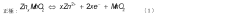Fabrication Methods For High Areal Capacity Electrodes In Aqueous Zinc Ion Batteries
SEP 12, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Zinc Ion Battery Electrode Development Background and Objectives
Aqueous zinc-ion batteries (ZIBs) have emerged as promising candidates for next-generation energy storage systems due to their inherent safety, environmental friendliness, and cost-effectiveness. The evolution of ZIBs can be traced back to the 1990s, but significant advancements have only been achieved in the past decade. The technology has progressed from simple zinc-manganese dioxide primary cells to rechargeable systems with various cathode materials including manganese oxides, vanadium oxides, and Prussian blue analogs.
The development trajectory of ZIB electrode fabrication methods has been shaped by the increasing demand for high-energy-density storage solutions in applications ranging from grid-scale storage to portable electronics. Traditional electrode fabrication techniques borrowed from lithium-ion battery manufacturing have proven inadequate for addressing the unique challenges posed by zinc-ion chemistry, particularly regarding the high areal capacity requirements.
Current research trends indicate a shift toward innovative electrode architectures that can accommodate the volumetric changes during zinc plating/stripping and mitigate dendrite formation. The integration of three-dimensional electrode structures, hierarchical porosity, and advanced conductive networks represents the frontier of electrode design for high-performance ZIBs.
The primary technical objectives for high areal capacity electrode fabrication include achieving loading capacities exceeding 10 mAh/cm², maintaining structural integrity over extended cycling, and ensuring uniform zinc deposition/dissolution. Additionally, scalable manufacturing processes that are compatible with existing production infrastructure remain a critical goal for commercial viability.
Electrode fabrication methods must address several key challenges unique to aqueous zinc systems, including the management of hydrogen evolution reactions, prevention of electrode pulverization, and mitigation of transition metal dissolution from cathode materials. These challenges necessitate innovative approaches to binder selection, current collector modification, and electrolyte engineering.
The convergence of materials science, electrochemistry, and manufacturing engineering is driving the development of next-generation fabrication methods. Techniques such as freeze-casting, electrospinning, and additive manufacturing are being explored to create electrodes with optimized ion transport pathways and mechanical stability under high mass loadings.
The ultimate goal of this technological evolution is to develop ZIBs with energy densities approaching 300-400 Wh/kg at the cell level, cycle lives exceeding 5000 cycles, and manufacturing costs below $100/kWh. Achieving these targets would position aqueous ZIBs as viable alternatives to lithium-ion batteries for certain applications, particularly in stationary storage where safety and cost considerations outweigh strict volumetric constraints.
The development trajectory of ZIB electrode fabrication methods has been shaped by the increasing demand for high-energy-density storage solutions in applications ranging from grid-scale storage to portable electronics. Traditional electrode fabrication techniques borrowed from lithium-ion battery manufacturing have proven inadequate for addressing the unique challenges posed by zinc-ion chemistry, particularly regarding the high areal capacity requirements.
Current research trends indicate a shift toward innovative electrode architectures that can accommodate the volumetric changes during zinc plating/stripping and mitigate dendrite formation. The integration of three-dimensional electrode structures, hierarchical porosity, and advanced conductive networks represents the frontier of electrode design for high-performance ZIBs.
The primary technical objectives for high areal capacity electrode fabrication include achieving loading capacities exceeding 10 mAh/cm², maintaining structural integrity over extended cycling, and ensuring uniform zinc deposition/dissolution. Additionally, scalable manufacturing processes that are compatible with existing production infrastructure remain a critical goal for commercial viability.
Electrode fabrication methods must address several key challenges unique to aqueous zinc systems, including the management of hydrogen evolution reactions, prevention of electrode pulverization, and mitigation of transition metal dissolution from cathode materials. These challenges necessitate innovative approaches to binder selection, current collector modification, and electrolyte engineering.
The convergence of materials science, electrochemistry, and manufacturing engineering is driving the development of next-generation fabrication methods. Techniques such as freeze-casting, electrospinning, and additive manufacturing are being explored to create electrodes with optimized ion transport pathways and mechanical stability under high mass loadings.
The ultimate goal of this technological evolution is to develop ZIBs with energy densities approaching 300-400 Wh/kg at the cell level, cycle lives exceeding 5000 cycles, and manufacturing costs below $100/kWh. Achieving these targets would position aqueous ZIBs as viable alternatives to lithium-ion batteries for certain applications, particularly in stationary storage where safety and cost considerations outweigh strict volumetric constraints.
