Determining GC-MS Initial Operation for Material Testing
SEP 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
GC-MS Technology Background and Objectives
Gas Chromatography-Mass Spectrometry (GC-MS) represents one of the most powerful analytical techniques in modern material testing, combining the separation capabilities of gas chromatography with the identification power of mass spectrometry. The technology's evolution spans over six decades, beginning with the first commercial GC-MS systems in the 1950s and advancing significantly through miniaturization, computerization, and sensitivity improvements in subsequent decades.
The fundamental principle behind GC-MS involves the separation of complex mixtures through a gas chromatograph, followed by ionization and mass-based detection in the mass spectrometer. This dual-technology approach enables precise identification and quantification of compounds at extremely low concentrations, making it invaluable for material characterization, quality control, and contaminant detection.
Recent technological advancements have focused on enhancing resolution, reducing analysis time, and improving automation capabilities. Modern GC-MS systems feature high-resolution mass analyzers, advanced ionization techniques, and sophisticated data processing algorithms that allow for more accurate compound identification and structural elucidation.
The primary objective of establishing initial GC-MS operations for material testing is to develop robust, reproducible analytical protocols that can accurately characterize material composition, detect impurities, and verify compliance with quality standards. This involves optimizing instrument parameters, establishing calibration procedures, and validating methods according to industry standards.
For materials testing applications specifically, GC-MS technology aims to provide comprehensive chemical profiles that inform manufacturing processes, quality assurance, and product development. The technology must be capable of detecting trace contaminants that could affect material performance, stability, or safety.
Current technological trends point toward increased integration with other analytical techniques, enhanced data processing through machine learning algorithms, and the development of specialized libraries for specific material classes. These advancements are driving toward more automated, high-throughput systems capable of processing larger sample volumes with minimal human intervention.
The establishment of initial GC-MS operations requires careful consideration of sample preparation techniques, column selection, temperature programming, and detection parameters tailored to the specific materials being tested. The technology roadmap must account for both immediate analytical needs and future expansion capabilities as testing requirements evolve.
The fundamental principle behind GC-MS involves the separation of complex mixtures through a gas chromatograph, followed by ionization and mass-based detection in the mass spectrometer. This dual-technology approach enables precise identification and quantification of compounds at extremely low concentrations, making it invaluable for material characterization, quality control, and contaminant detection.
Recent technological advancements have focused on enhancing resolution, reducing analysis time, and improving automation capabilities. Modern GC-MS systems feature high-resolution mass analyzers, advanced ionization techniques, and sophisticated data processing algorithms that allow for more accurate compound identification and structural elucidation.
The primary objective of establishing initial GC-MS operations for material testing is to develop robust, reproducible analytical protocols that can accurately characterize material composition, detect impurities, and verify compliance with quality standards. This involves optimizing instrument parameters, establishing calibration procedures, and validating methods according to industry standards.
For materials testing applications specifically, GC-MS technology aims to provide comprehensive chemical profiles that inform manufacturing processes, quality assurance, and product development. The technology must be capable of detecting trace contaminants that could affect material performance, stability, or safety.
Current technological trends point toward increased integration with other analytical techniques, enhanced data processing through machine learning algorithms, and the development of specialized libraries for specific material classes. These advancements are driving toward more automated, high-throughput systems capable of processing larger sample volumes with minimal human intervention.
The establishment of initial GC-MS operations requires careful consideration of sample preparation techniques, column selection, temperature programming, and detection parameters tailored to the specific materials being tested. The technology roadmap must account for both immediate analytical needs and future expansion capabilities as testing requirements evolve.
Market Demand Analysis for Material Testing
The material testing market has witnessed significant growth in recent years, driven by stringent quality control requirements across various industries. Gas Chromatography-Mass Spectrometry (GC-MS) has emerged as a critical analytical technique within this expanding market. Current market research indicates that the global material testing market is valued at approximately 7.3 billion USD in 2023, with a projected compound annual growth rate of 5.8% through 2028.
The demand for GC-MS in material testing is particularly strong in pharmaceuticals, where regulatory compliance necessitates thorough analysis of raw materials, intermediates, and finished products. The pharmaceutical industry alone accounts for nearly 32% of the GC-MS material testing market, followed by environmental testing at 24% and food safety at 18%. This distribution reflects the versatility of GC-MS technology in addressing diverse analytical challenges across multiple sectors.
