Modeling Ion Transport And Reaction Kinetics In Aqueous Zinc Ion Batteries
SEP 12, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Zinc Ion Battery Technology Evolution and Objectives
Aqueous zinc ion batteries (AZIBs) have emerged as a promising energy storage technology over the past decade, evolving from early conceptual designs to increasingly sophisticated systems with improved performance metrics. The development trajectory of AZIBs can be traced back to the 1980s when initial explorations into zinc-based electrochemical systems began, though significant advancements have primarily occurred since 2010 with the discovery of compatible cathode materials and electrolyte formulations.
The evolution of zinc ion battery technology has been driven by the fundamental advantages these systems offer: high theoretical capacity (820 mAh/g), abundant raw materials, inherent safety due to aqueous electrolytes, and potential cost-effectiveness compared to lithium-ion alternatives. Early research focused primarily on manganese oxide cathodes, but the technology landscape has expanded to include vanadium-based compounds, Prussian blue analogs, and organic cathode materials, each representing distinct evolutionary branches in the technology's development.
A critical milestone in AZIB development was the identification and mitigation of zinc dendrite formation, which historically limited cycle life and reliability. Subsequent innovations in electrolyte engineering, particularly the introduction of mild acidic electrolytes and various additives, have substantially improved the reversibility of zinc plating/stripping processes, marking a significant technological leap forward.
The modeling of ion transport and reaction kinetics represents the current frontier in AZIB research, with objectives centered on developing comprehensive mathematical frameworks that accurately describe the complex electrochemical processes occurring within these systems. These models aim to capture multiscale phenomena ranging from atomic-level interactions to macroscopic performance characteristics.
Primary technical objectives in this domain include: establishing predictive models for zinc ion diffusion in various electrolyte compositions; developing accurate representations of interfacial phenomena at electrode-electrolyte boundaries; creating multi-physics simulations that integrate electrochemical, thermal, and mechanical aspects; and formulating computational tools that can accelerate materials discovery and optimization for next-generation AZIBs.
The ultimate goal of these modeling efforts is to enable rational design principles for AZIB components, moving beyond empirical approaches to theory-guided development. This transition is expected to significantly accelerate the technology's maturation, potentially enabling performance metrics that rival or exceed current commercial battery technologies while maintaining the inherent advantages of aqueous zinc-based systems.
The evolution of zinc ion battery technology has been driven by the fundamental advantages these systems offer: high theoretical capacity (820 mAh/g), abundant raw materials, inherent safety due to aqueous electrolytes, and potential cost-effectiveness compared to lithium-ion alternatives. Early research focused primarily on manganese oxide cathodes, but the technology landscape has expanded to include vanadium-based compounds, Prussian blue analogs, and organic cathode materials, each representing distinct evolutionary branches in the technology's development.
A critical milestone in AZIB development was the identification and mitigation of zinc dendrite formation, which historically limited cycle life and reliability. Subsequent innovations in electrolyte engineering, particularly the introduction of mild acidic electrolytes and various additives, have substantially improved the reversibility of zinc plating/stripping processes, marking a significant technological leap forward.
The modeling of ion transport and reaction kinetics represents the current frontier in AZIB research, with objectives centered on developing comprehensive mathematical frameworks that accurately describe the complex electrochemical processes occurring within these systems. These models aim to capture multiscale phenomena ranging from atomic-level interactions to macroscopic performance characteristics.
Primary technical objectives in this domain include: establishing predictive models for zinc ion diffusion in various electrolyte compositions; developing accurate representations of interfacial phenomena at electrode-electrolyte boundaries; creating multi-physics simulations that integrate electrochemical, thermal, and mechanical aspects; and formulating computational tools that can accelerate materials discovery and optimization for next-generation AZIBs.
The ultimate goal of these modeling efforts is to enable rational design principles for AZIB components, moving beyond empirical approaches to theory-guided development. This transition is expected to significantly accelerate the technology's maturation, potentially enabling performance metrics that rival or exceed current commercial battery technologies while maintaining the inherent advantages of aqueous zinc-based systems.
Market Analysis for Aqueous Zinc Battery Systems
The global market for aqueous zinc ion batteries (AZIBs) is experiencing significant growth, driven by increasing demand for sustainable energy storage solutions. Current market valuations indicate that the aqueous battery sector is expanding at a compound annual growth rate of approximately 8-10%, with zinc-based systems capturing an increasing share due to their inherent safety advantages and cost-effectiveness compared to lithium-ion alternatives.
