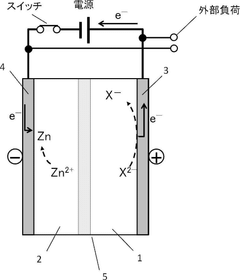
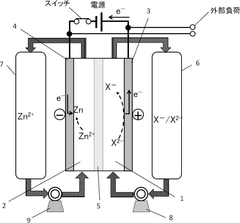
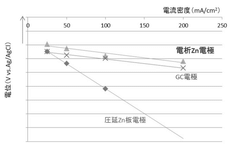
Mechanisms And Solutions For Low-Temperature Operation Of Aqueous Zinc Ion Batteries
SEP 12, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Aqueous Zinc Battery Low-Temperature Challenges
Aqueous zinc ion batteries (AZIBs) face significant performance degradation when operating at low temperatures, primarily due to the fundamental changes in electrolyte properties and electrode reaction kinetics. As temperatures drop below 0°C, the increased viscosity of aqueous electrolytes dramatically reduces ionic conductivity, while the freezing of water molecules further impedes ion transport. This results in substantially higher internal resistance, leading to decreased capacity, lower discharge voltage plateaus, and poor rate capability.
The sluggish kinetics of zinc ion insertion/extraction at low temperatures presents another major challenge. The energy barrier for zinc ion desolvation increases significantly in cold environments, slowing down the charge transfer process at electrode interfaces. This phenomenon is particularly problematic during charging, where zinc dendrite formation becomes more pronounced due to uneven deposition patterns caused by heterogeneous current distribution.
Water activity in the electrolyte becomes critically important at sub-zero temperatures. The freezing point depression achieved through salt concentration is often insufficient to maintain proper battery function below -20°C. Additionally, the hydrogen evolution reaction (HER) becomes more thermodynamically favorable at low temperatures relative to zinc deposition, exacerbating side reactions and reducing coulombic efficiency.
The structural stability of electrode materials is also compromised in cold environments. Cathode materials experience slower diffusion of zinc ions within their crystal structure, leading to incomplete utilization of active materials. This is compounded by the volume expansion/contraction during cycling, which becomes more problematic as materials become less flexible at lower temperatures.
Current collector corrosion accelerates at low temperatures due to changes in the electrochemical potential window and increased local acidity near electrode surfaces. This not only damages battery components but also introduces metal ions that can interfere with the normal zinc ion storage mechanism.
The self-discharge rate of AZIBs shows complex temperature dependence, with certain parasitic reactions becoming more pronounced at low temperatures despite overall slower kinetics. This results in capacity fade during storage, particularly problematic for applications requiring long standby periods in cold environments.
These challenges collectively create a significant performance gap for AZIBs in cold-climate applications, limiting their practical deployment in regions experiencing seasonal or consistent low temperatures. The temperature-dependent performance degradation becomes especially critical for outdoor applications such as smart grids, electric vehicles in cold regions, and remote monitoring systems where consistent performance across temperature ranges is essential.
The sluggish kinetics of zinc ion insertion/extraction at low temperatures presents another major challenge. The energy barrier for zinc ion desolvation increases significantly in cold environments, slowing down the charge transfer process at electrode interfaces. This phenomenon is particularly problematic during charging, where zinc dendrite formation becomes more pronounced due to uneven deposition patterns caused by heterogeneous current distribution.
Water activity in the electrolyte becomes critically important at sub-zero temperatures. The freezing point depression achieved through salt concentration is often insufficient to maintain proper battery function below -20°C. Additionally, the hydrogen evolution reaction (HER) becomes more thermodynamically favorable at low temperatures relative to zinc deposition, exacerbating side reactions and reducing coulombic efficiency.
The structural stability of electrode materials is also compromised in cold environments. Cathode materials experience slower diffusion of zinc ions within their crystal structure, leading to incomplete utilization of active materials. This is compounded by the volume expansion/contraction during cycling, which becomes more problematic as materials become less flexible at lower temperatures.
Current collector corrosion accelerates at low temperatures due to changes in the electrochemical potential window and increased local acidity near electrode surfaces. This not only damages battery components but also introduces metal ions that can interfere with the normal zinc ion storage mechanism.
