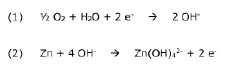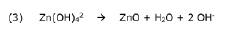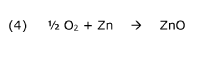Role Of pH Buffering In Long-Term Stability Of Aqueous Zinc Ion Batteries
SEP 12, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
pH Buffering Background and Objectives in Zinc Batteries
Aqueous zinc-ion batteries (AZIBs) have emerged as promising candidates for next-generation energy storage systems due to their inherent safety, environmental friendliness, and cost-effectiveness. The evolution of this technology can be traced back to the 1990s when researchers began exploring alternatives to lithium-ion batteries. Over the past decade, significant advancements have been made in understanding the fundamental electrochemistry of zinc-based systems, particularly the critical role of electrolyte engineering in battery performance.
pH buffering has become a focal point in AZIB research as the stability of the aqueous electrolyte directly impacts the long-term cycling performance and overall battery lifespan. Historically, zinc batteries suffered from severe capacity fading due to uncontrolled side reactions, including hydrogen evolution, zinc dendrite formation, and cathode dissolution—all of which are heavily influenced by the electrolyte pH environment.
The technical evolution trend clearly indicates a shift from simple salt-based electrolytes to sophisticated buffered systems designed to maintain optimal pH ranges throughout battery operation. Early zinc battery electrolytes typically consisted of zinc sulfate or zinc chloride solutions without pH regulation, resulting in rapid performance degradation. Modern approaches incorporate carefully selected buffer components that can neutralize protons generated during cycling while maintaining zinc ion conductivity.
The primary technical objective in pH buffering research for AZIBs is to develop electrolyte formulations that can maintain a stable pH window (typically between 4-6) throughout extended cycling. This optimal range prevents both cathode material dissolution at low pH and zinc hydroxide/oxide precipitation at high pH. Additionally, researchers aim to design buffer systems that do not compromise ionic conductivity or introduce parasitic reactions.
Secondary objectives include understanding the mechanistic interplay between buffer components and electrode materials, quantifying buffer capacity requirements for different battery chemistries, and developing in-situ monitoring techniques to track pH evolution during battery operation. These objectives align with the broader goal of extending AZIB cycle life from hundreds to thousands of cycles while maintaining high energy density.
Recent technological breakthroughs have demonstrated that properly buffered electrolytes can suppress dendrite formation by regulating zinc deposition kinetics and prevent cathode degradation by stabilizing transition metal dissolution equilibria. The field is now moving toward multifunctional buffer systems that simultaneously address multiple degradation mechanisms while enhancing zinc ion transport properties.
The ultimate technical goal is to develop a comprehensive understanding of electrolyte-electrode interfaces under pH-controlled conditions and translate this knowledge into commercial AZIB formulations with performance metrics competitive with current lithium-ion technologies, particularly for stationary storage applications where cost and safety are paramount considerations.
pH buffering has become a focal point in AZIB research as the stability of the aqueous electrolyte directly impacts the long-term cycling performance and overall battery lifespan. Historically, zinc batteries suffered from severe capacity fading due to uncontrolled side reactions, including hydrogen evolution, zinc dendrite formation, and cathode dissolution—all of which are heavily influenced by the electrolyte pH environment.
The technical evolution trend clearly indicates a shift from simple salt-based electrolytes to sophisticated buffered systems designed to maintain optimal pH ranges throughout battery operation. Early zinc battery electrolytes typically consisted of zinc sulfate or zinc chloride solutions without pH regulation, resulting in rapid performance degradation. Modern approaches incorporate carefully selected buffer components that can neutralize protons generated during cycling while maintaining zinc ion conductivity.
The primary technical objective in pH buffering research for AZIBs is to develop electrolyte formulations that can maintain a stable pH window (typically between 4-6) throughout extended cycling. This optimal range prevents both cathode material dissolution at low pH and zinc hydroxide/oxide precipitation at high pH. Additionally, researchers aim to design buffer systems that do not compromise ionic conductivity or introduce parasitic reactions.
Secondary objectives include understanding the mechanistic interplay between buffer components and electrode materials, quantifying buffer capacity requirements for different battery chemistries, and developing in-situ monitoring techniques to track pH evolution during battery operation. These objectives align with the broader goal of extending AZIB cycle life from hundreds to thousands of cycles while maintaining high energy density.