The commercial landscape for AZIBs is particularly promising in grid-scale energy storage applications, where safety considerations and long-term cost metrics outweigh energy density limitations. Market research indicates that utility companies are increasingly exploring zinc-based solutions for renewable energy integration, with several pilot projects demonstrating successful implementation across North America, Europe, and Asia.
Consumer electronics represents another emerging market segment for AZIBs, particularly in applications where safety is paramount. The non-flammable nature of aqueous electrolytes provides a compelling value proposition for devices used in sensitive environments such as healthcare, transportation, and industrial settings.
Regional analysis reveals that Asia-Pacific currently dominates the manufacturing landscape for zinc battery components, with China leading production capacity. However, North American and European markets are showing accelerated growth in adoption rates, supported by favorable regulatory frameworks promoting safer battery technologies and domestic supply chain development initiatives.
Market barriers for wider AZIB adoption include performance limitations related to dendrite formation and capacity fade during cycling—precisely the issues that advanced modeling of ion transport and reaction kinetics aims to address. Industry reports suggest that overcoming these technical challenges could unlock a market potential exceeding $5 billion by 2030.
Competitive analysis indicates that while traditional battery manufacturers are gradually incorporating aqueous zinc technology into their portfolios, several specialized startups focused exclusively on zinc-based chemistry have secured significant venture capital funding in recent years. These companies are leveraging advanced modeling techniques to optimize electrolyte compositions and electrode architectures.
End-user feedback highlights that improved cycle life and higher energy density remain the primary requirements for broader market acceptance. Market surveys indicate that achieving 2000+ cycles with energy densities above 100 Wh/kg would position AZIBs as viable alternatives in multiple applications currently dominated by lithium-ion technologies.
The economic value proposition of AZIBs is further strengthened by zinc's abundant global reserves and established recycling infrastructure, offering long-term price stability advantages over lithium and cobalt-based systems.
The commercial landscape for AZIBs is particularly promising in grid-scale energy storage applications, where safety considerations and long-term cost metrics outweigh energy density limitations. Market research indicates that utility companies are increasingly exploring zinc-based solutions for renewable energy integration, with several pilot projects demonstrating successful implementation across North America, Europe, and Asia.
Consumer electronics represents another emerging market segment for AZIBs, particularly in applications where safety is paramount. The non-flammable nature of aqueous electrolytes provides a compelling value proposition for devices used in sensitive environments such as healthcare, transportation, and industrial settings.
Regional analysis reveals that Asia-Pacific currently dominates the manufacturing landscape for zinc battery components, with China leading production capacity. However, North American and European markets are showing accelerated growth in adoption rates, supported by favorable regulatory frameworks promoting safer battery technologies and domestic supply chain development initiatives.
Market barriers for wider AZIB adoption include performance limitations related to dendrite formation and capacity fade during cycling—precisely the issues that advanced modeling of ion transport and reaction kinetics aims to address. Industry reports suggest that overcoming these technical challenges could unlock a market potential exceeding $5 billion by 2030.
Competitive analysis indicates that while traditional battery manufacturers are gradually incorporating aqueous zinc technology into their portfolios, several specialized startups focused exclusively on zinc-based chemistry have secured significant venture capital funding in recent years. These companies are leveraging advanced modeling techniques to optimize electrolyte compositions and electrode architectures.
End-user feedback highlights that improved cycle life and higher energy density remain the primary requirements for broader market acceptance. Market surveys indicate that achieving 2000+ cycles with energy densities above 100 Wh/kg would position AZIBs as viable alternatives in multiple applications currently dominated by lithium-ion technologies.
The economic value proposition of AZIBs is further strengthened by zinc's abundant global reserves and established recycling infrastructure, offering long-term price stability advantages over lithium and cobalt-based systems.
Current Challenges in Ion Transport Modeling
Despite significant advancements in aqueous zinc ion battery (AZIB) technology, modeling ion transport mechanisms remains one of the most challenging aspects in this field. Current models struggle to accurately capture the complex multi-physics phenomena occurring within these systems. The primary difficulty lies in simultaneously accounting for multiple transport mechanisms including diffusion, migration, and convection, while also considering the unique properties of zinc ions in aqueous electrolytes.