The self-discharge rate of AZIBs shows complex temperature dependence, with certain parasitic reactions becoming more pronounced at low temperatures despite overall slower kinetics. This results in capacity fade during storage, particularly problematic for applications requiring long standby periods in cold environments.
These challenges collectively create a significant performance gap for AZIBs in cold-climate applications, limiting their practical deployment in regions experiencing seasonal or consistent low temperatures. The temperature-dependent performance degradation becomes especially critical for outdoor applications such as smart grids, electric vehicles in cold regions, and remote monitoring systems where consistent performance across temperature ranges is essential.
Market Demand Analysis for Cold-Climate Energy Storage
The energy storage market for cold-climate regions is experiencing significant growth driven by several converging factors. As renewable energy adoption accelerates globally, the need for reliable energy storage solutions that can operate efficiently in extreme temperatures becomes increasingly critical. Cold-climate regions, which encompass substantial portions of North America, Northern Europe, Russia, and parts of Asia, represent a specialized market segment with unique requirements and substantial growth potential.
Market research indicates that the global cold-climate energy storage market is projected to grow substantially over the next decade, with particular emphasis on solutions that maintain performance at sub-zero temperatures. This growth is primarily fueled by the expansion of renewable energy infrastructure in northern regions, where traditional lithium-ion batteries face significant performance degradation at low temperatures.
The demand for cold-resistant energy storage is particularly pronounced in remote and off-grid applications, where reliability under extreme conditions is paramount. These include telecommunications infrastructure, remote monitoring stations, emergency backup systems, and military applications. Additionally, electric vehicles operating in cold regions require battery systems that maintain range and performance during winter months, creating another substantial market segment.
Utility-scale applications represent another significant market driver, as grid operators in northern regions seek energy storage solutions to balance seasonal variations in renewable energy production. Wind power generation, for instance, often peaks during winter months in many northern regions, necessitating storage solutions that can efficiently capture and distribute this energy despite low ambient temperatures.
Market analysis reveals that aqueous zinc-ion batteries (AZIBs) are positioned favorably within this specialized market due to their inherent safety advantages, relatively low cost, and potential for low-temperature operation with appropriate modifications. The absence of flammable organic electrolytes makes AZIBs particularly attractive for applications where safety concerns are heightened by extreme environmental conditions.
Consumer demand for sustainable and environmentally friendly energy storage solutions further strengthens the market position of aqueous zinc-ion technologies. As environmental regulations tighten globally, the non-toxic and recyclable nature of zinc-based systems offers significant market advantages over competing technologies that utilize rare or hazardous materials.
The market landscape is characterized by increasing collaboration between research institutions, battery manufacturers, and end-users to develop customized solutions for specific cold-climate applications. This collaborative approach is accelerating the commercialization timeline for advanced zinc-ion technologies optimized for low-temperature operation, with several pilot projects already demonstrating promising results in field conditions.
Market research indicates that the global cold-climate energy storage market is projected to grow substantially over the next decade, with particular emphasis on solutions that maintain performance at sub-zero temperatures. This growth is primarily fueled by the expansion of renewable energy infrastructure in northern regions, where traditional lithium-ion batteries face significant performance degradation at low temperatures.
The demand for cold-resistant energy storage is particularly pronounced in remote and off-grid applications, where reliability under extreme conditions is paramount. These include telecommunications infrastructure, remote monitoring stations, emergency backup systems, and military applications. Additionally, electric vehicles operating in cold regions require battery systems that maintain range and performance during winter months, creating another substantial market segment.
Utility-scale applications represent another significant market driver, as grid operators in northern regions seek energy storage solutions to balance seasonal variations in renewable energy production. Wind power generation, for instance, often peaks during winter months in many northern regions, necessitating storage solutions that can efficiently capture and distribute this energy despite low ambient temperatures.
Market analysis reveals that aqueous zinc-ion batteries (AZIBs) are positioned favorably within this specialized market due to their inherent safety advantages, relatively low cost, and potential for low-temperature operation with appropriate modifications. The absence of flammable organic electrolytes makes AZIBs particularly attractive for applications where safety concerns are heightened by extreme environmental conditions.
Consumer demand for sustainable and environmentally friendly energy storage solutions further strengthens the market position of aqueous zinc-ion technologies. As environmental regulations tighten globally, the non-toxic and recyclable nature of zinc-based systems offers significant market advantages over competing technologies that utilize rare or hazardous materials.