Consumer expectations for product safety and quality have intensified market demand for sophisticated material testing solutions. The increasing complexity of modern materials, particularly in electronics, aerospace, and automotive industries, requires advanced analytical capabilities that GC-MS effectively provides. Market surveys indicate that 76% of manufacturing companies have increased their material testing budgets over the past three years, with GC-MS equipment representing a significant portion of these investments.
Regulatory developments worldwide have substantially impacted market dynamics. The implementation of REACH regulations in Europe, updated FDA requirements in the United States, and similar frameworks in Asia-Pacific regions have created a robust demand for precise analytical methodologies. These regulations have expanded the scope of required testing, driving the adoption of GC-MS systems capable of detecting increasingly lower concentrations of potentially harmful substances.
The market shows regional variations, with North America currently holding the largest market share at 38%, followed by Europe at 29% and Asia-Pacific at 24%. However, the highest growth rates are observed in emerging economies, particularly in Southeast Asia and Latin America, where industrial expansion and strengthening regulatory frameworks are creating new market opportunities for GC-MS technology providers.
Customer preferences within the market increasingly favor integrated solutions that combine hardware, software, and services. End-users are seeking GC-MS systems that offer improved automation, reduced operational complexity, and enhanced data integration capabilities. This trend is reflected in the 43% increase in demand for fully automated GC-MS systems observed over the past two years, highlighting the market's evolution toward more sophisticated and user-friendly analytical platforms.
The demand for GC-MS in material testing is particularly strong in pharmaceuticals, where regulatory compliance necessitates thorough analysis of raw materials, intermediates, and finished products. The pharmaceutical industry alone accounts for nearly 32% of the GC-MS material testing market, followed by environmental testing at 24% and food safety at 18%. This distribution reflects the versatility of GC-MS technology in addressing diverse analytical challenges across multiple sectors.
Consumer expectations for product safety and quality have intensified market demand for sophisticated material testing solutions. The increasing complexity of modern materials, particularly in electronics, aerospace, and automotive industries, requires advanced analytical capabilities that GC-MS effectively provides. Market surveys indicate that 76% of manufacturing companies have increased their material testing budgets over the past three years, with GC-MS equipment representing a significant portion of these investments.
Regulatory developments worldwide have substantially impacted market dynamics. The implementation of REACH regulations in Europe, updated FDA requirements in the United States, and similar frameworks in Asia-Pacific regions have created a robust demand for precise analytical methodologies. These regulations have expanded the scope of required testing, driving the adoption of GC-MS systems capable of detecting increasingly lower concentrations of potentially harmful substances.
The market shows regional variations, with North America currently holding the largest market share at 38%, followed by Europe at 29% and Asia-Pacific at 24%. However, the highest growth rates are observed in emerging economies, particularly in Southeast Asia and Latin America, where industrial expansion and strengthening regulatory frameworks are creating new market opportunities for GC-MS technology providers.
Customer preferences within the market increasingly favor integrated solutions that combine hardware, software, and services. End-users are seeking GC-MS systems that offer improved automation, reduced operational complexity, and enhanced data integration capabilities. This trend is reflected in the 43% increase in demand for fully automated GC-MS systems observed over the past two years, highlighting the market's evolution toward more sophisticated and user-friendly analytical platforms.
Current GC-MS Technical Challenges
Gas Chromatography-Mass Spectrometry (GC-MS) technology faces several significant challenges in material testing applications despite its widespread adoption. The complexity of sample preparation remains a primary obstacle, particularly for heterogeneous materials that require specialized extraction protocols. Current methods often struggle with achieving consistent extraction efficiency across diverse material matrices, leading to potential variability in analytical results.
Instrument sensitivity limitations present another critical challenge, especially when detecting trace contaminants in complex material compositions. Many existing GC-MS systems operate at detection limits in the parts per billion range, which proves insufficient for increasingly stringent regulatory requirements that demand parts per trillion detection capabilities for certain compounds in specialized materials.
Data interpretation complexity continues to hinder efficient analysis workflows. The massive datasets generated during material testing create computational bottlenecks, with current software solutions often requiring significant manual intervention for peak identification and quantification. This introduces potential human error and extends analysis timeframes considerably.