Existing models often fail to adequately represent the concentration-dependent diffusion coefficients that characterize zinc ion movement in aqueous solutions. This becomes particularly problematic at high current densities where concentration gradients become steep and traditional Nernst-Planck equations may no longer provide accurate predictions. Furthermore, the strong electrostatic interactions between zinc ions and counter-ions create complex activity coefficient variations that most models oversimplify.
Another significant challenge is accurately modeling the electrode-electrolyte interface, where zinc deposition and dissolution occur. Current approaches struggle to capture the dynamic nature of this interface, particularly the evolution of surface morphology during cycling. The formation of dendrites, which significantly impacts transport pathways and overall battery performance, remains poorly represented in most computational frameworks.
The multi-scale nature of transport phenomena presents additional modeling difficulties. Processes ranging from atomic-level interactions to macroscopic fluid dynamics must be integrated coherently, but current models typically focus on limited scale ranges, missing critical cross-scale interactions. This limitation becomes particularly evident when attempting to predict long-term cycling behavior based on fundamental transport principles.
Water molecules in aqueous electrolytes introduce another layer of complexity through hydration effects and hydrogen bonding networks that influence zinc ion mobility. Most current models treat the solvent as a continuous medium with fixed properties, neglecting these molecular-level interactions that significantly affect transport kinetics.
Computational limitations further constrain modeling capabilities. Full molecular dynamics simulations that could potentially capture the complete transport behavior are prohibitively expensive for practical battery dimensions and realistic time scales. Meanwhile, continuum models that are computationally efficient often sacrifice accuracy by neglecting critical microscopic phenomena.
The integration of transport models with electrochemical reaction kinetics represents perhaps the most formidable challenge. The coupling between local ion concentrations, electric potential distributions, and reaction rates creates a highly non-linear system that is mathematically complex to solve, particularly when considering the spatial heterogeneity of real battery systems.
Existing models often fail to adequately represent the concentration-dependent diffusion coefficients that characterize zinc ion movement in aqueous solutions. This becomes particularly problematic at high current densities where concentration gradients become steep and traditional Nernst-Planck equations may no longer provide accurate predictions. Furthermore, the strong electrostatic interactions between zinc ions and counter-ions create complex activity coefficient variations that most models oversimplify.
Another significant challenge is accurately modeling the electrode-electrolyte interface, where zinc deposition and dissolution occur. Current approaches struggle to capture the dynamic nature of this interface, particularly the evolution of surface morphology during cycling. The formation of dendrites, which significantly impacts transport pathways and overall battery performance, remains poorly represented in most computational frameworks.
The multi-scale nature of transport phenomena presents additional modeling difficulties. Processes ranging from atomic-level interactions to macroscopic fluid dynamics must be integrated coherently, but current models typically focus on limited scale ranges, missing critical cross-scale interactions. This limitation becomes particularly evident when attempting to predict long-term cycling behavior based on fundamental transport principles.
Water molecules in aqueous electrolytes introduce another layer of complexity through hydration effects and hydrogen bonding networks that influence zinc ion mobility. Most current models treat the solvent as a continuous medium with fixed properties, neglecting these molecular-level interactions that significantly affect transport kinetics.
Computational limitations further constrain modeling capabilities. Full molecular dynamics simulations that could potentially capture the complete transport behavior are prohibitively expensive for practical battery dimensions and realistic time scales. Meanwhile, continuum models that are computationally efficient often sacrifice accuracy by neglecting critical microscopic phenomena.
The integration of transport models with electrochemical reaction kinetics represents perhaps the most formidable challenge. The coupling between local ion concentrations, electric potential distributions, and reaction rates creates a highly non-linear system that is mathematically complex to solve, particularly when considering the spatial heterogeneity of real battery systems.
State-of-the-Art Ion Transport Simulation Methods
01 Electrolyte modifications for enhanced ion transport
Various electrolyte modifications can significantly improve zinc ion transport in aqueous zinc ion batteries. These include using water-in-salt electrolytes to reduce water activity, adding specific salts to regulate the solvation structure, incorporating ionic liquids to modify the electrolyte-electrode interface, and using gel electrolytes to enhance ion conductivity while suppressing dendrite formation. These modifications help overcome the slow diffusion kinetics of zinc ions in traditional aqueous electrolytes.- Electrolyte modifications for enhanced ion transport: Various electrolyte modifications can significantly improve zinc ion transport in aqueous zinc batteries. These include using high-concentration electrolytes, adding specific salts or organic additives, and incorporating water-in-salt electrolytes. These modifications help to reduce water activity, suppress hydrogen evolution, form stable solid electrolyte interphases, and create favorable zinc ion solvation structures, all of which contribute to faster ion transport and improved electrochemical performance.
