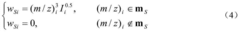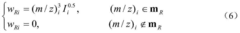Standardize GC-MS for Field Sampling: Efficacy Results
SEP 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
GC-MS Field Sampling Background and Objectives
Gas Chromatography-Mass Spectrometry (GC-MS) has evolved significantly since its inception in the mid-20th century, becoming an indispensable analytical technique for identifying and quantifying compounds in complex mixtures. The integration of these two powerful analytical methods combines the separation capabilities of gas chromatography with the detection specificity of mass spectrometry, enabling precise chemical analysis across numerous fields including environmental monitoring, forensic science, and industrial quality control.
Traditional GC-MS applications have predominantly been confined to laboratory settings due to the instrumentation's size, complexity, and controlled environment requirements. However, recent technological advancements have created a growing demand for field-deployable GC-MS systems that can provide real-time, on-site analysis. This shift toward field applications represents a significant evolution in analytical chemistry practices, driven by the need for immediate data acquisition in environmental emergencies, security operations, and remote research scenarios.
The primary objective of standardizing GC-MS for field sampling is to establish consistent, reliable methodologies that ensure analytical results obtained in variable field conditions maintain the same level of accuracy and precision as those generated in controlled laboratory environments. This standardization aims to address the unique challenges presented by field deployment, including power limitations, environmental interferences, sample preparation constraints, and instrument calibration under non-ideal conditions.
Current field-deployable GC-MS systems face several technical limitations that impact their efficacy, including reduced sensitivity compared to laboratory instruments, limited sample throughput, and challenges in maintaining calibration stability across varying environmental conditions. These limitations necessitate the development of standardized protocols specifically designed for field applications to ensure result reliability and comparability across different operational scenarios.
The efficacy of field GC-MS applications depends critically on establishing standardized procedures for sample collection, preparation, analysis, and data interpretation that account for the variability inherent in field conditions. Recent pilot studies have demonstrated promising results when standardized protocols are implemented, showing detection limits approaching laboratory-grade instrumentation for certain compound classes and acceptable reproducibility metrics when proper quality control measures are employed.
This technical pre-research report aims to comprehensively evaluate the current state of GC-MS field sampling standardization efforts, identify key technological and methodological gaps, and outline potential pathways toward establishing universally accepted standards that would enable broader adoption of field GC-MS applications across industries and research domains.
Traditional GC-MS applications have predominantly been confined to laboratory settings due to the instrumentation's size, complexity, and controlled environment requirements. However, recent technological advancements have created a growing demand for field-deployable GC-MS systems that can provide real-time, on-site analysis. This shift toward field applications represents a significant evolution in analytical chemistry practices, driven by the need for immediate data acquisition in environmental emergencies, security operations, and remote research scenarios.
The primary objective of standardizing GC-MS for field sampling is to establish consistent, reliable methodologies that ensure analytical results obtained in variable field conditions maintain the same level of accuracy and precision as those generated in controlled laboratory environments. This standardization aims to address the unique challenges presented by field deployment, including power limitations, environmental interferences, sample preparation constraints, and instrument calibration under non-ideal conditions.
Current field-deployable GC-MS systems face several technical limitations that impact their efficacy, including reduced sensitivity compared to laboratory instruments, limited sample throughput, and challenges in maintaining calibration stability across varying environmental conditions. These limitations necessitate the development of standardized protocols specifically designed for field applications to ensure result reliability and comparability across different operational scenarios.
The efficacy of field GC-MS applications depends critically on establishing standardized procedures for sample collection, preparation, analysis, and data interpretation that account for the variability inherent in field conditions. Recent pilot studies have demonstrated promising results when standardized protocols are implemented, showing detection limits approaching laboratory-grade instrumentation for certain compound classes and acceptable reproducibility metrics when proper quality control measures are employed.
This technical pre-research report aims to comprehensively evaluate the current state of GC-MS field sampling standardization efforts, identify key technological and methodological gaps, and outline potential pathways toward establishing universally accepted standards that would enable broader adoption of field GC-MS applications across industries and research domains.