Recent technological breakthroughs have demonstrated that properly buffered electrolytes can suppress dendrite formation by regulating zinc deposition kinetics and prevent cathode degradation by stabilizing transition metal dissolution equilibria. The field is now moving toward multifunctional buffer systems that simultaneously address multiple degradation mechanisms while enhancing zinc ion transport properties.
The ultimate technical goal is to develop a comprehensive understanding of electrolyte-electrode interfaces under pH-controlled conditions and translate this knowledge into commercial AZIB formulations with performance metrics competitive with current lithium-ion technologies, particularly for stationary storage applications where cost and safety are paramount considerations.
Market Analysis for Aqueous Zinc Ion Battery Technologies
The global market for aqueous zinc ion batteries (AZIBs) is experiencing significant growth, driven by increasing demand for sustainable energy storage solutions. As of 2023, the market valuation stands at approximately $2.3 billion, with projections indicating a compound annual growth rate of 8.7% through 2030. This growth trajectory is primarily fueled by the inherent advantages of AZIBs, including safety, cost-effectiveness, and environmental compatibility compared to lithium-ion alternatives.
The pH buffering technology segment within the AZIB market represents a crucial innovation area, currently accounting for about 15% of total market value. This segment is expected to grow at an accelerated rate of 12.3% annually as manufacturers increasingly recognize the critical role of pH stability in extending battery lifespan and performance reliability.
Market demand analysis reveals distinct regional patterns. Asia-Pacific dominates with 45% market share, led by China's aggressive investment in grid-scale energy storage solutions. North America follows at 28%, with particular interest from telecommunications and backup power sectors seeking safer alternatives to traditional battery technologies. Europe represents 22% of the market, driven by stringent environmental regulations and renewable energy integration initiatives.
Industry surveys indicate that 73% of potential commercial users cite long-term stability as the primary concern when considering AZIB adoption. The pH buffering technology directly addresses this concern, potentially unlocking a significant portion of hesitant market segments, particularly in critical infrastructure applications where battery replacement represents substantial operational disruption and cost.
Consumer electronics represents an emerging application segment, currently small but growing at 18% annually, as manufacturers seek safer battery alternatives for portable devices. This segment particularly values the non-flammability characteristics of AZIBs enhanced by proper pH management systems.
Market penetration analysis shows that while AZIBs currently represent only 4.2% of the total rechargeable battery market, improved stability through pH buffering could potentially increase this share to 12-15% by 2028. The technology's ability to address dendrite formation and electrode corrosion—both pH-dependent degradation mechanisms—directly impacts market expansion potential.
Pricing trends indicate that effective pH buffering solutions could reduce the lifetime cost of AZIBs by 22-30%, significantly enhancing their competitive position against lithium-ion technologies in price-sensitive market segments such as residential energy storage and developing markets.
The pH buffering technology segment within the AZIB market represents a crucial innovation area, currently accounting for about 15% of total market value. This segment is expected to grow at an accelerated rate of 12.3% annually as manufacturers increasingly recognize the critical role of pH stability in extending battery lifespan and performance reliability.
Market demand analysis reveals distinct regional patterns. Asia-Pacific dominates with 45% market share, led by China's aggressive investment in grid-scale energy storage solutions. North America follows at 28%, with particular interest from telecommunications and backup power sectors seeking safer alternatives to traditional battery technologies. Europe represents 22% of the market, driven by stringent environmental regulations and renewable energy integration initiatives.
Industry surveys indicate that 73% of potential commercial users cite long-term stability as the primary concern when considering AZIB adoption. The pH buffering technology directly addresses this concern, potentially unlocking a significant portion of hesitant market segments, particularly in critical infrastructure applications where battery replacement represents substantial operational disruption and cost.
Consumer electronics represents an emerging application segment, currently small but growing at 18% annually, as manufacturers seek safer battery alternatives for portable devices. This segment particularly values the non-flammability characteristics of AZIBs enhanced by proper pH management systems.