- Cathode material engineering for improved reaction kinetics: Engineering cathode materials at the nanoscale or with specific structures can significantly enhance reaction kinetics in aqueous zinc ion batteries. Strategies include creating hierarchical structures, introducing defects, doping with heteroatoms, and designing materials with expanded interlayer spacing. These approaches facilitate faster zinc ion diffusion, provide more active sites for redox reactions, and accommodate structural changes during cycling, resulting in improved rate capability and cycling stability.
- Interface optimization to reduce transport barriers: Optimizing the electrode-electrolyte interface is crucial for reducing transport barriers in aqueous zinc ion batteries. This can be achieved through surface coatings, interface engineering, and the use of functional interlayers. These modifications help to stabilize the interface, prevent side reactions, reduce charge transfer resistance, and create favorable pathways for zinc ion transport, ultimately enhancing the overall reaction kinetics and battery performance.
- Advanced characterization of ion transport mechanisms: Advanced in-situ and ex-situ characterization techniques provide deep insights into zinc ion transport and reaction mechanisms in aqueous batteries. These include synchrotron-based X-ray techniques, neutron diffraction, cryo-electron microscopy, and various spectroscopic methods. These techniques help to understand solvation structures, diffusion pathways, phase transformations, and degradation mechanisms, guiding the rational design of materials and electrolytes for improved battery performance.
- Novel cell designs and architectures: Innovative cell designs and architectures can significantly enhance ion transport and reaction kinetics in aqueous zinc batteries. These include 3D electrode structures, flow cell configurations, flexible/stretchable designs, and hybrid systems. Such designs help to shorten ion diffusion paths, improve electrolyte penetration, enhance mass transport, and accommodate volume changes during cycling, leading to batteries with higher power density and better rate performance.
02 Cathode material design for improved reaction kinetics
Advanced cathode materials with optimized structures can significantly enhance the reaction kinetics in aqueous zinc ion batteries. Strategies include developing layered materials with expanded interlayer spacing to facilitate zinc ion insertion/extraction, creating hierarchical porous structures to shorten ion diffusion paths, and incorporating conductive additives to improve electron transfer. These design approaches address the sluggish kinetics often observed at the cathode-electrolyte interface.Expand Specific Solutions03 Interface engineering to reduce energy barriers
Interface engineering techniques focus on modifying the electrode-electrolyte interfaces to reduce energy barriers for zinc ion transport. Methods include surface coating of electrodes with ion-conductive materials, creating artificial solid-electrolyte interphases, and introducing functional groups that facilitate ion transfer. These approaches help minimize interfacial resistance and side reactions, leading to improved cycling stability and rate performance of aqueous zinc ion batteries.Expand Specific Solutions04 Zinc anode stabilization techniques
Various techniques can be employed to stabilize the zinc anode and improve its reaction kinetics. These include using 3D structured zinc anodes to distribute current density more uniformly, adding specific additives to the electrolyte to regulate zinc deposition, applying protective coatings to prevent side reactions, and introducing nucleation sites to guide zinc plating. These approaches effectively address issues like dendrite formation and hydrogen evolution that typically plague zinc anodes in aqueous environments.Expand Specific Solutions05 Advanced characterization and modeling of ion transport mechanisms
Advanced characterization techniques and theoretical modeling provide deeper insights into zinc ion transport and reaction mechanisms. Methods include in-situ/operando spectroscopy to observe real-time ion movement, advanced microscopy to visualize structural changes, and computational simulations to predict ion diffusion pathways and energy barriers. These approaches help identify rate-limiting steps in the reaction kinetics and guide the rational design of materials and systems with enhanced performance.Expand Specific Solutions
Leading Research Groups and Industry Players
Aqueous zinc ion batteries are currently in an early growth phase, with the market expanding due to increasing demand for sustainable energy storage solutions. The technology is gaining traction as a promising alternative to lithium-ion batteries, with a projected market size reaching several billion dollars by 2030. Technical maturity remains moderate, with significant research activity from academic institutions like Jilin University, University of Waterloo, and City University of Hong Kong leading fundamental research on ion transport mechanisms. Commercial development is accelerating through companies such as Honeycomb Battery Co., Alsym Energy, and ZNL Energy AS, which are working to overcome challenges in electrode stability and electrolyte design. Major corporations including Samsung Electronics and PetroChina are also investing in this technology, indicating growing industrial interest in scaling these solutions for commercial applications.