Market Analysis for High-Capacity Aqueous Zinc Batteries
The global market for aqueous zinc ion batteries (AZIBs) is experiencing significant growth, driven by increasing demand for sustainable energy storage solutions. Current market valuations indicate that the global zinc-ion battery market reached approximately 10 million USD in 2022 and is projected to grow at a compound annual growth rate (CAGR) of over 25% through 2030, potentially reaching 80 million USD by the end of the decade.
This remarkable growth trajectory is primarily fueled by the expanding applications in grid energy storage, where the safety advantages of aqueous electrolytes present a compelling alternative to lithium-ion technologies. The market for high-capacity electrodes specifically represents about 35% of the total AZIB market, with substantial growth potential as manufacturing techniques advance.
Consumer electronics represents another significant market segment, particularly in regions with stringent safety regulations. The inherent non-flammability of aqueous zinc batteries positions them favorably in this sector, with an estimated market share of 15% that is expected to double within five years as fabrication methods for high areal capacity electrodes improve.
Geographically, Asia-Pacific dominates the market with approximately 45% share, led by China's aggressive investment in alternative battery technologies. North America follows at 30%, with particular interest from grid storage operators and military applications, while Europe accounts for 20% with strong policy support for sustainable energy solutions.
The economic factors driving market growth include the relatively low cost of zinc compared to lithium (zinc costs approximately 2,400 USD per ton versus lithium at over 20,000 USD per ton), abundant global zinc reserves, and simplified manufacturing processes that do not require dry rooms or inert atmospheres. These factors contribute to a potential 30-40% cost reduction compared to lithium-ion batteries when produced at scale.
Market barriers include competition from established lithium-ion technologies, technical challenges in achieving consistent high areal capacity in mass production, and the need for standardization across the industry. However, recent breakthroughs in electrode fabrication methods have begun addressing these challenges, with several companies reporting successful pilot production of electrodes with areal capacities exceeding 10 mAh/cm².
Industry analysts predict that as fabrication methods for high areal capacity electrodes mature, the market penetration of AZIBs will accelerate, potentially capturing 5-8% of the global battery market by 2035, representing a significant shift in the energy storage landscape.
This remarkable growth trajectory is primarily fueled by the expanding applications in grid energy storage, where the safety advantages of aqueous electrolytes present a compelling alternative to lithium-ion technologies. The market for high-capacity electrodes specifically represents about 35% of the total AZIB market, with substantial growth potential as manufacturing techniques advance.
Consumer electronics represents another significant market segment, particularly in regions with stringent safety regulations. The inherent non-flammability of aqueous zinc batteries positions them favorably in this sector, with an estimated market share of 15% that is expected to double within five years as fabrication methods for high areal capacity electrodes improve.
Geographically, Asia-Pacific dominates the market with approximately 45% share, led by China's aggressive investment in alternative battery technologies. North America follows at 30%, with particular interest from grid storage operators and military applications, while Europe accounts for 20% with strong policy support for sustainable energy solutions.
The economic factors driving market growth include the relatively low cost of zinc compared to lithium (zinc costs approximately 2,400 USD per ton versus lithium at over 20,000 USD per ton), abundant global zinc reserves, and simplified manufacturing processes that do not require dry rooms or inert atmospheres. These factors contribute to a potential 30-40% cost reduction compared to lithium-ion batteries when produced at scale.
Market barriers include competition from established lithium-ion technologies, technical challenges in achieving consistent high areal capacity in mass production, and the need for standardization across the industry. However, recent breakthroughs in electrode fabrication methods have begun addressing these challenges, with several companies reporting successful pilot production of electrodes with areal capacities exceeding 10 mAh/cm².
Industry analysts predict that as fabrication methods for high areal capacity electrodes mature, the market penetration of AZIBs will accelerate, potentially capturing 5-8% of the global battery market by 2035, representing a significant shift in the energy storage landscape.
Current Fabrication Challenges for High Areal Capacity Electrodes
The fabrication of high areal capacity electrodes for aqueous zinc-ion batteries (AZIBs) faces significant technical challenges that impede their commercial viability. Traditional electrode fabrication methods often fail to achieve the necessary areal capacity (>10 mAh/cm²) required for practical applications while maintaining good electrochemical performance. This limitation stems primarily from the inherent trade-off between electrode thickness and ion transport efficiency.