Market penetration analysis shows that while AZIBs currently represent only 4.2% of the total rechargeable battery market, improved stability through pH buffering could potentially increase this share to 12-15% by 2028. The technology's ability to address dendrite formation and electrode corrosion—both pH-dependent degradation mechanisms—directly impacts market expansion potential.
Pricing trends indicate that effective pH buffering solutions could reduce the lifetime cost of AZIBs by 22-30%, significantly enhancing their competitive position against lithium-ion technologies in price-sensitive market segments such as residential energy storage and developing markets.
Current Challenges in Zinc Battery Electrolyte Stability
Aqueous zinc ion batteries (AZIBs) face significant electrolyte stability challenges that hinder their widespread commercial adoption. The primary issue stems from the inherent properties of zinc metal anodes in aqueous environments, where parasitic hydrogen evolution reactions (HER) occur due to the low hydrogen evolution potential of zinc (-0.76V vs. SHE). This reaction not only consumes water from the electrolyte but also leads to pH fluctuations that accelerate corrosion and dendrite formation.
The conventional zinc sulfate (ZnSO₄) electrolyte systems suffer from rapid capacity fading during cycling, primarily attributed to the acidification of the electrolyte. As discharge-charge cycles progress, the pH value typically decreases from near-neutral to highly acidic conditions (pH < 3), creating an increasingly corrosive environment for the zinc anode. This acidification accelerates zinc dissolution, promotes dendrite growth, and ultimately leads to internal short circuits and battery failure.
Another critical challenge is the formation of zinc hydroxide species at the electrode-electrolyte interface. During cycling, localized pH increases near the electrode surface can trigger the precipitation of Zn(OH)₂ and ZnO, forming passivation layers that impede ion transport and increase internal resistance. These insulating layers contribute significantly to capacity loss and voltage hysteresis during operation.
The presence of dissolved oxygen in aqueous electrolytes further complicates stability issues by participating in oxidation reactions with the zinc anode. This oxygen reduction reaction (ORR) accelerates corrosion and contributes to self-discharge, particularly during storage periods. Current electrolyte formulations have not adequately addressed this oxygen sensitivity problem.
Water activity in the electrolyte presents another fundamental challenge. High water activity expands the electrochemical stability window but simultaneously increases the risk of parasitic reactions. Conversely, reduced water activity through high-concentration electrolytes improves stability but compromises ionic conductivity and rate capability. This trade-off remains unresolved in current electrolyte designs.
The lack of effective additives for long-term stabilization represents a significant gap in current technology. While various organic and inorganic additives have been explored to suppress hydrogen evolution and dendrite formation, most provide only temporary improvements and fail to maintain effectiveness over extended cycling. The degradation of these additives over time results in progressive performance decline.
Temperature sensitivity further exacerbates electrolyte stability issues, with accelerated side reactions at elevated temperatures and reduced ionic conductivity at lower temperatures. This narrow operational temperature window severely limits the practical applications of AZIBs in real-world environments where temperature fluctuations are common.
The conventional zinc sulfate (ZnSO₄) electrolyte systems suffer from rapid capacity fading during cycling, primarily attributed to the acidification of the electrolyte. As discharge-charge cycles progress, the pH value typically decreases from near-neutral to highly acidic conditions (pH < 3), creating an increasingly corrosive environment for the zinc anode. This acidification accelerates zinc dissolution, promotes dendrite growth, and ultimately leads to internal short circuits and battery failure.
Another critical challenge is the formation of zinc hydroxide species at the electrode-electrolyte interface. During cycling, localized pH increases near the electrode surface can trigger the precipitation of Zn(OH)₂ and ZnO, forming passivation layers that impede ion transport and increase internal resistance. These insulating layers contribute significantly to capacity loss and voltage hysteresis during operation.
The presence of dissolved oxygen in aqueous electrolytes further complicates stability issues by participating in oxidation reactions with the zinc anode. This oxygen reduction reaction (ORR) accelerates corrosion and contributes to self-discharge, particularly during storage periods. Current electrolyte formulations have not adequately addressed this oxygen sensitivity problem.
Water activity in the electrolyte presents another fundamental challenge. High water activity expands the electrochemical stability window but simultaneously increases the risk of parasitic reactions. Conversely, reduced water activity through high-concentration electrolytes improves stability but compromises ionic conductivity and rate capability. This trade-off remains unresolved in current electrolyte designs.