Jilin University
Technical Solution: Jilin University has developed a comprehensive multi-scale modeling framework for aqueous zinc ion batteries (AZIBs) that integrates molecular dynamics simulations with continuum-level transport models. Their approach focuses on accurately capturing the solvation structure of Zn2+ ions in various electrolyte compositions and correlating these structures with macroscopic transport properties. The research team has implemented advanced computational methods to model the desolvation process at electrode interfaces, which is critical for understanding the rate-limiting steps in zinc ion insertion/extraction. Their models incorporate the effects of pH, electrolyte concentration, and temperature on reaction kinetics, enabling precise prediction of battery performance under various operating conditions. The university has also developed specialized algorithms to account for zinc dendrite formation mechanisms, which has been a significant challenge in AZIB development.
Strengths: Strong integration of atomistic and continuum modeling approaches provides comprehensive insights across multiple scales. Their models effectively predict dendrite formation, a critical failure mode in zinc batteries. Weaknesses: Computational models require extensive validation with experimental data, which may limit immediate practical application. The approach may be too academically focused for direct industrial implementation.
The University of Waterloo
Technical Solution: The University of Waterloo has pioneered a data-driven modeling approach for aqueous zinc ion batteries that combines electrochemical impedance spectroscopy (EIS) with machine learning algorithms to characterize ion transport phenomena. Their technical solution incorporates a novel phase-field model that captures the spatiotemporal evolution of zinc deposition/dissolution processes at the electrode-electrolyte interface. The research team has developed a unique mathematical framework that accounts for the complex interplay between electrolyte composition, surface energy, and interfacial kinetics. Their models specifically address the challenges of passivation layer formation and its impact on long-term cycling stability. The university has also created advanced numerical methods to solve the coupled partial differential equations that describe the multi-physics nature of zinc ion transport in aqueous electrolytes, enabling accurate prediction of concentration gradients and potential distributions within the battery system.
Strengths: Advanced integration of experimental data with computational models enhances predictive accuracy. Their phase-field approach effectively captures the dynamic nature of electrode-electrolyte interfaces. Weaknesses: The computational complexity of their models may require significant computing resources, limiting real-time applications. The approach may need further refinement to account for all degradation mechanisms in practical battery systems.
Critical Patents in Zinc Ion Battery Modeling
Method of preparing vse2 material and electrochemical whole cell and electrochemical symmetric cell using the same
PatentPendingUS20240072255A1
Innovation
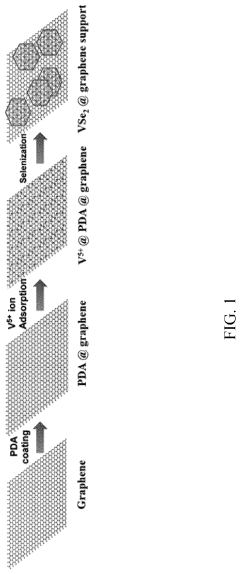
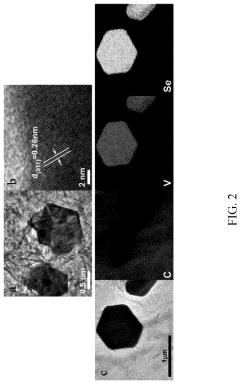
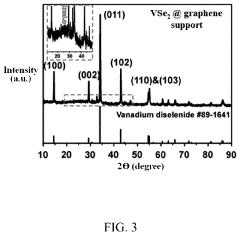
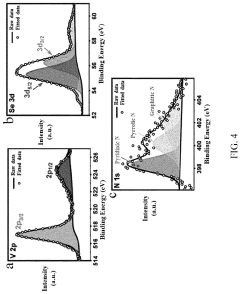
- A method involving the preparation of VSe2 material with a graphene support composite using chemical vapor deposition and polydopamine confinement, applied as a functional layer on the Zn anode to promote aligned (002) zinc deposition and inhibit hydrogen evolution reactions.