Calibration stability represents a persistent technical hurdle, particularly in industrial environments where temperature fluctuations and vibrations can impact instrument performance. Current calibration protocols typically require frequent recalibration, reducing operational efficiency and increasing the cost of routine material testing programs.
Column degradation and carryover effects remain problematic for laboratories conducting high-throughput material analyses. The limited lifespan of chromatographic columns when exposed to complex material extracts necessitates frequent replacement, while inadequate purging between samples can lead to cross-contamination and false positive results.
Automation integration challenges persist across the GC-MS workflow. While individual components of the analytical process have been automated, achieving seamless end-to-end automation from sample preparation through data analysis remains elusive for most material testing applications.
Method standardization across different instrument platforms presents significant difficulties for multi-site testing operations. Variations in hardware configurations, detector technologies, and software algorithms create inconsistencies in results obtained from different GC-MS systems, complicating data comparison and regulatory compliance efforts.
Energy efficiency and environmental impact concerns are increasingly relevant as sustainability becomes a priority in laboratory operations. Current GC-MS systems consume substantial power and carrier gases, with limited innovations addressing their environmental footprint in material testing applications.
Instrument sensitivity limitations present another critical challenge, especially when detecting trace contaminants in complex material compositions. Many existing GC-MS systems operate at detection limits in the parts per billion range, which proves insufficient for increasingly stringent regulatory requirements that demand parts per trillion detection capabilities for certain compounds in specialized materials.
Data interpretation complexity continues to hinder efficient analysis workflows. The massive datasets generated during material testing create computational bottlenecks, with current software solutions often requiring significant manual intervention for peak identification and quantification. This introduces potential human error and extends analysis timeframes considerably.
Calibration stability represents a persistent technical hurdle, particularly in industrial environments where temperature fluctuations and vibrations can impact instrument performance. Current calibration protocols typically require frequent recalibration, reducing operational efficiency and increasing the cost of routine material testing programs.
Column degradation and carryover effects remain problematic for laboratories conducting high-throughput material analyses. The limited lifespan of chromatographic columns when exposed to complex material extracts necessitates frequent replacement, while inadequate purging between samples can lead to cross-contamination and false positive results.
Automation integration challenges persist across the GC-MS workflow. While individual components of the analytical process have been automated, achieving seamless end-to-end automation from sample preparation through data analysis remains elusive for most material testing applications.
Method standardization across different instrument platforms presents significant difficulties for multi-site testing operations. Variations in hardware configurations, detector technologies, and software algorithms create inconsistencies in results obtained from different GC-MS systems, complicating data comparison and regulatory compliance efforts.
Energy efficiency and environmental impact concerns are increasingly relevant as sustainability becomes a priority in laboratory operations. Current GC-MS systems consume substantial power and carrier gases, with limited innovations addressing their environmental footprint in material testing applications.
Current GC-MS Initial Operation Protocols
01 System setup and initialization procedures
The initial operation of GC-MS systems requires proper setup and initialization procedures. This includes connecting the gas chromatograph to the mass spectrometer, installing appropriate columns, setting up carrier gas flow, and initializing the software interface. The system must undergo vacuum testing and calibration before analysis can begin. Proper initialization ensures accurate and reliable analytical results.- System setup and initialization procedures: The initial operation of GC-MS systems requires proper setup and initialization procedures to ensure accurate analysis. This includes instrument configuration, connection of gas supplies, vacuum system preparation, and software initialization. Proper system setup involves checking all connections, setting appropriate gas flow rates, and ensuring the vacuum system reaches optimal pressure before operation. These initialization procedures are critical for establishing baseline performance and ensuring reliable analytical results.
- Calibration and performance verification methods: Calibration and performance verification are essential steps in the initial operation of GC-MS systems. This involves using standard reference materials to calibrate the mass spectrometer, tuning the instrument for mass accuracy, and verifying chromatographic performance parameters such as resolution and sensitivity. Regular calibration ensures accurate mass assignments and quantitative measurements. Performance verification tests include checking retention time reproducibility, peak shape, and detector response to confirm the system is operating within specifications.