The lack of effective additives for long-term stabilization represents a significant gap in current technology. While various organic and inorganic additives have been explored to suppress hydrogen evolution and dendrite formation, most provide only temporary improvements and fail to maintain effectiveness over extended cycling. The degradation of these additives over time results in progressive performance decline.
Temperature sensitivity further exacerbates electrolyte stability issues, with accelerated side reactions at elevated temperatures and reduced ionic conductivity at lower temperatures. This narrow operational temperature window severely limits the practical applications of AZIBs in real-world environments where temperature fluctuations are common.
Current pH Buffering Solutions for Aqueous Zinc Batteries
01 Electrolyte modifications for stability enhancement
Various electrolyte modifications can significantly improve the long-term stability of aqueous zinc ion batteries. These include using high-concentration electrolytes, adding specific salts or organic additives, and employing gel electrolytes. These modifications help suppress hydrogen evolution, reduce zinc dendrite formation, and mitigate electrode corrosion, which are major factors affecting battery stability during extended cycling.- Electrolyte modifications for stability enhancement: Various electrolyte modifications can significantly improve the long-term stability of aqueous zinc ion batteries. These include using specific salt concentrations, adding organic or inorganic additives, and employing water-in-salt electrolytes to suppress hydrogen evolution and zinc dendrite formation. Modified electrolytes can effectively reduce side reactions at electrode interfaces, minimize water activity, and create stable solid electrolyte interphases, all contributing to extended cycle life and improved performance retention.
- Advanced cathode materials design: Innovative cathode materials play a crucial role in enhancing the long-term stability of aqueous zinc ion batteries. These include manganese-based oxides with modified structures, vanadium-based compounds with expanded interlayers, Prussian blue analogs, and organic cathodes with optimized zinc ion storage sites. Strategic design approaches focus on preventing cathode dissolution, accommodating structural changes during cycling, and facilitating reversible zinc ion insertion/extraction processes to maintain capacity over extended cycling.
- Zinc anode protection strategies: Protecting the zinc anode is essential for achieving long-term stability in aqueous zinc ion batteries. Effective strategies include surface coatings with polymers or inorganic materials, introducing artificial solid electrolyte interphases, using 3D structured zinc anodes, and incorporating zinc alloys instead of pure zinc. These approaches help suppress dendrite formation, prevent side reactions with the electrolyte, reduce hydrogen evolution, and maintain uniform zinc plating/stripping behavior over numerous cycles.
- Separator and interface engineering: Engineering the separator and electrode interfaces significantly improves the long-term stability of aqueous zinc ion batteries. Advanced separators with modified pore structures, functional coatings, or composite designs can regulate zinc ion transport, inhibit dendrite penetration, and prevent short circuits. Interface engineering techniques include creating artificial interphases between electrodes and electrolytes, introducing buffer layers, and optimizing the electrode-electrolyte contact to minimize parasitic reactions and maintain stable cycling performance.
- Novel cell designs and system integration: Innovative cell designs and system integration approaches enhance the long-term stability of aqueous zinc ion batteries. These include developing quasi-solid-state or gel electrolyte systems, implementing flow battery configurations for zinc-based chemistries, creating flexible or printable battery architectures, and designing self-healing components. Advanced battery management systems with optimized charging protocols and temperature control further extend cycle life by preventing conditions that accelerate degradation mechanisms.