Conventional slurry-casting techniques, widely used in battery manufacturing, encounter severe limitations when applied to thick electrodes for AZIBs. As electrode thickness increases beyond 100-200 μm, the extended ion diffusion pathways result in significant kinetic barriers, leading to capacity fading and poor rate capability. Additionally, the binder systems traditionally employed (such as PVDF) create excessive tortuosity in the electrode structure, further hindering zinc ion transport.
Mass loading presents another critical challenge. While increasing active material loading seems straightforward, it often leads to mechanical instability. Thick electrodes tend to crack during drying processes and delaminate from current collectors during cycling, especially under the mechanical stresses induced by zinc plating/stripping. This mechanical degradation accelerates capacity fade and shortens battery lifespan.
The electronic conductivity network in high-loading electrodes poses additional complications. Conventional conductive additives like carbon black become ineffective at maintaining percolation networks throughout thick electrode structures. This results in underutilization of active materials, particularly in regions distant from the current collector, severely limiting the practical energy density achievable.
Electrolyte penetration represents a fundamental barrier to high areal capacity electrodes. Thick electrodes with traditional pore structures often suffer from incomplete electrolyte wetting, creating "dead zones" where active materials remain electrochemically inactive. This issue is particularly pronounced in aqueous systems where electrolyte viscosity and surface tension properties differ significantly from organic electrolytes.
Interfacial resistance between electrode components increases disproportionately with electrode thickness. The numerous interfaces between active materials, conductive additives, and binder materials create multiple barriers to charge transfer, resulting in high internal resistance and poor power performance in thick electrodes.
Manufacturing scalability presents perhaps the most significant industrial challenge. While laboratory-scale fabrication methods can sometimes achieve high areal capacities, these approaches often involve complex procedures incompatible with existing battery manufacturing infrastructure. The development of scalable fabrication methods that can be integrated into current production lines remains a significant hurdle for commercialization.
Conventional slurry-casting techniques, widely used in battery manufacturing, encounter severe limitations when applied to thick electrodes for AZIBs. As electrode thickness increases beyond 100-200 μm, the extended ion diffusion pathways result in significant kinetic barriers, leading to capacity fading and poor rate capability. Additionally, the binder systems traditionally employed (such as PVDF) create excessive tortuosity in the electrode structure, further hindering zinc ion transport.
Mass loading presents another critical challenge. While increasing active material loading seems straightforward, it often leads to mechanical instability. Thick electrodes tend to crack during drying processes and delaminate from current collectors during cycling, especially under the mechanical stresses induced by zinc plating/stripping. This mechanical degradation accelerates capacity fade and shortens battery lifespan.
The electronic conductivity network in high-loading electrodes poses additional complications. Conventional conductive additives like carbon black become ineffective at maintaining percolation networks throughout thick electrode structures. This results in underutilization of active materials, particularly in regions distant from the current collector, severely limiting the practical energy density achievable.
Electrolyte penetration represents a fundamental barrier to high areal capacity electrodes. Thick electrodes with traditional pore structures often suffer from incomplete electrolyte wetting, creating "dead zones" where active materials remain electrochemically inactive. This issue is particularly pronounced in aqueous systems where electrolyte viscosity and surface tension properties differ significantly from organic electrolytes.
Interfacial resistance between electrode components increases disproportionately with electrode thickness. The numerous interfaces between active materials, conductive additives, and binder materials create multiple barriers to charge transfer, resulting in high internal resistance and poor power performance in thick electrodes.
Manufacturing scalability presents perhaps the most significant industrial challenge. While laboratory-scale fabrication methods can sometimes achieve high areal capacities, these approaches often involve complex procedures incompatible with existing battery manufacturing infrastructure. The development of scalable fabrication methods that can be integrated into current production lines remains a significant hurdle for commercialization.