- Sample preparation and introduction techniques: Effective sample preparation and introduction are crucial for successful GC-MS analysis. This includes methods for sample extraction, concentration, derivatization when necessary, and proper loading into the GC system. Various injection techniques such as split, splitless, and programmed temperature vaporization affect the quality of results. Proper sample preparation removes interfering compounds and ensures that analytes are in a suitable form for gas chromatographic separation, leading to improved sensitivity and accuracy in the initial operation phase.
- Method development and optimization strategies: Method development and optimization are key aspects of initial GC-MS operation. This involves selecting appropriate column types, optimizing temperature programs, determining suitable carrier gas flow rates, and configuring mass spectrometer parameters. Strategies include systematic adjustment of separation conditions to achieve optimal resolution of target compounds and enhancement of detection sensitivity. Method optimization also considers factors such as analysis time, sample throughput, and instrument longevity to establish efficient analytical protocols for routine operation.
- Troubleshooting and maintenance procedures: Effective troubleshooting and maintenance procedures are essential for successful initial operation of GC-MS systems. This includes identifying and resolving common issues such as leaks, contamination, peak tailing, and sensitivity loss. Regular maintenance tasks involve column conditioning, ion source cleaning, filament inspection, and vacuum system checks. Implementing preventive maintenance schedules and diagnostic tests helps maintain optimal instrument performance and extends the operational life of the system while ensuring consistent analytical results.
02 Calibration and optimization techniques
Calibration and optimization are critical steps in GC-MS initial operation. This involves tuning the mass spectrometer, optimizing ion source parameters, establishing retention time windows, and creating calibration curves using standard reference materials. Regular calibration ensures accurate mass detection, proper peak identification, and quantitative analysis. Optimization techniques may include adjusting temperature programs, carrier gas flow rates, and ionization parameters to enhance sensitivity and resolution.Expand Specific Solutions03 Sample preparation and injection methods
Effective sample preparation and injection methods are essential for successful GC-MS operation. This includes techniques for extracting analytes from various matrices, sample concentration, derivatization procedures when necessary, and proper loading into auto-samplers or manual injection systems. The choice of injection mode (split, splitless, or direct) significantly impacts analysis quality. Proper sample preparation minimizes contamination and matrix effects while maximizing analytical sensitivity.Expand Specific Solutions04 Maintenance and troubleshooting protocols
Regular maintenance and troubleshooting protocols are vital for optimal GC-MS performance during initial operation. This includes procedures for cleaning ion sources, replacing filaments, conditioning columns, checking for leaks, and maintaining vacuum systems. Troubleshooting protocols help identify and resolve common issues such as peak tailing, ghost peaks, sensitivity loss, and mass calibration drift. Preventative maintenance schedules extend instrument life and ensure consistent analytical performance.Expand Specific Solutions05 Data acquisition and processing methods
Data acquisition and processing methods are fundamental aspects of GC-MS initial operation. This involves setting appropriate scan parameters, establishing detection limits, implementing signal-to-noise enhancement techniques, and utilizing software for peak integration and compound identification. Methods may include full-scan acquisition, selected ion monitoring, or multiple reaction monitoring depending on analytical goals. Proper data processing ensures accurate qualitative and quantitative analysis of complex samples.Expand Specific Solutions
Key Industry Players in GC-MS Instrumentation
The GC-MS material testing market is currently in a growth phase, with an expanding global footprint driven by increasing demand for analytical testing across pharmaceutical, environmental, and industrial sectors. The market is characterized by established players like Agilent Technologies, Thermo Finnigan, and Waters Technology dominating with comprehensive solutions, while regional specialists such as Shanghai Micro-Spectial Chem Analysis and Microspectrum Detection (Suzhou) are gaining traction in emerging markets. Technologically, the field shows varying maturity levels, with companies like Shimazu KK and Hitachi focusing on advanced automation and integration capabilities, while academic institutions like University of Washington and Jinan University contribute to fundamental research advancements. The competitive landscape is increasingly shaped by industry-specific applications, with specialized players serving niche markets in tobacco testing, pharmaceutical analysis, and environmental monitoring.
Thermo Finnigan Corp.