02 Advanced electrode materials design
Innovative electrode materials play a crucial role in enhancing the long-term stability of aqueous zinc ion batteries. This includes developing manganese-based cathodes with specific crystal structures, carbon-based composite materials, and vanadium-based compounds. These materials offer improved structural stability during repeated zinc ion insertion/extraction, reducing capacity fading and extending cycle life.Expand Specific Solutions03 Protective coatings and interface engineering
Applying protective coatings on electrodes and engineering the electrode-electrolyte interface can significantly enhance the long-term stability of aqueous zinc ion batteries. These approaches include using polymer coatings, inorganic protective layers, and surface modifications that prevent side reactions, control zinc deposition behavior, and maintain electrode integrity during extended cycling.Expand Specific Solutions04 Novel battery architectures and configurations
Innovative battery designs and configurations can address stability challenges in aqueous zinc ion batteries. These include three-dimensional electrode structures, dual-salt systems, and hybrid electrolyte configurations. Such designs help manage volume changes during cycling, improve ion transport pathways, and enhance overall electrochemical performance and longevity.Expand Specific Solutions05 Additives and stabilizing agents
Incorporating specific additives and stabilizing agents into aqueous zinc ion batteries can significantly improve their long-term stability. These include metal ion additives, organic compounds, and pH regulators that suppress side reactions, stabilize the zinc anode, and maintain electrolyte properties over extended cycling. These additives help address issues like hydrogen evolution, electrode passivation, and electrolyte degradation.Expand Specific Solutions
Leading Research Groups and Companies in Zinc Battery Development
The aqueous zinc ion battery market is currently in an early growth phase, characterized by increasing research focus on pH buffering mechanisms for long-term stability. The global market size is expanding rapidly, driven by demand for safe, cost-effective energy storage solutions. From a technical maturity perspective, the field remains developmental with significant innovation opportunities. Leading academic institutions like Jilin University, University of Adelaide, and KAIST are advancing fundamental research, while companies including Urban Electric Power, ZNL Energy, and NGK Insulators are commercializing solutions. Murata Manufacturing and Sumitomo Metal Mining bring materials expertise, while Toyota Central R&D Labs contributes system integration capabilities. The competitive landscape features collaboration between research institutions and industrial players to overcome electrolyte stability challenges.
Jilin University
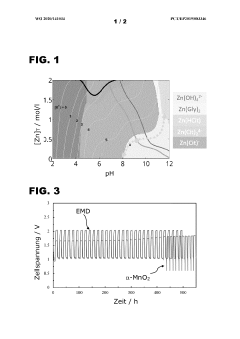
Technical Solution: Jilin University has developed an innovative pH buffering system for aqueous zinc ion batteries (AZIBs) that utilizes a dual-buffer approach combining organic and inorganic components. Their research focuses on maintaining electrolyte pH stability within the optimal range of 3.8-4.2 to prevent both zinc dendrite formation at higher pH and excessive hydrogen evolution at lower pH. The university's technical solution incorporates specially designed zwitterionic compounds that can donate or accept protons depending on the local pH environment, effectively creating a self-regulating system. Additionally, they've engineered porous separator materials with buffer-containing microchannels that provide continuous pH regulation throughout the battery's operational lifetime. Their approach has demonstrated significant improvements in cycling stability, with test cells maintaining over 80% capacity after 1000 cycles, compared to non-buffered systems that typically fail after 200-300 cycles.
Strengths: Their dual-buffer approach provides superior long-term pH stability across a wide range of operating conditions and current densities. The integration of buffer compounds directly into separator materials creates a spatially distributed buffering effect that addresses localized pH variations near electrode surfaces. Weakness: The specialized buffer compounds may increase production costs, and the complex chemistry requires precise manufacturing controls to ensure consistent performance.
Toyota Central R&D Labs, Inc.
Technical Solution: Toyota Central R&D Labs has developed a proprietary pH buffering technology for aqueous zinc ion batteries focused on automotive applications. Their approach centers on a multi-layer electrolyte system with gradient buffering capabilities that can withstand the demanding charge-discharge cycles required in vehicle applications. The technology employs specially formulated zinc-compatible phosphate and acetate buffer systems that maintain optimal pH ranges (4.0-5.0) even under high current densities experienced during rapid charging and discharging. Toyota's research has shown that controlling the zinc ion activity through precise pH management significantly reduces parasitic side reactions at the electrode-electrolyte interface. Their system incorporates temperature-responsive buffer components that automatically adjust buffering capacity based on operating conditions, addressing one of the key challenges in automotive battery applications where temperature fluctuations are common. Testing has demonstrated that their buffered electrolyte systems can achieve over 2000 stable cycles at 80% depth of discharge, representing a 3-4x improvement over conventional non-buffered aqueous zinc systems.
Strengths: The temperature-adaptive buffering system is particularly well-suited for automotive applications with varying environmental conditions. Their multi-layer approach provides excellent protection against localized pH extremes at electrode surfaces. Weakness: The complex formulation requires sophisticated manufacturing processes and may present challenges for cost-effective mass production compared to simpler electrolyte systems.