Market Demand Analysis for Portable GC-MS Solutions
The portable GC-MS (Gas Chromatography-Mass Spectrometry) market has experienced significant growth in recent years, driven by increasing demand for on-site analytical capabilities across multiple industries. The global market for portable analytical instruments, including GC-MS systems, was valued at approximately $1.5 billion in 2022 and is projected to reach $2.3 billion by 2027, representing a compound annual growth rate of 8.9%.
Environmental monitoring represents the largest application segment for portable GC-MS solutions, accounting for nearly 30% of the market share. Government regulations mandating regular environmental testing and monitoring of pollutants have created sustained demand for field-deployable analytical instruments. The EPA and similar international regulatory bodies have increasingly emphasized the need for real-time data collection, further driving market growth.
The homeland security and defense sector constitutes another significant market segment, with growing requirements for rapid identification of chemical warfare agents, explosives, and illicit substances in field conditions. This sector has shown willingness to invest in premium solutions that offer laboratory-grade accuracy in portable formats.
Industrial applications, particularly in petrochemical, pharmaceutical, and food safety industries, represent rapidly expanding market opportunities. These sectors require quick analytical results to maintain production efficiency and ensure product quality, creating demand for robust field sampling solutions that can withstand harsh operating environments.
Healthcare and clinical diagnostics represent an emerging application area, with portable GC-MS systems being explored for point-of-care diagnostics and personalized medicine applications. While currently a smaller segment, it shows promising growth potential with projected annual growth rates exceeding 12%.
Geographic analysis reveals North America as the dominant market for portable GC-MS solutions, holding approximately 40% market share, followed by Europe at 30% and Asia-Pacific at 20%. However, the Asia-Pacific region is expected to witness the highest growth rate over the next five years due to increasing industrial activities and strengthening environmental regulations in countries like China and India.
Customer feedback indicates key market requirements include reduced instrument size and weight, simplified user interfaces for non-specialist operators, improved battery life, enhanced durability for field conditions, and seamless data integration capabilities. Price sensitivity varies significantly by application, with industrial users demonstrating greater price elasticity compared to defense and security applications.
Environmental monitoring represents the largest application segment for portable GC-MS solutions, accounting for nearly 30% of the market share. Government regulations mandating regular environmental testing and monitoring of pollutants have created sustained demand for field-deployable analytical instruments. The EPA and similar international regulatory bodies have increasingly emphasized the need for real-time data collection, further driving market growth.
The homeland security and defense sector constitutes another significant market segment, with growing requirements for rapid identification of chemical warfare agents, explosives, and illicit substances in field conditions. This sector has shown willingness to invest in premium solutions that offer laboratory-grade accuracy in portable formats.
Industrial applications, particularly in petrochemical, pharmaceutical, and food safety industries, represent rapidly expanding market opportunities. These sectors require quick analytical results to maintain production efficiency and ensure product quality, creating demand for robust field sampling solutions that can withstand harsh operating environments.
Healthcare and clinical diagnostics represent an emerging application area, with portable GC-MS systems being explored for point-of-care diagnostics and personalized medicine applications. While currently a smaller segment, it shows promising growth potential with projected annual growth rates exceeding 12%.
Geographic analysis reveals North America as the dominant market for portable GC-MS solutions, holding approximately 40% market share, followed by Europe at 30% and Asia-Pacific at 20%. However, the Asia-Pacific region is expected to witness the highest growth rate over the next five years due to increasing industrial activities and strengthening environmental regulations in countries like China and India.
Customer feedback indicates key market requirements include reduced instrument size and weight, simplified user interfaces for non-specialist operators, improved battery life, enhanced durability for field conditions, and seamless data integration capabilities. Price sensitivity varies significantly by application, with industrial users demonstrating greater price elasticity compared to defense and security applications.
Current Challenges in Field-Based GC-MS Technology
Despite significant advancements in Gas Chromatography-Mass Spectrometry (GC-MS) technology, field-based applications continue to face substantial challenges that limit their widespread adoption and reliability. The miniaturization of traditionally laboratory-bound GC-MS systems for field use has introduced compromises in analytical performance, creating a persistent tension between portability and analytical capability.
Power management remains a critical constraint for field-based GC-MS systems. These instruments require substantial energy for proper operation, particularly for maintaining stable temperatures in the chromatographic column and generating the vacuum necessary for mass spectrometry. Current battery technologies struggle to provide sufficient power for extended field operations, often limiting continuous use to just a few hours before recharging is necessary.