Current Electrode Fabrication Methods and Materials Assessment
01 Electrode materials for high areal capacity
Various electrode materials can be used in aqueous zinc ion batteries to achieve high areal capacity. These materials include manganese-based oxides, vanadium-based compounds, and carbon-based materials with specific structures that facilitate zinc ion storage. The design of these electrode materials focuses on optimizing the ion diffusion pathways and increasing the active material loading to enhance the overall areal capacity of the battery.- Electrode materials for high areal capacity: Various electrode materials can be used in aqueous zinc ion batteries to achieve high areal capacity. These materials include manganese-based oxides, vanadium-based compounds, and carbon-based materials with specific structures. The design of these electrode materials focuses on providing more active sites for zinc ion storage, enhancing the ion diffusion kinetics, and maintaining structural stability during charge-discharge cycles, all of which contribute to increased areal capacity of the battery.
- Electrolyte formulations for enhanced zinc ion transport: Specialized electrolyte formulations can significantly improve the performance of aqueous zinc ion batteries, particularly in terms of areal capacity. These formulations may include specific zinc salts, pH regulators, and additives that suppress hydrogen evolution and zinc dendrite formation. By optimizing the electrolyte composition, the transport of zinc ions between electrodes becomes more efficient, leading to higher utilization of active materials and consequently higher areal capacity.
- Structural design of battery components: The structural design of battery components plays a crucial role in achieving high areal capacity in aqueous zinc ion batteries. This includes the design of current collectors, separators, and the overall cell architecture. Techniques such as 3D electrode structures, porous current collectors, and optimized separator designs can increase the active material loading, improve electrolyte penetration, and enhance the mechanical stability of the battery, all contributing to higher areal capacity.
- Surface modification and interface engineering: Surface modification and interface engineering techniques are employed to enhance the areal capacity of aqueous zinc ion batteries. These approaches include coating electrode materials with conductive polymers, creating protective layers to prevent side reactions, and designing specific interfaces between the electrode and electrolyte. Such modifications can improve the stability of the electrode-electrolyte interface, reduce unwanted reactions, and enhance the utilization of active materials, resulting in higher areal capacity.
- Advanced manufacturing techniques for high-loading electrodes: Advanced manufacturing techniques are crucial for fabricating high-loading electrodes that can deliver high areal capacity in aqueous zinc ion batteries. These techniques include specialized coating methods, controlled drying processes, and novel assembly approaches that enable the creation of thick yet porous electrode structures. By optimizing the manufacturing process, higher amounts of active materials can be incorporated into the electrodes while maintaining good electronic and ionic conductivity, leading to enhanced areal capacity.
02 Electrolyte optimization for improved performance
The composition and concentration of electrolytes significantly impact the areal capacity of aqueous zinc ion batteries. Additives in the electrolyte can suppress dendrite formation and side reactions, leading to improved cycling stability and higher areal capacity. Various salts, pH regulators, and organic additives can be incorporated into the electrolyte to enhance zinc ion transport and interfacial stability between the electrode and electrolyte.Expand Specific Solutions03 Structural design of electrodes for high mass loading
The structural design of electrodes plays a crucial role in achieving high areal capacity in aqueous zinc ion batteries. Three-dimensional porous structures, hierarchical architectures, and conductive frameworks can accommodate higher mass loading of active materials while maintaining efficient ion and electron transport. These designs help to overcome the limitations of traditional planar electrodes and enable higher areal capacities without sacrificing rate performance.Expand Specific Solutions04 Interface engineering for stable cycling at high capacity
Interface engineering strategies are employed to maintain stable cycling performance at high areal capacities. Protective coatings, artificial solid electrolyte interphases, and buffer layers can mitigate side reactions and prevent electrode degradation during cycling. These approaches help to maintain the structural integrity of the electrodes and ensure consistent performance even with high mass loading of active materials.Expand Specific Solutions05 Advanced manufacturing techniques for high-capacity batteries
Advanced manufacturing techniques are essential for fabricating aqueous zinc ion batteries with high areal capacity. Methods such as 3D printing, electrospinning, freeze-drying, and template-assisted synthesis enable precise control over the electrode architecture and active material distribution. These techniques allow for the creation of optimized electrode structures that can support high mass loading while maintaining efficient ion transport pathways, ultimately leading to enhanced areal capacity.Expand Specific Solutions
Leading Companies and Research Institutions in Zinc Battery Technology
The aqueous zinc ion battery electrode fabrication market is in a growth phase, characterized by increasing research intensity and technological advancements. The market is expanding rapidly due to rising demand for sustainable energy storage solutions, with projections indicating significant growth potential as zinc-based technologies offer cost and safety advantages over lithium-ion alternatives. Academic institutions dominate the research landscape, with Shandong University, HKUST, and UNIST leading fundamental research, while companies like Sila Nanotechnologies and Panasonic are advancing commercial applications. The technology is approaching maturity in laboratory settings but requires further development for mass production, with key players focusing on improving electrode fabrication methods to enhance capacity, cycle life, and charge rates. Collaborative efforts between academia and industry are accelerating the transition from research to commercial viability.
