GC-MS Organic Chemistry: Enabling Analysis for New Drugs
SEP 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
GC-MS Technology Evolution and Research Objectives
Gas Chromatography-Mass Spectrometry (GC-MS) has evolved significantly since its inception in the 1950s, transforming from a specialized analytical technique to an indispensable tool in pharmaceutical research and development. The integration of gas chromatography's separation capabilities with mass spectrometry's identification power created a synergistic analytical platform that revolutionized organic chemistry analysis.
The evolution of GC-MS technology has been marked by several pivotal advancements. Early systems featured magnetic sector mass analyzers with limited sensitivity and resolution. The 1970s witnessed the introduction of quadrupole mass analyzers, which offered improved reliability and accessibility. The 1980s and 1990s brought computerized data systems and automated sample handling, significantly enhancing throughput and reproducibility.
Recent technological breakthroughs include the development of high-resolution time-of-flight (TOF) mass analyzers, tandem MS capabilities, and comprehensive two-dimensional gas chromatography (GCxGC). These innovations have dramatically improved detection limits, compound identification accuracy, and the ability to analyze complex mixtures—critical factors in pharmaceutical development.
In the pharmaceutical context, GC-MS has become essential for drug discovery, development, and quality control. The technique enables researchers to identify and quantify trace compounds, characterize metabolites, monitor reaction progress, and ensure product purity. Its application spans from initial lead compound identification through preclinical testing to manufacturing quality assurance.
Current research objectives in GC-MS technology for pharmaceutical applications focus on several key areas. Enhancing sensitivity and selectivity remains paramount, particularly for detecting compounds at sub-nanogram levels in complex biological matrices. Researchers aim to develop more efficient ionization techniques and improved mass analyzers to address this challenge.
Miniaturization represents another significant research direction, with efforts to create portable, field-deployable GC-MS systems that maintain laboratory-grade performance. Such systems could revolutionize point-of-care diagnostics and on-site quality control in pharmaceutical manufacturing.
Data processing and interpretation present ongoing challenges, particularly with the massive datasets generated by modern high-resolution instruments. Machine learning and artificial intelligence approaches are being explored to automate compound identification, predict fragmentation patterns, and extract meaningful insights from complex chromatographic and spectral data.
The ultimate research objective is to establish GC-MS as a comprehensive analytical platform that seamlessly integrates with other techniques in a holistic approach to drug discovery and development. This includes coupling with liquid chromatography, nuclear magnetic resonance, and various spectroscopic methods to provide complementary structural information and enhance analytical capabilities.
The evolution of GC-MS technology has been marked by several pivotal advancements. Early systems featured magnetic sector mass analyzers with limited sensitivity and resolution. The 1970s witnessed the introduction of quadrupole mass analyzers, which offered improved reliability and accessibility. The 1980s and 1990s brought computerized data systems and automated sample handling, significantly enhancing throughput and reproducibility.
Recent technological breakthroughs include the development of high-resolution time-of-flight (TOF) mass analyzers, tandem MS capabilities, and comprehensive two-dimensional gas chromatography (GCxGC). These innovations have dramatically improved detection limits, compound identification accuracy, and the ability to analyze complex mixtures—critical factors in pharmaceutical development.
In the pharmaceutical context, GC-MS has become essential for drug discovery, development, and quality control. The technique enables researchers to identify and quantify trace compounds, characterize metabolites, monitor reaction progress, and ensure product purity. Its application spans from initial lead compound identification through preclinical testing to manufacturing quality assurance.
Current research objectives in GC-MS technology for pharmaceutical applications focus on several key areas. Enhancing sensitivity and selectivity remains paramount, particularly for detecting compounds at sub-nanogram levels in complex biological matrices. Researchers aim to develop more efficient ionization techniques and improved mass analyzers to address this challenge.
Miniaturization represents another significant research direction, with efforts to create portable, field-deployable GC-MS systems that maintain laboratory-grade performance. Such systems could revolutionize point-of-care diagnostics and on-site quality control in pharmaceutical manufacturing.
Data processing and interpretation present ongoing challenges, particularly with the massive datasets generated by modern high-resolution instruments. Machine learning and artificial intelligence approaches are being explored to automate compound identification, predict fragmentation patterns, and extract meaningful insights from complex chromatographic and spectral data.
The ultimate research objective is to establish GC-MS as a comprehensive analytical platform that seamlessly integrates with other techniques in a holistic approach to drug discovery and development. This includes coupling with liquid chromatography, nuclear magnetic resonance, and various spectroscopic methods to provide complementary structural information and enhance analytical capabilities.