Environmental factors significantly impact field-based GC-MS performance. Temperature fluctuations, humidity variations, dust, and vibration can all compromise analytical precision and instrument longevity. Most portable systems lack robust environmental control mechanisms, resulting in decreased reliability compared to laboratory counterparts, particularly in extreme conditions such as high humidity tropical environments or arid desert settings.
Sample preparation presents another major hurdle. Laboratory GC-MS typically benefits from sophisticated sample preparation protocols that are difficult to replicate in field conditions. The absence of proper sample preparation facilities can lead to contamination, matrix effects, and reduced analytical sensitivity, ultimately compromising data quality and interpretation.
Calibration and standardization pose persistent challenges for field applications. Laboratory instruments benefit from regular calibration against certified standards under controlled conditions. In contrast, field instruments often experience calibration drift due to environmental variations and physical movement, requiring more frequent recalibration that may not always be feasible during extended field campaigns.
Data processing capabilities remain limited in most portable systems. While laboratory GC-MS workstations offer sophisticated software for data analysis, interpretation, and database comparison, field units typically provide more basic analytical capabilities. This limitation can delay comprehensive analysis until data can be transferred to more powerful computing systems, reducing the immediate value of field analysis.
Method transferability between laboratory and field instruments presents significant standardization challenges. Methods developed and validated on laboratory systems often require substantial modification for field instruments due to differences in column specifications, detector sensitivity, and operational parameters. This lack of standardization complicates the comparison of results between field and laboratory analyses, raising questions about data compatibility and scientific validity.
Human factors also contribute to field-based GC-MS challenges. These systems typically require specialized training for operation under non-ideal conditions, and the complexity of troubleshooting instrumental issues in remote locations demands a higher level of expertise than laboratory settings where technical support is readily available.
Power management remains a critical constraint for field-based GC-MS systems. These instruments require substantial energy for proper operation, particularly for maintaining stable temperatures in the chromatographic column and generating the vacuum necessary for mass spectrometry. Current battery technologies struggle to provide sufficient power for extended field operations, often limiting continuous use to just a few hours before recharging is necessary.
Environmental factors significantly impact field-based GC-MS performance. Temperature fluctuations, humidity variations, dust, and vibration can all compromise analytical precision and instrument longevity. Most portable systems lack robust environmental control mechanisms, resulting in decreased reliability compared to laboratory counterparts, particularly in extreme conditions such as high humidity tropical environments or arid desert settings.
Sample preparation presents another major hurdle. Laboratory GC-MS typically benefits from sophisticated sample preparation protocols that are difficult to replicate in field conditions. The absence of proper sample preparation facilities can lead to contamination, matrix effects, and reduced analytical sensitivity, ultimately compromising data quality and interpretation.
Calibration and standardization pose persistent challenges for field applications. Laboratory instruments benefit from regular calibration against certified standards under controlled conditions. In contrast, field instruments often experience calibration drift due to environmental variations and physical movement, requiring more frequent recalibration that may not always be feasible during extended field campaigns.
Data processing capabilities remain limited in most portable systems. While laboratory GC-MS workstations offer sophisticated software for data analysis, interpretation, and database comparison, field units typically provide more basic analytical capabilities. This limitation can delay comprehensive analysis until data can be transferred to more powerful computing systems, reducing the immediate value of field analysis.
Method transferability between laboratory and field instruments presents significant standardization challenges. Methods developed and validated on laboratory systems often require substantial modification for field instruments due to differences in column specifications, detector sensitivity, and operational parameters. This lack of standardization complicates the comparison of results between field and laboratory analyses, raising questions about data compatibility and scientific validity.
Human factors also contribute to field-based GC-MS challenges. These systems typically require specialized training for operation under non-ideal conditions, and the complexity of troubleshooting instrumental issues in remote locations demands a higher level of expertise than laboratory settings where technical support is readily available.