Key Mechanisms of pH-Dependent Zinc Electrode Passivation
Buffer composition comprising a first and a second buffer component
PatentWO2022223804A1
Innovation
- A buffer composition comprising a first water-soluble or water-dispersible source of magnesium or zinc ions and a second alkali carbonate or alkali bicarbonate, with a specific molar ratio, is used to maintain the pH of aqueous and solid articles below 12, preventing alkalization and optical property degradation.
Electrolyte for a zinc battery
PatentWO2020141034A1
Innovation
- An aqueous electrolyte solution comprising organic compounds that form soluble complexes with zinc ions and provide pH buffering, replacing inorganic salts to prevent undesirable zinc salt precipitation and promote zinc oxide formation, using carboxylic acids, aminocarboxylic acids, and heterocyclic compounds to maintain a stable pH range.
Environmental Impact of Zinc Battery Materials
The environmental impact of zinc battery materials in aqueous zinc ion batteries (AZIBs) requires careful consideration, particularly in relation to pH buffering systems. The extraction and processing of zinc, a key component in these batteries, has significant environmental implications. Mining operations for zinc ore typically involve land disturbance, habitat destruction, and potential acid mine drainage, which can contaminate surrounding water bodies and soil with heavy metals.
When examining the buffering agents used in AZIBs, many common pH regulators such as phosphates and carbonates require energy-intensive manufacturing processes that contribute to carbon emissions. However, compared to lithium-ion batteries, the environmental footprint of zinc extraction is generally lower, as zinc is more abundant and requires less energy to process than lithium and cobalt.
The long-term stability improvements achieved through pH buffering in AZIBs offer environmental benefits by extending battery life cycles. This reduces the frequency of battery replacement and consequently decreases waste generation. Studies indicate that well-buffered zinc batteries can achieve up to 3-4 times longer operational lifespans than non-buffered alternatives, significantly reducing the environmental burden of manufacturing replacement units.
Water usage presents another environmental consideration for aqueous zinc batteries. While these systems require water as an electrolyte component, the amount needed is relatively small compared to water requirements for extracting and processing other battery materials. The pH buffering agents themselves typically have minimal direct water footprint, though their production may involve water-intensive processes.
End-of-life management of zinc batteries presents both challenges and opportunities. Zinc is highly recyclable, with recovery rates potentially exceeding 90% through established hydrometallurgical processes. The buffering compounds, depending on their chemical composition, may either facilitate or complicate recycling efforts. Phosphate-based buffers, for instance, can be recovered and repurposed for agricultural applications, creating potential circular economy opportunities.
Toxicity concerns vary among different buffering agents. While zinc itself has moderate environmental toxicity at high concentrations, many common buffering compounds such as acetates and citrates are biodegradable and present minimal environmental hazards. However, certain more exotic buffering systems containing heavy metals or persistent organic compounds require careful handling and disposal protocols to prevent environmental contamination.
When examining the buffering agents used in AZIBs, many common pH regulators such as phosphates and carbonates require energy-intensive manufacturing processes that contribute to carbon emissions. However, compared to lithium-ion batteries, the environmental footprint of zinc extraction is generally lower, as zinc is more abundant and requires less energy to process than lithium and cobalt.
The long-term stability improvements achieved through pH buffering in AZIBs offer environmental benefits by extending battery life cycles. This reduces the frequency of battery replacement and consequently decreases waste generation. Studies indicate that well-buffered zinc batteries can achieve up to 3-4 times longer operational lifespans than non-buffered alternatives, significantly reducing the environmental burden of manufacturing replacement units.
Water usage presents another environmental consideration for aqueous zinc batteries. While these systems require water as an electrolyte component, the amount needed is relatively small compared to water requirements for extracting and processing other battery materials. The pH buffering agents themselves typically have minimal direct water footprint, though their production may involve water-intensive processes.
End-of-life management of zinc batteries presents both challenges and opportunities. Zinc is highly recyclable, with recovery rates potentially exceeding 90% through established hydrometallurgical processes. The buffering compounds, depending on their chemical composition, may either facilitate or complicate recycling efforts. Phosphate-based buffers, for instance, can be recovered and repurposed for agricultural applications, creating potential circular economy opportunities.