Environmental Impact and Sustainability Assessment
Aqueous zinc ion batteries (AZIBs) represent a promising sustainable energy storage technology, yet their environmental implications require thorough assessment. The manufacturing processes for AZIBs involve significantly lower energy consumption compared to lithium-ion counterparts, primarily due to the aqueous electrolyte preparation which eliminates energy-intensive dry room requirements. This energy reduction translates to approximately 20-30% lower carbon emissions during production phases.
The raw material acquisition for zinc-based batteries presents notable sustainability advantages. Zinc is abundantly available in the earth's crust (approximately 75 ppm), ranking 24th in element abundance globally. Unlike lithium, zinc mining operations generally create less ecological disruption and require less water consumption. The established zinc recycling infrastructure, with global recovery rates exceeding 70% in developed economies, further enhances the sustainability profile of these systems.
Water utilization in AZIB manufacturing requires careful consideration. While the aqueous nature of these batteries reduces organic solvent usage, water quality management remains essential. Current modeling approaches have begun incorporating water footprint assessments, indicating that AZIBs require approximately 35-45% less water throughout their lifecycle compared to conventional battery technologies when accounting for both direct and virtual water consumption.
Toxicity profiles of zinc-based systems demonstrate favorable characteristics compared to heavy metal alternatives. Zinc, as an essential micronutrient, presents lower environmental persistence and bioaccumulation risks than cobalt or nickel. However, modeling studies indicate potential aquatic toxicity concerns if electrolyte leakage occurs. Advanced ion transport models now incorporate environmental fate parameters to predict zinc mobility in various environmental compartments following potential release scenarios.
End-of-life management represents a critical sustainability factor. Current reaction kinetics models are being expanded to include degradation pathways relevant to recycling processes. These models suggest that approximately 85-95% of zinc materials can be theoretically recovered and reused, significantly reducing the need for primary resource extraction. The simplicity of aqueous systems facilitates separation processes during recycling, requiring approximately 40% less energy than equivalent processes for organic electrolyte-based batteries.
Life cycle assessment (LCA) studies incorporating ion transport and reaction kinetics data indicate that AZIBs could achieve 50-60% lower global warming potential compared to conventional lithium-ion technologies when evaluated on a full cradle-to-grave basis. These environmental advantages become particularly pronounced in grid-scale applications where battery weight constraints are less restrictive and the safety benefits of non-flammable aqueous electrolytes provide additional sustainability value through reduced risk management requirements.
The raw material acquisition for zinc-based batteries presents notable sustainability advantages. Zinc is abundantly available in the earth's crust (approximately 75 ppm), ranking 24th in element abundance globally. Unlike lithium, zinc mining operations generally create less ecological disruption and require less water consumption. The established zinc recycling infrastructure, with global recovery rates exceeding 70% in developed economies, further enhances the sustainability profile of these systems.
Water utilization in AZIB manufacturing requires careful consideration. While the aqueous nature of these batteries reduces organic solvent usage, water quality management remains essential. Current modeling approaches have begun incorporating water footprint assessments, indicating that AZIBs require approximately 35-45% less water throughout their lifecycle compared to conventional battery technologies when accounting for both direct and virtual water consumption.
Toxicity profiles of zinc-based systems demonstrate favorable characteristics compared to heavy metal alternatives. Zinc, as an essential micronutrient, presents lower environmental persistence and bioaccumulation risks than cobalt or nickel. However, modeling studies indicate potential aquatic toxicity concerns if electrolyte leakage occurs. Advanced ion transport models now incorporate environmental fate parameters to predict zinc mobility in various environmental compartments following potential release scenarios.
End-of-life management represents a critical sustainability factor. Current reaction kinetics models are being expanded to include degradation pathways relevant to recycling processes. These models suggest that approximately 85-95% of zinc materials can be theoretically recovered and reused, significantly reducing the need for primary resource extraction. The simplicity of aqueous systems facilitates separation processes during recycling, requiring approximately 40% less energy than equivalent processes for organic electrolyte-based batteries.
Life cycle assessment (LCA) studies incorporating ion transport and reaction kinetics data indicate that AZIBs could achieve 50-60% lower global warming potential compared to conventional lithium-ion technologies when evaluated on a full cradle-to-grave basis. These environmental advantages become particularly pronounced in grid-scale applications where battery weight constraints are less restrictive and the safety benefits of non-flammable aqueous electrolytes provide additional sustainability value through reduced risk management requirements.