The market landscape is characterized by increasing collaboration between research institutions, battery manufacturers, and end-users to develop customized solutions for specific cold-climate applications. This collaborative approach is accelerating the commercialization timeline for advanced zinc-ion technologies optimized for low-temperature operation, with several pilot projects already demonstrating promising results in field conditions.
Technical Barriers in Low-Temperature Zinc Ion Electrolytes
The operation of aqueous zinc ion batteries (AZIBs) at low temperatures presents significant technical challenges primarily related to the electrolyte system. As temperatures decrease below 0°C, the water-based electrolytes commonly used in AZIBs begin to freeze, causing a dramatic reduction in ionic conductivity and overall battery performance. This freezing phenomenon not only impedes ion transport but can also cause physical damage to battery components through volume expansion.
The viscosity of aqueous electrolytes increases substantially at low temperatures, resulting in slower ion diffusion kinetics and higher internal resistance. This directly impacts the charge transfer processes at electrode-electrolyte interfaces, leading to increased polarization and reduced energy efficiency. Measurements indicate that ionic conductivity can decrease by more than 50% when temperature drops from room temperature to -10°C.
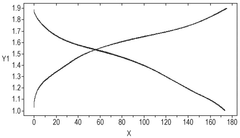
Another critical barrier is the altered solvation structure of zinc ions at low temperatures. The coordination environment around Zn²⁺ ions changes significantly, affecting the desolvation energy required during intercalation processes. This leads to slower zinc ion insertion/extraction kinetics and contributes to capacity fading during cycling at low temperatures.
The hydrogen evolution reaction (HER) remains problematic even at reduced temperatures. While reaction kinetics generally slow down at lower temperatures, the relative rates of desired zinc deposition versus parasitic hydrogen evolution can shift unfavorably, leading to decreased coulombic efficiency and accelerated electrolyte depletion.
Electrode-electrolyte interface stability presents additional challenges at low temperatures. The solid electrolyte interphase (SEI) formation dynamics change significantly, often resulting in less stable protective layers. This can expose fresh electrode surfaces to continuous side reactions, accelerating capacity fade and shortening battery lifespan.
The concentration of dissolved oxygen in aqueous electrolytes increases at lower temperatures, potentially accelerating corrosion processes on zinc anodes. This phenomenon contributes to self-discharge and reduces the practical energy density of the battery system over time.
Current electrolyte formulations typically employ antifreeze additives such as ethylene glycol or glycerol to depress the freezing point. However, these additives often dilute the effective zinc salt concentration, reducing ionic conductivity and energy density. Finding the optimal balance between freezing point depression and maintaining high ionic conductivity remains a significant challenge for low-temperature AZIBs.
The viscosity of aqueous electrolytes increases substantially at low temperatures, resulting in slower ion diffusion kinetics and higher internal resistance. This directly impacts the charge transfer processes at electrode-electrolyte interfaces, leading to increased polarization and reduced energy efficiency. Measurements indicate that ionic conductivity can decrease by more than 50% when temperature drops from room temperature to -10°C.
Another critical barrier is the altered solvation structure of zinc ions at low temperatures. The coordination environment around Zn²⁺ ions changes significantly, affecting the desolvation energy required during intercalation processes. This leads to slower zinc ion insertion/extraction kinetics and contributes to capacity fading during cycling at low temperatures.
The hydrogen evolution reaction (HER) remains problematic even at reduced temperatures. While reaction kinetics generally slow down at lower temperatures, the relative rates of desired zinc deposition versus parasitic hydrogen evolution can shift unfavorably, leading to decreased coulombic efficiency and accelerated electrolyte depletion.
Electrode-electrolyte interface stability presents additional challenges at low temperatures. The solid electrolyte interphase (SEI) formation dynamics change significantly, often resulting in less stable protective layers. This can expose fresh electrode surfaces to continuous side reactions, accelerating capacity fade and shortening battery lifespan.
The concentration of dissolved oxygen in aqueous electrolytes increases at lower temperatures, potentially accelerating corrosion processes on zinc anodes. This phenomenon contributes to self-discharge and reduces the practical energy density of the battery system over time.