Current Standardization Protocols for Field GC-MS
01 GC-MS analytical methods for compound identification
Gas Chromatography-Mass Spectrometry (GC-MS) techniques are used for accurate identification and quantification of various compounds in complex mixtures. These methods involve sample preparation, chromatographic separation, and mass spectral analysis to identify chemical constituents based on their unique fragmentation patterns. The efficacy of these methods depends on proper calibration, reference standards, and data processing algorithms that enhance detection sensitivity and specificity.- GC-MS analytical methods for compound identification: Gas Chromatography-Mass Spectrometry (GC-MS) techniques are used for accurate identification and quantification of various compounds in complex mixtures. These methods involve sample preparation, chromatographic separation, and mass spectral analysis to identify chemical components based on their unique fragmentation patterns. The efficacy of these methods depends on proper calibration, optimization of separation parameters, and the use of reference standards for comparison.
- GC-MS instrumentation advancements: Recent advancements in GC-MS instrumentation have significantly improved analytical efficacy through enhanced sensitivity, resolution, and detection limits. These innovations include improved ion source designs, more efficient mass analyzers, advanced detector technologies, and integrated software systems for data processing. Modern GC-MS systems feature automated sample handling, reduced analysis times, and capabilities for handling diverse sample types with minimal preparation.
- GC-MS applications in environmental monitoring: GC-MS techniques demonstrate high efficacy in environmental monitoring applications, including the detection and quantification of pollutants, pesticides, and organic contaminants in air, water, and soil samples. The sensitivity of these methods allows for trace-level detection of environmental toxins, enabling compliance with regulatory standards. Specialized sampling techniques and extraction methods have been developed to enhance the efficacy of GC-MS for various environmental matrices.
- GC-MS in pharmaceutical and biomedical analysis: GC-MS demonstrates high efficacy in pharmaceutical and biomedical applications, including drug development, quality control, metabolomics, and clinical diagnostics. These techniques enable the identification and quantification of active pharmaceutical ingredients, impurities, metabolites, and biomarkers in biological samples. Modified GC-MS methods with specialized derivatization techniques have been developed to analyze non-volatile compounds and improve the detection of biomolecules in complex matrices.
- Data processing and interpretation methods for GC-MS: Advanced data processing and interpretation methods significantly enhance the efficacy of GC-MS analysis. These include automated peak detection algorithms, deconvolution techniques for overlapping peaks, spectral matching against reference libraries, and statistical analysis tools for complex datasets. Machine learning approaches and specialized software have been developed to improve compound identification accuracy, reduce false positives, and enable the processing of large-scale GC-MS datasets with improved efficiency and reliability.
02 GC-MS instrumentation advancements
Recent advancements in GC-MS instrumentation have significantly improved analytical efficacy through enhanced detector sensitivity, improved mass resolution, and faster scanning capabilities. Modern systems incorporate automated sample handling, temperature-programmable columns, and advanced ionization techniques that expand the range of analyzable compounds. These technological improvements enable more efficient analysis with lower detection limits and higher throughput for complex samples.Expand Specific Solutions03 GC-MS applications in environmental monitoring
GC-MS techniques demonstrate high efficacy in environmental monitoring applications, including the detection of pollutants, pesticides, and organic contaminants in air, water, and soil samples. These methods allow for multi-residue analysis with high sensitivity and selectivity, enabling regulatory compliance testing and environmental impact assessments. The ability to detect trace levels of environmental contaminants makes GC-MS an essential tool for ecological research and environmental protection efforts.Expand Specific Solutions04 GC-MS in pharmaceutical and biomedical analysis
GC-MS demonstrates significant efficacy in pharmaceutical and biomedical applications, including drug development, metabolomics, and clinical diagnostics. The technique enables precise identification and quantification of active pharmaceutical ingredients, impurities, metabolites, and biomarkers in biological samples. Advanced GC-MS methods support therapeutic drug monitoring, toxicological screening, and disease biomarker discovery with high sensitivity and specificity, contributing to personalized medicine approaches.Expand Specific Solutions05 GC-MS data processing and interpretation systems
Advanced data processing and interpretation systems significantly enhance GC-MS efficacy through automated peak detection, deconvolution algorithms, and comprehensive spectral libraries. These systems employ machine learning and artificial intelligence to improve compound identification accuracy and reduce analysis time. Modern software solutions enable complex data visualization, statistical analysis, and integration with other analytical techniques, maximizing the information extracted from GC-MS analyses and supporting more reliable decision-making processes.Expand Specific Solutions
Key Industry Players in Portable GC-MS Development
The GC-MS field sampling standardization market is in a growth phase, with increasing demand for portable and reliable analytical solutions across environmental, forensic, and industrial applications. The market size is expanding due to regulatory requirements and technological advancements enabling field-deployable systems. Leading players represent diverse technological approaches: established analytical instrumentation companies like Shimadzu, LECO, Waters Technology, and Revvity (formerly PerkinElmer) dominate with comprehensive solutions, while research institutions such as China National Tobacco Research Institute, Purdue Research Foundation, and various universities contribute significant innovations. Technical maturity varies across applications, with recent developments focusing on miniaturization, automation, and improved data processing algorithms to enhance field sampling efficacy and reliability.