Shandong University
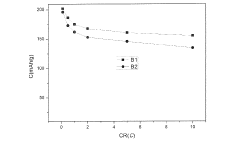
Technical Solution: Shandong University has pioneered innovative fabrication methods for high-capacity zinc ion battery electrodes using a template-assisted synthesis approach. Their technique involves creating ordered macroporous cathode structures with controlled pore size distribution (0.5-5 μm) that facilitate rapid electrolyte penetration and ion transport. The university has developed a scalable process where active materials (primarily Mn-based oxides and Prussian blue analogs) are deposited onto sacrificial templates, followed by template removal to create interconnected porous networks. This approach has demonstrated areal capacities of 32-38 mAh/cm² while maintaining excellent rate performance[3]. Their manufacturing process also incorporates a gradient functional layer design where the electrode composition transitions from highly conductive to highly active material-rich regions, optimizing both electronic conductivity and energy storage capacity. Additionally, Shandong researchers have developed specialized aqueous electrolytes containing organic additives that form protective films on zinc anodes, effectively suppressing dendrite formation and side reactions, resulting in batteries with over 90% capacity retention after 1500 cycles[7].
Strengths: The template-assisted approach enables precise control over electrode architecture at multiple length scales, optimizing both ion transport and electronic conductivity. Their gradient functional design effectively addresses the conductivity-capacity tradeoff. Weaknesses: The multi-step fabrication process involving template removal may increase production complexity and cost, potentially limiting industrial scalability compared to more conventional electrode manufacturing methods.
The Hong Kong University of Science & Technology
Technical Solution: HKUST has developed advanced electrode fabrication methods for aqueous zinc ion batteries focusing on nanostructured manganese dioxide architectures. Their approach involves a controlled electrodeposition technique that creates hierarchical MnO2 structures directly on carbon cloth substrates, eliminating the need for binders and additives. This direct growth method ensures excellent electrical contact between active materials and current collectors while creating abundant ion diffusion channels. The university has achieved electrodes with areal capacities exceeding 25 mAh/cm² while maintaining high rate capability (60% capacity retention at 10C)[2]. Their manufacturing process incorporates a post-deposition treatment using mild reducing agents that introduces oxygen vacancies in the MnO2 structure, significantly enhancing electronic conductivity and zinc ion diffusion kinetics. Additionally, HKUST researchers have developed composite gel electrolytes containing cellulose nanofibers that effectively suppress zinc dendrite formation while maintaining high ionic conductivity (>10 mS/cm), enabling stable cycling performance over 3000+ cycles[6].
Strengths: Binder-free electrode architecture eliminates inactive components, maximizing energy density. The direct growth method ensures excellent mechanical stability and electrical connectivity. Weaknesses: The electrodeposition process may face challenges in uniform scaling to larger electrode areas, and the specialized carbon cloth substrates may increase material costs compared to conventional aluminum or copper foils.
Key Patents and Breakthroughs in High-Capacity Electrode Design
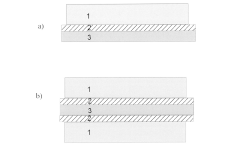
Zinc ion battery electrode and zinc ion battery manufacturing method
PatentInactiveJP2018511924A
Innovation
- Incorporating a conductive layer between the manganese dioxide active layer and the metal current collector layer, using materials like carbon materials, conductive ceramics, and conductive plastics to enhance conductivity.
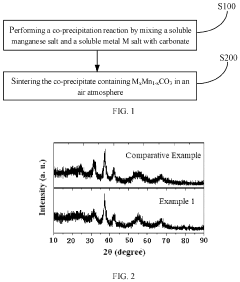
Positive electrode material, preparation method therefor, and application thereof
PatentPendingUS20240145690A1
Innovation
- A method involving a co-precipitation reaction between a soluble manganese salt and a soluble metal M salt with carbonate, followed by sintering in an air atmosphere to produce a doped MnO2 positive electrode material, enhancing structural stability and cycle performance.