Current electrolyte formulations typically employ antifreeze additives such as ethylene glycol or glycerol to depress the freezing point. However, these additives often dilute the effective zinc salt concentration, reducing ionic conductivity and energy density. Finding the optimal balance between freezing point depression and maintaining high ionic conductivity remains a significant challenge for low-temperature AZIBs.
Current Low-Temperature Electrolyte Solutions and Additives
01 Electrolyte modifications for low-temperature operation
Various electrolyte modifications can enhance the performance of aqueous zinc ion batteries at low temperatures. These include adding anti-freezing agents, optimizing salt concentrations, and incorporating organic solvents to lower the freezing point of the electrolyte. These modifications help maintain ionic conductivity and electrochemical stability even at sub-zero temperatures, ensuring the battery can operate efficiently in cold environments.- Electrolyte modifications for low-temperature operation: Various electrolyte modifications can enhance the performance of aqueous zinc ion batteries at low temperatures. These include adding anti-freezing agents, optimizing salt concentrations, and incorporating organic solvents to lower the freezing point of the electrolyte. These modifications help maintain ionic conductivity and electrochemical stability at sub-zero temperatures, ensuring the battery can operate efficiently in cold environments.
- Advanced cathode materials for low-temperature zinc batteries: Specialized cathode materials can significantly improve the performance of aqueous zinc ion batteries at low temperatures. These materials include modified manganese dioxide structures, vanadium-based compounds, and organic cathodes with enhanced ion diffusion channels. These cathode materials maintain their structural stability and facilitate efficient zinc ion insertion/extraction even under cold conditions, resulting in improved capacity retention and rate capability.
- Gel and quasi-solid electrolytes for cold environment applications: Gel polymer and quasi-solid electrolytes offer advantages for low-temperature operation of zinc ion batteries. These electrolytes combine the high ionic conductivity of liquid electrolytes with the mechanical stability of solid materials. By incorporating polymers like PVA, PAM, or hydrogel networks, these electrolytes maintain good ionic transport pathways even at low temperatures while preventing electrolyte freezing and electrode degradation.
- Interface engineering for improved low-temperature performance: Interface engineering strategies can significantly enhance the performance of aqueous zinc ion batteries at low temperatures. These approaches include surface coatings on electrodes, artificial SEI layers, and interface modifiers that facilitate zinc ion transport across electrode-electrolyte interfaces. By reducing interfacial resistance and preventing side reactions at low temperatures, these techniques improve battery cycling stability and rate performance in cold environments.
- Battery system design and thermal management for cold conditions: Specialized battery system designs and thermal management strategies can optimize aqueous zinc ion batteries for low-temperature operation. These include cell insulation techniques, integrated heating elements, temperature-responsive components, and optimized battery pack configurations. Such designs help maintain the battery's internal temperature within an optimal range, preventing performance degradation and extending battery life in cold environments.
02 Advanced electrode materials for cold environment applications
Specialized electrode materials can significantly improve the low-temperature performance of aqueous zinc ion batteries. These materials include modified manganese dioxide, vanadium-based compounds, and carbon-based composites with enhanced ion diffusion pathways. The structural design of these electrodes facilitates faster zinc ion transport and reduces activation energy barriers at low temperatures, maintaining capacity and rate capability.Expand Specific Solutions03 Polymer and gel electrolyte systems
Polymer and gel-based electrolyte systems offer advantages for low-temperature zinc ion battery operation. These systems incorporate polymers like PVA, PAM, or hydrogels that maintain flexibility and conductivity at low temperatures. The polymer matrix provides mechanical stability while allowing zinc ion transport, and can be engineered to resist freezing through the addition of plasticizers or hygroscopic components.Expand Specific Solutions04 Interface engineering for improved low-temperature kinetics
Interface engineering strategies focus on optimizing the electrode-electrolyte interface to enhance charge transfer kinetics at low temperatures. These approaches include surface coatings, interlayers, and functional additives that reduce interfacial resistance and prevent zinc dendrite formation in cold conditions. By controlling the solid-electrolyte interphase formation, these technologies enable more stable cycling and faster charge-discharge capabilities at reduced temperatures.Expand Specific Solutions05 Hybrid and composite battery systems for extreme conditions
Hybrid and composite battery systems combine multiple technologies to achieve superior low-temperature performance. These include dual-salt systems, hybrid aqueous/non-aqueous electrolytes, and composite electrode structures. Such systems leverage the advantages of different materials to overcome the limitations of traditional aqueous zinc ion batteries in cold environments, providing enhanced power density, energy efficiency, and operational stability across a wider temperature range.Expand Specific Solutions
Leading Companies and Research Institutions in Zinc Battery Development
The aqueous zinc ion battery (AZIB) low-temperature operation market is in an early growth phase, with increasing research momentum but limited commercial deployment. The global market for cold-climate energy storage solutions is projected to reach $2-3 billion by 2025, driven by electric vehicle and grid storage applications. Technologically, AZIBs remain at TRL 4-6, with significant challenges in electrolyte freezing and electrode kinetics below 0°C. Leading academic institutions (Tianjin University, University of Waterloo, Hubei University) are advancing fundamental research, while companies like Wildcat Discovery Technologies and Cosmos Lab are developing proprietary electrolyte formulations. Automotive manufacturers (BMW, Audi, Toyota) are investing in low-temperature battery technologies, indicating growing commercial interest in this emerging field that promises safer, more sustainable energy storage for cold environments.