Toxicity concerns vary among different buffering agents. While zinc itself has moderate environmental toxicity at high concentrations, many common buffering compounds such as acetates and citrates are biodegradable and present minimal environmental hazards. However, certain more exotic buffering systems containing heavy metals or persistent organic compounds require careful handling and disposal protocols to prevent environmental contamination.
Scalability and Manufacturing Considerations
The scalability and manufacturing considerations for pH buffering systems in aqueous zinc ion batteries (AZIBs) present significant challenges for commercial deployment. Current laboratory-scale demonstrations of pH buffering techniques must be evaluated through the lens of mass production feasibility and cost-effectiveness to determine their industrial viability.
Manufacturing pH-buffered electrolytes at scale requires precise control of chemical compositions and mixing processes. The integration of buffering agents such as acetate, phosphate, or borate compounds demands specialized equipment capable of maintaining homogeneity across large production volumes. Variations in buffer concentration can lead to inconsistent battery performance, necessitating robust quality control protocols throughout the manufacturing pipeline.
Raw material sourcing represents another critical consideration. While common buffering agents like sodium acetate are relatively inexpensive and abundant, more specialized buffers may face supply chain constraints when scaled to industrial production levels. Manufacturers must evaluate buffer selection not only based on electrochemical performance but also on long-term supply security and price stability.
Process integration presents additional challenges when incorporating pH buffering into existing battery manufacturing lines. The introduction of buffering agents may require modifications to mixing, filling, and sealing equipment. Furthermore, the interaction between buffering compounds and other battery components during mass production must be thoroughly investigated to prevent unforeseen complications in assembly processes.
Environmental and safety considerations also impact scalability. Some buffering systems may introduce additional waste streams or require special handling procedures during manufacturing. Regulatory compliance across different markets must be evaluated, particularly for novel buffer formulations that may require safety certifications or environmental impact assessments.
Cost analysis reveals that while buffering agents themselves may represent a minor component of overall battery cost, the associated manufacturing complexities could significantly impact production economics. Automated quality control systems for monitoring pH stability throughout the manufacturing process and battery lifetime would add capital expenditure but might be essential for ensuring consistent performance.
The shelf life of pre-mixed buffered electrolytes presents another manufacturing challenge. Production facilities must determine whether to prepare buffered solutions on-demand or develop stabilized formulations suitable for storage. This decision impacts facility design, production scheduling, and ultimately the economics of large-scale manufacturing operations.
Manufacturing pH-buffered electrolytes at scale requires precise control of chemical compositions and mixing processes. The integration of buffering agents such as acetate, phosphate, or borate compounds demands specialized equipment capable of maintaining homogeneity across large production volumes. Variations in buffer concentration can lead to inconsistent battery performance, necessitating robust quality control protocols throughout the manufacturing pipeline.
Raw material sourcing represents another critical consideration. While common buffering agents like sodium acetate are relatively inexpensive and abundant, more specialized buffers may face supply chain constraints when scaled to industrial production levels. Manufacturers must evaluate buffer selection not only based on electrochemical performance but also on long-term supply security and price stability.
Process integration presents additional challenges when incorporating pH buffering into existing battery manufacturing lines. The introduction of buffering agents may require modifications to mixing, filling, and sealing equipment. Furthermore, the interaction between buffering compounds and other battery components during mass production must be thoroughly investigated to prevent unforeseen complications in assembly processes.
Environmental and safety considerations also impact scalability. Some buffering systems may introduce additional waste streams or require special handling procedures during manufacturing. Regulatory compliance across different markets must be evaluated, particularly for novel buffer formulations that may require safety certifications or environmental impact assessments.
Cost analysis reveals that while buffering agents themselves may represent a minor component of overall battery cost, the associated manufacturing complexities could significantly impact production economics. Automated quality control systems for monitoring pH stability throughout the manufacturing process and battery lifetime would add capital expenditure but might be essential for ensuring consistent performance.
The shelf life of pre-mixed buffered electrolytes presents another manufacturing challenge. Production facilities must determine whether to prepare buffered solutions on-demand or develop stabilized formulations suitable for storage. This decision impacts facility design, production scheduling, and ultimately the economics of large-scale manufacturing operations.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!