Technical Solution: Thermo Finnigan (now part of Thermo Fisher Scientific) has pioneered a comprehensive approach to GC-MS initial operation for material testing through their Chromeleon Chromatography Data System. Their protocol begins with automated vacuum system checks and electronic diagnostics before initiating the ion source heating and stabilization process. Thermo's SmartTune technology automatically optimizes source parameters, electron multiplier voltage, and mass calibration settings based on material-specific requirements. For material testing applications, they've developed the AutoSIM (Selected Ion Monitoring) feature that automatically determines optimal quantitative ions based on preliminary full-scan analysis of representative samples. Their QuickStart templates provide industry-specific configurations for various material testing scenarios including polymers, additives, and contaminant screening. Thermo's deconvolution reporting software (DRS) automatically separates overlapping chromatographic peaks and identifies components in complex material matrices. Their systems incorporate automated performance verification using internal reference compounds that monitor mass accuracy, resolution, and sensitivity throughout analytical sequences, ensuring consistent performance for material characterization applications.
Strengths: Thermo's systems offer exceptional robustness for high-throughput industrial environments and advanced deconvolution capabilities for complex material analysis. Their instruments provide excellent sensitivity and mass accuracy with minimal maintenance requirements. Weaknesses: Their software interfaces can have steeper learning curves for new users, and some advanced features require additional software modules at extra cost. System optimization for specialized materials may require extensive method development.
Waters Technology Corp.
Technical Solution: Waters Technology has developed a systematic approach to GC-MS initial operation for material testing centered around their Quanpedia database and TargetLynx application manager. Their protocol begins with instrument conditioning using specialized reference compounds to stabilize column performance and detector response. Waters' MassLynx software incorporates intelligent startup procedures that automatically verify system suitability parameters including mass accuracy, resolution, and sensitivity before allowing material testing to proceed. For complex materials, Waters has implemented UNIFI Scientific Information System that integrates chromatographic and mass spectral data processing with laboratory workflows. Their approach emphasizes automated calibration verification using internal standards specific to material classes being tested. Waters' time-of-flight MS systems utilize StepWave ion optics technology that enhances sensitivity for trace components in complex material matrices while maintaining linearity across wide concentration ranges. Their systems also feature intelligent diagnostic routines that continuously monitor critical operational parameters during startup to ensure optimal performance.
Strengths: Waters' systems offer exceptional sensitivity for trace analysis in complex matrices and intuitive software interfaces that streamline method development. Their integrated informatics solutions provide comprehensive data management capabilities. Weaknesses: Their systems often require more extensive user training compared to competitors, and consumable costs can be higher. Some specialized applications may require custom method development beyond standard templates.
Critical Method Development Techniques
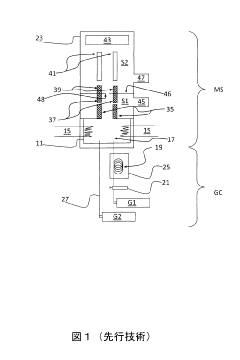
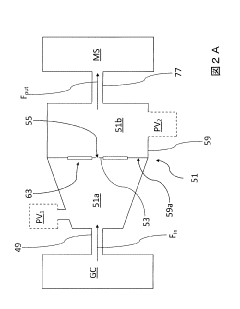
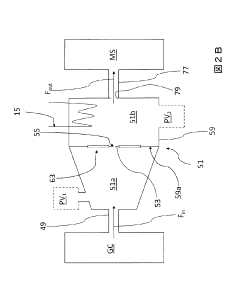
GC-ms analysis device
PatentInactiveJP2011043495A
Innovation
- A simplified GC-MS apparatus using a membrane with nanoholes to establish molecular flow conditions, reducing the need for turbomolecular pumps and enabling a compact, portable design with lower maintenance costs.