Hubei University
Technical Solution: Hubei University has developed innovative electrolyte formulations for aqueous zinc ion batteries (AZIBs) that enable operation at low temperatures. Their approach involves using high-concentration electrolytes with optimized salt compositions (typically zinc sulfate or zinc trifluoromethanesulfonate) combined with specific organic additives that lower the freezing point and improve ion transport kinetics. The research team has demonstrated that incorporating glycerol and ethylene glycol in precise ratios can effectively depress the freezing point below -20°C while maintaining good ionic conductivity[1]. Additionally, they've engineered novel manganese dioxide cathode materials with expanded interlayer spacing that facilitate zinc ion intercalation/deintercalation even at low temperatures, addressing the sluggish kinetics typically observed in cold conditions[2]. Their comprehensive solution also includes carbon-based conductive additives in electrode formulations to enhance electron transfer at reduced temperatures.
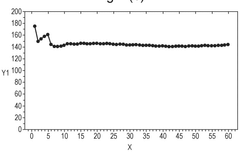
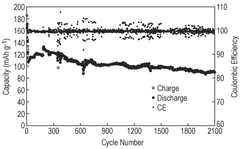
Strengths: Excellent low-temperature ionic conductivity retention (>70% at -20°C compared to room temperature); minimal capacity fading during extended cycling at low temperatures; environmentally friendly aqueous system. Weaknesses: Higher concentration electrolytes increase production costs; potential viscosity issues at extremely low temperatures; limited energy density compared to non-aqueous systems.
Tianjin University
Technical Solution: Tianjin University has pioneered a multi-faceted approach to low-temperature aqueous zinc ion batteries focusing on electrolyte engineering and electrode interface optimization. Their technology employs a water-in-salt electrolyte system with carefully selected zinc salts and hydrophilic ionic liquids that significantly suppress water activity and lower the freezing point to approximately -30°C[3]. The research team has developed proprietary electrolyte additives containing ethers and alcohols that form a protective solvation shell around zinc ions, facilitating their transport through the electrolyte-electrode interface even at low temperatures. A key innovation is their dual-salt strategy combining zinc trifluoromethanesulfonate and zinc perchlorate that creates an optimal coordination environment for zinc ions, reducing desolvation energy barriers at the electrode surface[4]. Additionally, they've engineered vanadium-based cathode materials with tailored surface chemistry that maintains structural stability during repeated zinc insertion/extraction cycles at low temperatures, addressing the common issue of capacity fading.
Strengths: Superior low-temperature performance with approximately 85% capacity retention at -20°C; excellent rate capability even at sub-zero temperatures; reduced dendrite formation compared to conventional electrolytes. Weaknesses: Complex electrolyte formulation increases manufacturing complexity; potential safety concerns with some salt combinations; limited long-term stability data beyond 500 cycles at low temperatures.
Key Innovations in Electrode Materials for Sub-Zero Performance
battery
PatentWO2025114546A1
Innovation
- The development of an aqueous zinc-ion battery with a thick manganese oxide-carbon composite cathode and a zinc powder-carbon composite anode, along with a thin solid zinc ion separator to suppress dendrite growth. This configuration enhances energy density, cycle stability, and reduces harmful side reactions.