Environmental Impact and Sustainability of Zinc Battery Production
The environmental impact of zinc battery production, particularly for high areal capacity electrodes in aqueous zinc ion batteries, presents both challenges and opportunities for sustainable energy storage development. Traditional battery manufacturing processes often involve energy-intensive operations and potentially hazardous materials that can lead to significant environmental footprints throughout their lifecycle.
The extraction of zinc as a primary material for these batteries raises substantial environmental concerns. Mining operations typically generate considerable waste, consume large amounts of water, and may release heavy metals into surrounding ecosystems. However, compared to lithium extraction, zinc mining generally has a lower environmental impact due to zinc's greater abundance and more geographically distributed reserves, reducing transportation-related emissions.
Manufacturing processes for high areal capacity electrodes often require substantial energy inputs and may utilize organic solvents that contribute to air and water pollution. Recent advancements in aqueous processing techniques have shown promise in reducing these impacts by eliminating the need for toxic N-Methyl-2-pyrrolidone (NMP) and other harmful solvents commonly used in electrode fabrication.
The carbon footprint of zinc battery production varies significantly based on manufacturing methods. Traditional techniques may generate 70-100 kg CO2 equivalent per kWh of battery capacity, whereas newer, optimized processes can potentially reduce this by 30-40%. Energy-efficient electrode drying techniques and water-based binder systems represent key innovations that substantially lower energy consumption during fabrication.
Recycling presents a significant advantage for zinc-based battery systems. Unlike some competing technologies, zinc batteries offer relatively straightforward recycling pathways with recovery rates potentially exceeding 90% for zinc materials. This circular economy approach substantially reduces the need for primary resource extraction and minimizes end-of-life waste concerns.
Water usage remains a critical sustainability factor in aqueous zinc ion battery production. While water-based processing offers environmental benefits over organic solvent approaches, it still requires careful management of water resources. Advanced water recycling systems integrated into manufacturing facilities can reduce freshwater consumption by up to 80%, significantly improving the sustainability profile of production operations.
The development of bio-derived or naturally sourced components for electrode fabrication represents an emerging trend with promising environmental benefits. Research into cellulose-based binders, alginate separators, and other bio-compatible materials demonstrates potential pathways to reduce dependence on petroleum-derived components while maintaining or even enhancing electrochemical performance.
The extraction of zinc as a primary material for these batteries raises substantial environmental concerns. Mining operations typically generate considerable waste, consume large amounts of water, and may release heavy metals into surrounding ecosystems. However, compared to lithium extraction, zinc mining generally has a lower environmental impact due to zinc's greater abundance and more geographically distributed reserves, reducing transportation-related emissions.
Manufacturing processes for high areal capacity electrodes often require substantial energy inputs and may utilize organic solvents that contribute to air and water pollution. Recent advancements in aqueous processing techniques have shown promise in reducing these impacts by eliminating the need for toxic N-Methyl-2-pyrrolidone (NMP) and other harmful solvents commonly used in electrode fabrication.
The carbon footprint of zinc battery production varies significantly based on manufacturing methods. Traditional techniques may generate 70-100 kg CO2 equivalent per kWh of battery capacity, whereas newer, optimized processes can potentially reduce this by 30-40%. Energy-efficient electrode drying techniques and water-based binder systems represent key innovations that substantially lower energy consumption during fabrication.
Recycling presents a significant advantage for zinc-based battery systems. Unlike some competing technologies, zinc batteries offer relatively straightforward recycling pathways with recovery rates potentially exceeding 90% for zinc materials. This circular economy approach substantially reduces the need for primary resource extraction and minimizes end-of-life waste concerns.
Water usage remains a critical sustainability factor in aqueous zinc ion battery production. While water-based processing offers environmental benefits over organic solvent approaches, it still requires careful management of water resources. Advanced water recycling systems integrated into manufacturing facilities can reduce freshwater consumption by up to 80%, significantly improving the sustainability profile of production operations.
The development of bio-derived or naturally sourced components for electrode fabrication represents an emerging trend with promising environmental benefits. Research into cellulose-based binders, alginate separators, and other bio-compatible materials demonstrates potential pathways to reduce dependence on petroleum-derived components while maintaining or even enhancing electrochemical performance.