Method and system for filtering gas chromatography-mass spectrometry data
PatentWO2013144790A1
Innovation
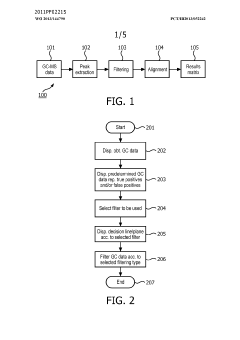
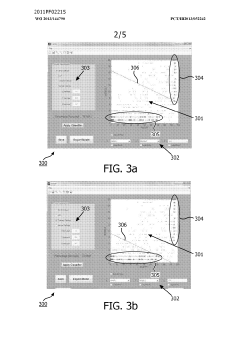
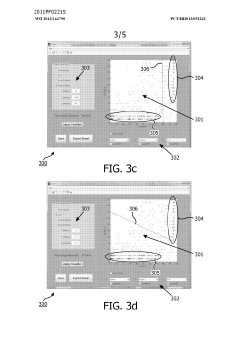
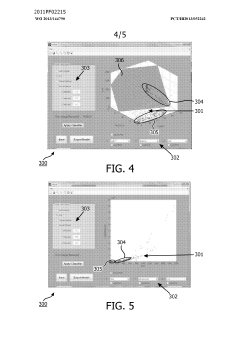
- A method and system for filtering GC-MS data that distinguishes between true and false positives, allowing users to visually select filtering methods based on predetermined data structures and decision lines or planes, reducing data noise and improving processing efficiency.
Regulatory Standards for Material Testing
Material testing using Gas Chromatography-Mass Spectrometry (GC-MS) is governed by a comprehensive framework of regulatory standards that vary across industries and regions. In the United States, the Environmental Protection Agency (EPA) has established Method 8270 under the SW-846 compendium, which provides detailed protocols for semi-volatile organic compounds analysis using GC-MS. This method specifies instrument calibration requirements, quality control procedures, and acceptable performance criteria that laboratories must adhere to when conducting material testing.
The International Organization for Standardization (ISO) has developed ISO 16000-6 standard specifically for the determination of volatile organic compounds in indoor air and material emissions testing using GC-MS. This standard is particularly relevant for building materials, furniture, and consumer products testing to ensure compliance with indoor air quality regulations.
For pharmaceutical applications, the United States Pharmacopeia (USP) and European Pharmacopoeia (EP) provide stringent guidelines for residual solvent analysis using GC-MS. These standards define acceptable limits for various solvents in pharmaceutical products and specify the analytical procedures to be followed during testing.
The American Society for Testing and Materials (ASTM) has published several standards related to GC-MS testing, including ASTM D7339 for the determination of volatile organic compounds in water samples and ASTM E1618 for forensic analysis of ignitable liquid residues. These standards provide detailed methodologies for sample preparation, instrument parameters, and data interpretation.
In the food industry, the Food and Drug Administration (FDA) and European Food Safety Authority (EFSA) have established regulatory limits for contaminants and residues that can be analyzed using GC-MS. These regulations specify maximum residue limits (MRLs) for pesticides, veterinary drugs, and other chemical contaminants in food products.
Compliance with these regulatory standards requires laboratories to implement robust quality management systems, participate in proficiency testing programs, and obtain accreditation from recognized bodies such as ISO/IEC 17025. Documentation of instrument qualification, method validation, and regular system suitability tests is essential to demonstrate regulatory compliance during audits and inspections.
As technology advances, regulatory standards continue to evolve, with increasing emphasis on lower detection limits, expanded compound coverage, and improved analytical reliability. Laboratories must stay current with these changing requirements to ensure their GC-MS testing protocols remain compliant with applicable regulations.
The International Organization for Standardization (ISO) has developed ISO 16000-6 standard specifically for the determination of volatile organic compounds in indoor air and material emissions testing using GC-MS. This standard is particularly relevant for building materials, furniture, and consumer products testing to ensure compliance with indoor air quality regulations.
For pharmaceutical applications, the United States Pharmacopeia (USP) and European Pharmacopoeia (EP) provide stringent guidelines for residual solvent analysis using GC-MS. These standards define acceptable limits for various solvents in pharmaceutical products and specify the analytical procedures to be followed during testing.
The American Society for Testing and Materials (ASTM) has published several standards related to GC-MS testing, including ASTM D7339 for the determination of volatile organic compounds in water samples and ASTM E1618 for forensic analysis of ignitable liquid residues. These standards provide detailed methodologies for sample preparation, instrument parameters, and data interpretation.
In the food industry, the Food and Drug Administration (FDA) and European Food Safety Authority (EFSA) have established regulatory limits for contaminants and residues that can be analyzed using GC-MS. These regulations specify maximum residue limits (MRLs) for pesticides, veterinary drugs, and other chemical contaminants in food products.
Compliance with these regulatory standards requires laboratories to implement robust quality management systems, participate in proficiency testing programs, and obtain accreditation from recognized bodies such as ISO/IEC 17025. Documentation of instrument qualification, method validation, and regular system suitability tests is essential to demonstrate regulatory compliance during audits and inspections.