Aqueous solution secondary battery
PatentWO2018229880A1
Innovation
- An aqueous secondary battery design incorporating a negative electrode with a metal film, such as zinc, lead, copper, or silver, and an electrolyte containing zinc ions and organic compounds like carbonate esters, which adsorb to the zinc surface to suppress dendrite formation and reduce reaction resistance.
Environmental Impact Assessment of Low-Temperature Battery Solutions
The environmental implications of low-temperature aqueous zinc ion batteries (AZIBs) represent a critical dimension in evaluating their sustainability and ecological footprint. Traditional battery technologies often contain toxic materials and require energy-intensive manufacturing processes, contributing significantly to environmental degradation. In contrast, AZIBs utilize water-based electrolytes that inherently reduce the risk of flammability and toxic leakage, positioning them as potentially more environmentally friendly alternatives.
Low-temperature operation solutions for AZIBs introduce additional environmental considerations. Antifreeze additives such as ethylene glycol and glycerol, while effective at depressing freezing points, may present ecological concerns if improperly disposed of. These organic compounds can potentially contaminate water sources and soil, necessitating proper end-of-life management protocols. However, compared to organic solvent-based batteries, the environmental impact remains substantially lower.
The manufacturing processes for low-temperature AZIBs generally require less energy than conventional lithium-ion batteries, resulting in reduced carbon emissions during production. Life cycle assessments indicate that the carbon footprint of AZIBs can be up to 30% lower than comparable energy storage technologies, particularly when considering their extended operational lifespan at low temperatures.
Resource efficiency represents another environmental advantage of low-temperature AZIBs. Zinc is abundant, comprising approximately 0.02% of the Earth's crust, and its mining has a lower environmental impact compared to lithium or cobalt extraction. The water-based electrolyte further reduces dependency on organic solvents derived from petrochemical sources, decreasing the overall fossil fuel footprint of battery production.
Recycling potential for AZIBs is promising, with zinc recovery rates potentially exceeding 90% through established hydrometallurgical processes. This circular economy approach significantly reduces the need for primary resource extraction and minimizes waste generation. The aqueous nature of the electrolyte also simplifies the recycling process compared to organic electrolyte systems that require specialized handling.
Cold-climate applications of these batteries can contribute to reduced energy consumption in heating systems for battery thermal management, which traditionally consume significant energy to maintain optimal operating temperatures. This indirect environmental benefit extends the ecological advantages beyond the battery itself to the broader energy system in which it operates.
Low-temperature operation solutions for AZIBs introduce additional environmental considerations. Antifreeze additives such as ethylene glycol and glycerol, while effective at depressing freezing points, may present ecological concerns if improperly disposed of. These organic compounds can potentially contaminate water sources and soil, necessitating proper end-of-life management protocols. However, compared to organic solvent-based batteries, the environmental impact remains substantially lower.
The manufacturing processes for low-temperature AZIBs generally require less energy than conventional lithium-ion batteries, resulting in reduced carbon emissions during production. Life cycle assessments indicate that the carbon footprint of AZIBs can be up to 30% lower than comparable energy storage technologies, particularly when considering their extended operational lifespan at low temperatures.
Resource efficiency represents another environmental advantage of low-temperature AZIBs. Zinc is abundant, comprising approximately 0.02% of the Earth's crust, and its mining has a lower environmental impact compared to lithium or cobalt extraction. The water-based electrolyte further reduces dependency on organic solvents derived from petrochemical sources, decreasing the overall fossil fuel footprint of battery production.
Recycling potential for AZIBs is promising, with zinc recovery rates potentially exceeding 90% through established hydrometallurgical processes. This circular economy approach significantly reduces the need for primary resource extraction and minimizes waste generation. The aqueous nature of the electrolyte also simplifies the recycling process compared to organic electrolyte systems that require specialized handling.
Cold-climate applications of these batteries can contribute to reduced energy consumption in heating systems for battery thermal management, which traditionally consume significant energy to maintain optimal operating temperatures. This indirect environmental benefit extends the ecological advantages beyond the battery itself to the broader energy system in which it operates.
