaining high surface area for efficient ion transport.
Composite electrode materials combining organic and inorganic components have shown synergistic effects in mitigating self-discharge. Polymer-reinforced zinc electrodes demonstrate enhanced mechanical stability and reduced surface reactivity, while carbon-based composites provide improved conductivity and reduced internal resistance, both factors that contribute to lower self-discharge rates in practical applications.
Environmental Impact and Lifecycle Assessment
The environmental impact of aqueous zinc ion batteries (AZIBs) represents a critical dimension in their overall assessment as a sustainable energy storage solution. Unlike lithium-ion batteries that rely on organic electrolytes with high flammability and toxicity concerns, AZIBs utilize water-based electrolytes that significantly reduce fire hazards and environmental risks during production, operation, and disposal phases.
Life cycle assessment (LCA) studies indicate that the carbon footprint of AZIBs is potentially 25-30% lower than conventional lithium-ion batteries, primarily due to the abundance of zinc resources and less energy-intensive manufacturing processes. The raw material extraction phase for zinc demonstrates lower environmental impact scores across categories including acidification potential, eutrophication potential, and human toxicity compared to lithium and cobalt mining operations.
Self-discharge mechanisms in AZIBs present unique environmental considerations. The parasitic reactions that cause capacity loss often involve hydrogen evolution and zinc corrosion, which may release minimal amounts of hydrogen gas during long-term storage. While these emissions are generally negligible from a safety perspective, they represent energy inefficiency that indirectly increases the environmental burden through more frequent charging cycles and reduced battery lifespan.
Mitigation strategies for self-discharge in AZIBs offer environmental co-benefits beyond performance improvements. Electrolyte additives that suppress hydrogen evolution not only extend battery life but also reduce the frequency of replacement, thereby decreasing waste generation. Similarly, electrode surface modifications that minimize side reactions contribute to resource conservation by maximizing material utilization efficiency throughout the battery lifecycle.
End-of-life management for AZIBs presents distinct advantages over other battery technologies. The recyclability rate for zinc exceeds 80% in established recycling streams, with recovery processes requiring significantly less energy than comparable processes for lithium-ion batteries. Additionally, the aqueous electrolytes facilitate safer and more straightforward recycling procedures with reduced requirements for specialized handling equipment or hazardous waste protocols.
Regulatory frameworks increasingly recognize these environmental advantages, with several jurisdictions developing specific provisions for aqueous battery systems within broader battery directives. The European Battery Directive revision, for instance, proposes lower environmental compliance thresholds for water-based battery technologies, potentially accelerating market adoption of AZIBs as manufacturers seek to meet stricter sustainability requirements.
Life cycle assessment (LCA) studies indicate that the carbon footprint of AZIBs is potentially 25-30% lower than conventional lithium-ion batteries, primarily due to the abundance of zinc resources and less energy-intensive manufacturing processes. The raw material extraction phase for zinc demonstrates lower environmental impact scores across categories including acidification potential, eutrophication potential, and human toxicity compared to lithium and cobalt mining operations.
Self-discharge mechanisms in AZIBs present unique environmental considerations. The parasitic reactions that cause capacity loss often involve hydrogen evolution and zinc corrosion, which may release minimal amounts of hydrogen gas during long-term storage. While these emissions are generally negligible from a safety perspective, they represent energy inefficiency that indirectly increases the environmental burden through more frequent charging cycles and reduced battery lifespan.
Mitigation strategies for self-discharge in AZIBs offer environmental co-benefits beyond performance improvements. Electrolyte additives that suppress hydrogen evolution not only extend battery life but also reduce the frequency of replacement, thereby decreasing waste generation. Similarly, electrode surface modifications that minimize side reactions contribute to resource conservation by maximizing material utilization efficiency throughout the battery lifecycle.
End-of-life management for AZIBs presents distinct advantages over other battery technologies. The recyclability rate for zinc exceeds 80% in established recycling streams, with recovery processes requiring significantly less energy than comparable processes for lithium-ion batteries. Additionally, the aqueous electrolytes facilitate safer and more straightforward recycling procedures with reduced requirements for specialized handling equipment or hazardous waste protocols.
Regulatory frameworks increasingly recognize these environmental advantages, with several jurisdictions developing specific provisions for aqueous battery systems within broader battery directives. The European Battery Directive revision, for instance, proposes lower environmental compliance thresholds for water-based battery technologies, potentially accelerating market adoption of AZIBs as manufacturers seek to meet stricter sustainability requirements.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!