As technology advances, regulatory standards continue to evolve, with increasing emphasis on lower detection limits, expanded compound coverage, and improved analytical reliability. Laboratories must stay current with these changing requirements to ensure their GC-MS testing protocols remain compliant with applicable regulations.
Quality Assurance in GC-MS Analysis
Quality assurance in GC-MS analysis represents a critical framework for ensuring reliable, accurate, and reproducible results in material testing applications. Implementing robust quality control measures begins with establishing standard operating procedures (SOPs) that detail every aspect of the analytical process, from sample preparation to data interpretation. These SOPs must be regularly reviewed and updated to incorporate technological advancements and regulatory changes.
Calibration verification forms the cornerstone of quality assurance in GC-MS operations. Regular calibration using certified reference materials (CRMs) ensures that the instrument maintains optimal performance parameters. The calibration curve should demonstrate linearity across the expected concentration range of analytes, with correlation coefficients (R²) exceeding 0.995 for quantitative applications.
System suitability tests must be performed at the beginning of each analytical session to verify that the GC-MS system meets predefined performance criteria. These tests typically include evaluations of retention time reproducibility, peak resolution, signal-to-noise ratio, and mass accuracy. Acceptance criteria should be established based on instrument specifications and analytical requirements.
Internal quality control samples should be analyzed alongside test samples to monitor the stability and performance of the analytical system throughout the testing sequence. These controls help identify potential drift or systematic errors that may affect result integrity. Control charts tracking key performance indicators provide visual representation of system stability over time.
Method validation represents another essential component of quality assurance, encompassing assessments of accuracy, precision, specificity, linearity, range, limit of detection (LOD), limit of quantification (LOQ), and robustness. For material testing applications, matrix-matched validation is particularly important to account for potential matrix effects.
Proficiency testing through participation in interlaboratory comparison programs offers external verification of analytical capabilities. These programs provide valuable insights into method performance relative to peer laboratories and help identify areas for improvement.
Documentation and traceability must be maintained throughout the analytical process. This includes comprehensive records of instrument maintenance, calibration history, analyst training, and all raw data associated with sample analysis. Electronic data systems should incorporate appropriate security features to prevent unauthorized modifications.
Regular preventive maintenance schedules, coupled with performance verification after maintenance activities, help minimize instrument downtime and ensure consistent analytical performance. This proactive approach to instrument care significantly contributes to the overall quality assurance framework.
Calibration verification forms the cornerstone of quality assurance in GC-MS operations. Regular calibration using certified reference materials (CRMs) ensures that the instrument maintains optimal performance parameters. The calibration curve should demonstrate linearity across the expected concentration range of analytes, with correlation coefficients (R²) exceeding 0.995 for quantitative applications.
System suitability tests must be performed at the beginning of each analytical session to verify that the GC-MS system meets predefined performance criteria. These tests typically include evaluations of retention time reproducibility, peak resolution, signal-to-noise ratio, and mass accuracy. Acceptance criteria should be established based on instrument specifications and analytical requirements.
Internal quality control samples should be analyzed alongside test samples to monitor the stability and performance of the analytical system throughout the testing sequence. These controls help identify potential drift or systematic errors that may affect result integrity. Control charts tracking key performance indicators provide visual representation of system stability over time.
Method validation represents another essential component of quality assurance, encompassing assessments of accuracy, precision, specificity, linearity, range, limit of detection (LOD), limit of quantification (LOQ), and robustness. For material testing applications, matrix-matched validation is particularly important to account for potential matrix effects.
Proficiency testing through participation in interlaboratory comparison programs offers external verification of analytical capabilities. These programs provide valuable insights into method performance relative to peer laboratories and help identify areas for improvement.
Documentation and traceability must be maintained throughout the analytical process. This includes comprehensive records of instrument maintenance, calibration history, analyst training, and all raw data associated with sample analysis. Electronic data systems should incorporate appropriate security features to prevent unauthorized modifications.
Regular preventive maintenance schedules, coupled with performance verification after maintenance activities, help minimize instrument downtime and ensure consistent analytical performance. This proactive approach to instrument care significantly contributes to the overall quality assurance framework.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!