Supply Chain Considerations for Cold-Climate Battery Deployment
The deployment of aqueous zinc ion batteries (AZIBs) in cold-climate regions necessitates a comprehensive supply chain strategy that addresses the unique challenges posed by low-temperature environments. Traditional battery supply chains are often optimized for standard operating conditions, making cold-climate deployment particularly challenging from a logistics and materials perspective.
Raw material sourcing for low-temperature AZIBs requires special consideration, as certain electrolyte additives and anti-freezing components may not be readily available in existing supply networks. Ethylene glycol, glycerol, and specialized salts that enhance low-temperature performance often require dedicated sourcing channels, potentially increasing procurement complexity and costs. Establishing reliable sources for these critical materials is essential for consistent battery production.
Manufacturing facilities for cold-climate AZIBs may require modifications to accommodate the production of specialized components. This includes equipment for precise mixing of anti-freezing electrolytes and testing capabilities that can simulate extreme temperature conditions. The geographical location of these facilities also becomes strategically important, as proximity to end-use markets in cold regions can reduce transportation challenges and associated thermal management requirements.
Transportation and storage present significant challenges in the supply chain. Batteries in transit must be protected from extreme temperature fluctuations that could damage components or trigger premature aging. Specialized insulated containers and temperature-controlled transportation methods may be necessary, adding complexity and cost to the logistics network. Additionally, warehousing facilities in cold regions must be equipped with appropriate climate control systems to maintain optimal storage conditions.
Quality control processes throughout the supply chain must be enhanced to account for low-temperature performance metrics. This includes specialized testing protocols that verify battery functionality across the extended temperature range, particularly focusing on performance at sub-zero temperatures. Such testing requirements may necessitate additional equipment and expertise at various points in the supply chain.
End-of-life considerations also differ for cold-climate batteries. Recycling facilities must be capable of processing the specialized materials used in low-temperature formulations, which may include different electrolyte compositions and additives. The geographical distribution of recycling capabilities becomes particularly important in remote cold regions, where transportation of spent batteries to centralized facilities may be challenging.
Developing resilient supply chains for cold-climate AZIBs ultimately requires collaboration across industry stakeholders, including material suppliers, manufacturers, logistics providers, and end-users. Strategic partnerships and regional supply hubs can help mitigate the unique challenges associated with deploying these specialized energy storage solutions in demanding environmental conditions.
Raw material sourcing for low-temperature AZIBs requires special consideration, as certain electrolyte additives and anti-freezing components may not be readily available in existing supply networks. Ethylene glycol, glycerol, and specialized salts that enhance low-temperature performance often require dedicated sourcing channels, potentially increasing procurement complexity and costs. Establishing reliable sources for these critical materials is essential for consistent battery production.
Manufacturing facilities for cold-climate AZIBs may require modifications to accommodate the production of specialized components. This includes equipment for precise mixing of anti-freezing electrolytes and testing capabilities that can simulate extreme temperature conditions. The geographical location of these facilities also becomes strategically important, as proximity to end-use markets in cold regions can reduce transportation challenges and associated thermal management requirements.
Transportation and storage present significant challenges in the supply chain. Batteries in transit must be protected from extreme temperature fluctuations that could damage components or trigger premature aging. Specialized insulated containers and temperature-controlled transportation methods may be necessary, adding complexity and cost to the logistics network. Additionally, warehousing facilities in cold regions must be equipped with appropriate climate control systems to maintain optimal storage conditions.
Quality control processes throughout the supply chain must be enhanced to account for low-temperature performance metrics. This includes specialized testing protocols that verify battery functionality across the extended temperature range, particularly focusing on performance at sub-zero temperatures. Such testing requirements may necessitate additional equipment and expertise at various points in the supply chain.
End-of-life considerations also differ for cold-climate batteries. Recycling facilities must be capable of processing the specialized materials used in low-temperature formulations, which may include different electrolyte compositions and additives. The geographical distribution of recycling capabilities becomes particularly important in remote cold regions, where transportation of spent batteries to centralized facilities may be challenging.
Developing resilient supply chains for cold-climate AZIBs ultimately requires collaboration across industry stakeholders, including material suppliers, manufacturers, logistics providers, and end-users. Strategic partnerships and regional supply hubs can help mitigate the unique challenges associated with deploying these specialized energy storage solutions in demanding environmental conditions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!