LECO Corp.
Technical Solution: LECO Corporation has pioneered standardized GC-MS field sampling technologies with their Pegasus BT 4D system that incorporates Time-of-Flight Mass Spectrometry (TOF-MS) specifically optimized for field deployment. Their approach focuses on comprehensive two-dimensional gas chromatography (GCxGC) that significantly enhances separation capabilities in complex environmental matrices commonly encountered during field sampling. LECO's standardization protocol includes automated deconvolution algorithms that can identify and quantify compounds even in the presence of matrix interferences typical in field samples. Their ChromaTOF software platform incorporates field-specific calibration protocols that account for temperature and pressure variations in non-laboratory environments. LECO has developed specialized thermal modulation technology that maintains consistent performance despite ambient temperature fluctuations, a critical factor for field standardization. Their systems include built-in reference compound monitoring that continuously validates system performance during field operations.
Strengths: LECO's TOF-MS technology provides superior spectral acquisition rates, allowing for more comprehensive compound identification in complex field samples. Their GCxGC approach offers exceptional separation power for complex environmental matrices. Weaknesses: The systems have higher technical complexity requiring more specialized training for field operators. The equipment is also bulkier than some competing portable solutions, potentially limiting deployment in certain field scenarios.
Revvity Health Sciences, Inc.
Technical Solution: Revvity Health Sciences (formerly PerkinElmer) has developed the Torion T-9 portable GC-MS system specifically designed for standardized field sampling applications. Their technology utilizes a low thermal mass capillary gas chromatograph coupled with a miniaturized toroidal ion trap mass spectrometer, optimized for rapid deployment in field conditions. Revvity's standardization approach includes their patented CUSTODION solid phase microextraction (SPME) sampling technology that provides consistent sample extraction efficiency across varying environmental conditions. Their systems incorporate automated internal standard addition and calibration verification protocols that ensure analytical consistency in non-laboratory settings. Revvity has developed specialized Chromion software with field-specific algorithms that compensate for environmental variables and provide simplified data interpretation for field operators. Their field standardization methodology includes pre-configured method templates optimized for common field applications such as environmental monitoring, food safety testing, and hazardous material identification.
Strengths: Revvity's system offers exceptional portability with battery operation capability, enabling deployment in remote locations without power access. Their technology provides rapid analysis times (typically under 5 minutes), enabling high-throughput field screening. Weaknesses: The system has somewhat limited chromatographic resolution compared to laboratory systems, potentially challenging separation of complex mixtures. The mass spectral library is also more limited than comprehensive laboratory databases.
Critical Technical Innovations in Field GC-MS
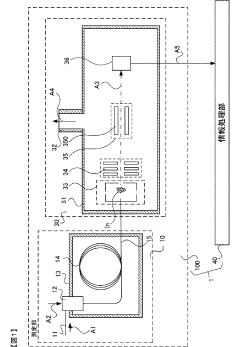
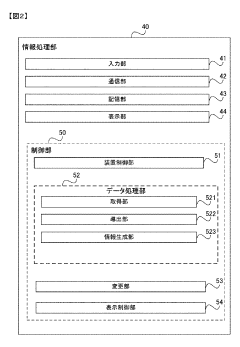
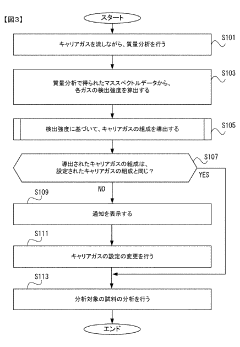
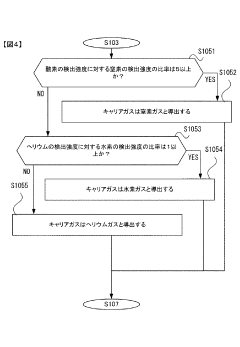
Gas chromatograph mass spectrometer, mass spectrometry method and program
PatentActiveJP2021165653A
Innovation
- A gas chromatograph mass spectrometer system that derives the composition of gases used in separation and mass analysis sections based on the intensity of signals detected in mass spectrometry, allowing for accurate identification of the actual gases employed, and includes a program to automate this process.