Safety Standards and Performance Benchmarking
The development of safety standards for aqueous zinc ion batteries (AZIBs) represents a critical aspect of their commercialization pathway. Unlike lithium-ion batteries, which have well-established safety protocols, AZIBs require specialized standards that account for their unique aqueous chemistry and zinc metal anode behaviors. Current safety evaluations typically focus on thermal stability, electrolyte leakage prevention, and dendrite formation mitigation—all of which must be quantitatively assessed through standardized testing procedures.
Performance benchmarking for AZIBs necessitates comprehensive metrics that extend beyond traditional parameters. While energy density, power density, and cycle life remain fundamental, additional metrics specific to zinc-based systems must be incorporated, including zinc plating efficiency, dendrite suppression capability, and pH stability during extended cycling. The International Electrotechnical Commission (IEC) and ASTM International have begun developing preliminary testing protocols, though these remain in early stages compared to lithium-ion battery standards.
Computational modeling plays an essential role in establishing these benchmarks by predicting safety thresholds and performance limitations. Models that accurately capture zinc ion transport and reaction kinetics can identify potential failure modes before they manifest in physical testing. This predictive capability is particularly valuable for establishing accelerated testing protocols that can reliably simulate years of operational degradation within compressed timeframes.
Industry consortia, including the Zinc Battery Initiative and the Consortium for Battery Innovation, have proposed standardized testing methodologies specifically designed for aqueous zinc systems. These include protocols for evaluating self-discharge rates, shelf-life in various environmental conditions, and resistance to capacity fade during partial state-of-charge cycling—all critical parameters for grid storage applications where AZIBs show particular promise.
The benchmarking landscape is further complicated by the diversity of AZIB chemistries, with manganese oxide, vanadium-based, and Prussian blue analog cathodes each requiring tailored performance metrics. Computational models must therefore be adaptable to various material combinations while maintaining predictive accuracy. Recent round-robin testing among leading research institutions has demonstrated that variations in testing protocols can lead to significant discrepancies in reported performance, highlighting the urgent need for standardization.
Environmental considerations also factor into modern benchmarking standards, with metrics increasingly incorporating sustainability parameters such as water consumption during manufacturing, recyclability of components, and environmental impact of electrolyte disposal. These factors are particularly relevant for AZIBs, which are often positioned as environmentally superior alternatives to lithium-ion technologies.
Performance benchmarking for AZIBs necessitates comprehensive metrics that extend beyond traditional parameters. While energy density, power density, and cycle life remain fundamental, additional metrics specific to zinc-based systems must be incorporated, including zinc plating efficiency, dendrite suppression capability, and pH stability during extended cycling. The International Electrotechnical Commission (IEC) and ASTM International have begun developing preliminary testing protocols, though these remain in early stages compared to lithium-ion battery standards.
Computational modeling plays an essential role in establishing these benchmarks by predicting safety thresholds and performance limitations. Models that accurately capture zinc ion transport and reaction kinetics can identify potential failure modes before they manifest in physical testing. This predictive capability is particularly valuable for establishing accelerated testing protocols that can reliably simulate years of operational degradation within compressed timeframes.
Industry consortia, including the Zinc Battery Initiative and the Consortium for Battery Innovation, have proposed standardized testing methodologies specifically designed for aqueous zinc systems. These include protocols for evaluating self-discharge rates, shelf-life in various environmental conditions, and resistance to capacity fade during partial state-of-charge cycling—all critical parameters for grid storage applications where AZIBs show particular promise.
The benchmarking landscape is further complicated by the diversity of AZIB chemistries, with manganese oxide, vanadium-based, and Prussian blue analog cathodes each requiring tailored performance metrics. Computational models must therefore be adaptable to various material combinations while maintaining predictive accuracy. Recent round-robin testing among leading research institutions has demonstrated that variations in testing protocols can lead to significant discrepancies in reported performance, highlighting the urgent need for standardization.
Environmental considerations also factor into modern benchmarking standards, with metrics increasingly incorporating sustainability parameters such as water consumption during manufacturing, recyclability of components, and environmental impact of electrolyte disposal. These factors are particularly relevant for AZIBs, which are often positioned as environmentally superior alternatives to lithium-ion technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!