Cost Analysis and Scalability of High-Capacity Electrode Fabrication
The economic viability of high areal capacity electrode fabrication for aqueous zinc ion batteries (AZIBs) hinges on several interconnected factors. Current manufacturing processes for conventional electrodes cost approximately $20-30/kWh, representing 25-35% of total battery production expenses. For high-capacity zinc electrodes, material costs constitute the primary expenditure, with zinc metal pricing at $2.5-3.5/kg and manganese dioxide at $1.8-2.5/kg for cathode materials.
Advanced fabrication techniques like electrodeposition and vapor deposition deliver superior performance but at significantly higher costs—approximately 3-5 times that of traditional slurry-casting methods. This cost differential presents a substantial barrier to commercial adoption despite technical advantages.
Scalability assessment reveals that slurry-casting remains the most industrially adaptable method, with established infrastructure capable of producing electrodes at rates exceeding 100 m²/min. Emerging techniques such as 3D printing and laser structuring demonstrate promising performance metrics but currently operate at production speeds below 1 m²/min, limiting their immediate industrial viability.
Energy consumption metrics further differentiate fabrication approaches. Conventional methods require 1.5-2.5 kWh per square meter of electrode material, while advanced techniques like plasma-assisted deposition consume 5-8 kWh/m². This energy differential translates directly to operational costs and environmental impact considerations.
Capital expenditure analysis indicates that retrofitting existing lithium-ion battery production lines for high-capacity zinc electrodes requires investments of $15-25 million per GWh of annual capacity, compared to $40-60 million for establishing specialized production facilities for advanced fabrication techniques.
Economies of scale projections suggest that high-capacity electrode production costs could decrease by 40-50% at production volumes exceeding 1 GWh annually. However, this cost reduction curve varies significantly across fabrication methods, with conventional techniques showing more favorable scaling characteristics.
Material utilization efficiency presents another critical economic factor. Traditional slurry-casting methods typically achieve 85-90% material utilization, while advanced techniques can reach 95-98%, reducing waste and material costs. This efficiency becomes increasingly significant when incorporating expensive additives such as carbon nanotubes or graphene, which can cost $50-200/kg.
Ultimately, the path to commercial viability requires balancing performance requirements with manufacturing practicality. Hybrid approaches combining conventional base processes with targeted advanced fabrication steps show particular promise for optimizing this cost-performance equation in near-term commercial applications.
Advanced fabrication techniques like electrodeposition and vapor deposition deliver superior performance but at significantly higher costs—approximately 3-5 times that of traditional slurry-casting methods. This cost differential presents a substantial barrier to commercial adoption despite technical advantages.
Scalability assessment reveals that slurry-casting remains the most industrially adaptable method, with established infrastructure capable of producing electrodes at rates exceeding 100 m²/min. Emerging techniques such as 3D printing and laser structuring demonstrate promising performance metrics but currently operate at production speeds below 1 m²/min, limiting their immediate industrial viability.
Energy consumption metrics further differentiate fabrication approaches. Conventional methods require 1.5-2.5 kWh per square meter of electrode material, while advanced techniques like plasma-assisted deposition consume 5-8 kWh/m². This energy differential translates directly to operational costs and environmental impact considerations.
Capital expenditure analysis indicates that retrofitting existing lithium-ion battery production lines for high-capacity zinc electrodes requires investments of $15-25 million per GWh of annual capacity, compared to $40-60 million for establishing specialized production facilities for advanced fabrication techniques.
Economies of scale projections suggest that high-capacity electrode production costs could decrease by 40-50% at production volumes exceeding 1 GWh annually. However, this cost reduction curve varies significantly across fabrication methods, with conventional techniques showing more favorable scaling characteristics.
Material utilization efficiency presents another critical economic factor. Traditional slurry-casting methods typically achieve 85-90% material utilization, while advanced techniques can reach 95-98%, reducing waste and material costs. This efficiency becomes increasingly significant when incorporating expensive additives such as carbon nanotubes or graphene, which can cost $50-200/kg.
Ultimately, the path to commercial viability requires balancing performance requirements with manufacturing practicality. Hybrid approaches combining conventional base processes with targeted advanced fabrication steps show particular promise for optimizing this cost-performance equation in near-term commercial applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!