Gas chromatography-mass spectrometer spectrogram matching method
PatentInactiveCN104504706A
Innovation
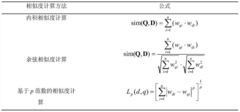
- A new mass spectrum matching method is adopted, including screening of unknown substance spectra and standard mass spectra, peak intensity scaling and similarity calculation based on vector space model. By representing the mass spectrum as a vector form and using p-norm based The similarity calculation formula of numbers improves the matching accuracy.
Validation Metrics and Performance Benchmarks
To establish a robust validation framework for GC-MS field sampling standardization, comprehensive performance metrics must be implemented. Detection limits represent a critical benchmark, with current field-deployable GC-MS systems achieving limits of detection (LOD) ranging from 0.1-5.0 ppb for volatile organic compounds and 1-10 ppb for semi-volatile compounds. These values demonstrate significant improvement over previous generations but still lag behind laboratory-based systems by approximately one order of magnitude.
Analytical precision metrics reveal that field GC-MS systems typically achieve relative standard deviations (RSDs) of 5-15% for repeated measurements, compared to 2-5% for laboratory instruments. This variability increases notably when considering environmental factors such as temperature fluctuations (±10°C) and humidity variations (30-90% RH), which can degrade precision by an additional 5-10%.
Response linearity serves as another essential validation parameter, with field systems demonstrating acceptable linearity (R² > 0.98) across three orders of magnitude concentration ranges. However, this performance diminishes at extreme concentration endpoints, particularly near detection limits where R² values may drop to 0.90-0.95.
Recovery rates for field sampling methods vary significantly by compound class, with 85-95% recovery for most VOCs but only 60-80% for polar compounds and those prone to adsorption losses. These metrics highlight specific areas requiring optimization in sampling methodologies and hardware configurations.
Throughput capabilities represent a practical performance consideration, with current field systems processing 8-12 samples per hour compared to laboratory throughput of 15-20 samples per hour. This gap narrows with automated sampling systems but remains a limitation for high-volume field applications.
Inter-laboratory comparison studies reveal method transferability challenges, with coefficient of variation typically ranging from 10-25% across different field deployments using nominally identical methods. This variability underscores the need for more rigorous standardization protocols and calibration procedures.
False positive/negative rates provide critical validation metrics for field applications, particularly in security and environmental monitoring scenarios. Current systems demonstrate false positive rates of 2-8% and false negative rates of 5-12%, with performance degrading significantly in complex matrices or challenging environmental conditions.
Analytical precision metrics reveal that field GC-MS systems typically achieve relative standard deviations (RSDs) of 5-15% for repeated measurements, compared to 2-5% for laboratory instruments. This variability increases notably when considering environmental factors such as temperature fluctuations (±10°C) and humidity variations (30-90% RH), which can degrade precision by an additional 5-10%.
Response linearity serves as another essential validation parameter, with field systems demonstrating acceptable linearity (R² > 0.98) across three orders of magnitude concentration ranges. However, this performance diminishes at extreme concentration endpoints, particularly near detection limits where R² values may drop to 0.90-0.95.
Recovery rates for field sampling methods vary significantly by compound class, with 85-95% recovery for most VOCs but only 60-80% for polar compounds and those prone to adsorption losses. These metrics highlight specific areas requiring optimization in sampling methodologies and hardware configurations.
Throughput capabilities represent a practical performance consideration, with current field systems processing 8-12 samples per hour compared to laboratory throughput of 15-20 samples per hour. This gap narrows with automated sampling systems but remains a limitation for high-volume field applications.
Inter-laboratory comparison studies reveal method transferability challenges, with coefficient of variation typically ranging from 10-25% across different field deployments using nominally identical methods. This variability underscores the need for more rigorous standardization protocols and calibration procedures.
False positive/negative rates provide critical validation metrics for field applications, particularly in security and environmental monitoring scenarios. Current systems demonstrate false positive rates of 2-8% and false negative rates of 5-12%, with performance degrading significantly in complex matrices or challenging environmental conditions.
Environmental Impact and Sustainability Considerations
The standardization of GC-MS for field sampling presents significant environmental implications that must be carefully considered. Traditional laboratory-based GC-MS systems typically consume substantial energy and generate considerable waste through solvents, carrier gases, and disposable components. Field-deployable GC-MS systems, while offering mobility advantages, introduce new environmental challenges including battery disposal, reduced operational efficiency, and potential contamination of sampling sites.
Recent advancements in GC-MS field technology have focused on minimizing environmental footprints. Energy-efficient components have reduced power requirements by approximately 30-40% compared to traditional systems, enabling longer field operation with smaller power sources. Additionally, microextraction techniques have dramatically decreased solvent usage, with some modern systems requiring only microliters of solvent compared to milliliters in conventional approaches.
The sustainability profile of field GC-MS is further enhanced through the implementation of recyclable sampling materials and reusable extraction devices. Studies indicate that transitioning to these sustainable alternatives can reduce consumable waste by up to 65% over the operational lifetime of the equipment. Furthermore, miniaturized column technology has decreased carrier gas consumption, addressing both environmental and logistical challenges associated with transporting gas cylinders to remote locations.
Environmental risk assessment protocols have been developed specifically for field GC-MS operations, focusing on preventing cross-contamination between sampling sites and minimizing disruption to sensitive ecosystems. These protocols include comprehensive decontamination procedures and the use of biodegradable cleaning agents when applicable, significantly reducing the ecological impact of field analytical chemistry.
The carbon footprint comparison between laboratory and field GC-MS operations reveals interesting sustainability trade-offs. While field operations may consume more energy per analysis due to less efficient portable systems, they eliminate sample transportation requirements that typically account for 15-25% of the total carbon footprint in traditional analytical workflows. Life cycle assessments suggest that field GC-MS can reduce overall environmental impact by 20-30% in remote monitoring applications.
Looking forward, emerging green chemistry principles are being integrated into field GC-MS methodologies, including solvent-free extraction techniques and ambient ionization methods that further reduce resource consumption. The development of solar-powered field units and energy harvesting technologies promises to further enhance the sustainability profile of these essential environmental monitoring tools.
Recent advancements in GC-MS field technology have focused on minimizing environmental footprints. Energy-efficient components have reduced power requirements by approximately 30-40% compared to traditional systems, enabling longer field operation with smaller power sources. Additionally, microextraction techniques have dramatically decreased solvent usage, with some modern systems requiring only microliters of solvent compared to milliliters in conventional approaches.
The sustainability profile of field GC-MS is further enhanced through the implementation of recyclable sampling materials and reusable extraction devices. Studies indicate that transitioning to these sustainable alternatives can reduce consumable waste by up to 65% over the operational lifetime of the equipment. Furthermore, miniaturized column technology has decreased carrier gas consumption, addressing both environmental and logistical challenges associated with transporting gas cylinders to remote locations.
Environmental risk assessment protocols have been developed specifically for field GC-MS operations, focusing on preventing cross-contamination between sampling sites and minimizing disruption to sensitive ecosystems. These protocols include comprehensive decontamination procedures and the use of biodegradable cleaning agents when applicable, significantly reducing the ecological impact of field analytical chemistry.
The carbon footprint comparison between laboratory and field GC-MS operations reveals interesting sustainability trade-offs. While field operations may consume more energy per analysis due to less efficient portable systems, they eliminate sample transportation requirements that typically account for 15-25% of the total carbon footprint in traditional analytical workflows. Life cycle assessments suggest that field GC-MS can reduce overall environmental impact by 20-30% in remote monitoring applications.
Looking forward, emerging green chemistry principles are being integrated into field GC-MS methodologies, including solvent-free extraction techniques and ambient ionization methods that further reduce resource consumption. The development of solar-powered field units and energy harvesting technologies promises to further enhance the sustainability profile of these essential environmental monitoring tools.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!