Pharmaceutical Market Demand for GC-MS Analysis
The pharmaceutical industry's demand for Gas Chromatography-Mass Spectrometry (GC-MS) analysis has experienced significant growth over the past decade, driven primarily by increasing regulatory requirements and the expanding complexity of drug development processes. The global pharmaceutical analytical testing outsourcing market, where GC-MS plays a crucial role, was valued at approximately $6.1 billion in 2021 and is projected to reach $11.4 billion by 2028, representing a compound annual growth rate of 8.3%.
Drug discovery and development processes have become increasingly dependent on advanced analytical techniques, with GC-MS emerging as an essential tool for compound identification, purity assessment, and metabolite analysis. Pharmaceutical companies are particularly leveraging GC-MS capabilities for impurity profiling, where the technique's high sensitivity allows detection of trace contaminants down to parts per billion levels, critical for meeting stringent regulatory standards set by FDA, EMA, and other global authorities.
The market demand is further amplified by the rising prevalence of chronic diseases worldwide, which has accelerated the need for novel therapeutic agents. Cancer, diabetes, and cardiovascular diseases collectively drive approximately 65% of pharmaceutical R&D efforts, creating substantial demand for analytical technologies that can facilitate faster drug development cycles while maintaining quality standards.
Small molecule drug development continues to represent the largest segment utilizing GC-MS analysis, accounting for approximately 58% of the total market demand. This dominance stems from GC-MS's particular suitability for analyzing volatile and semi-volatile compounds commonly encountered in small molecule drug candidates. However, the biologics sector is showing the fastest growth rate at 12.7% annually, as manufacturers increasingly adopt GC-MS for specific applications in protein-based therapeutics.
Geographically, North America commands the largest market share at 42%, followed by Europe at 28% and Asia-Pacific at 22%. The Asia-Pacific region, particularly China and India, is experiencing the most rapid growth due to increasing outsourcing of pharmaceutical manufacturing and the expansion of domestic drug development capabilities in these countries.
Contract Research Organizations (CROs) represent a significant driver of GC-MS adoption, as pharmaceutical companies increasingly outsource analytical testing to specialized service providers. This trend is expected to continue, with approximately 70% of pharmaceutical companies planning to increase their analytical testing outsourcing over the next five years, creating sustained demand for advanced GC-MS instrumentation and expertise.
Drug discovery and development processes have become increasingly dependent on advanced analytical techniques, with GC-MS emerging as an essential tool for compound identification, purity assessment, and metabolite analysis. Pharmaceutical companies are particularly leveraging GC-MS capabilities for impurity profiling, where the technique's high sensitivity allows detection of trace contaminants down to parts per billion levels, critical for meeting stringent regulatory standards set by FDA, EMA, and other global authorities.
The market demand is further amplified by the rising prevalence of chronic diseases worldwide, which has accelerated the need for novel therapeutic agents. Cancer, diabetes, and cardiovascular diseases collectively drive approximately 65% of pharmaceutical R&D efforts, creating substantial demand for analytical technologies that can facilitate faster drug development cycles while maintaining quality standards.
Small molecule drug development continues to represent the largest segment utilizing GC-MS analysis, accounting for approximately 58% of the total market demand. This dominance stems from GC-MS's particular suitability for analyzing volatile and semi-volatile compounds commonly encountered in small molecule drug candidates. However, the biologics sector is showing the fastest growth rate at 12.7% annually, as manufacturers increasingly adopt GC-MS for specific applications in protein-based therapeutics.
Geographically, North America commands the largest market share at 42%, followed by Europe at 28% and Asia-Pacific at 22%. The Asia-Pacific region, particularly China and India, is experiencing the most rapid growth due to increasing outsourcing of pharmaceutical manufacturing and the expansion of domestic drug development capabilities in these countries.
Contract Research Organizations (CROs) represent a significant driver of GC-MS adoption, as pharmaceutical companies increasingly outsource analytical testing to specialized service providers. This trend is expected to continue, with approximately 70% of pharmaceutical companies planning to increase their analytical testing outsourcing over the next five years, creating sustained demand for advanced GC-MS instrumentation and expertise.
Current GC-MS Capabilities and Technical Limitations
Gas Chromatography-Mass Spectrometry (GC-MS) represents one of the most powerful analytical techniques in organic chemistry, particularly for drug discovery and development. Current GC-MS systems offer exceptional sensitivity, with detection limits in the picogram to femtogram range, enabling the identification of trace compounds critical in pharmaceutical research. Modern instruments typically achieve mass resolution of 1,000-10,000 FWHM (Full Width at Half Maximum), allowing for discrimination between compounds with similar molecular weights.
The separation capabilities of contemporary GC-MS systems are remarkable, with high-efficiency capillary columns providing theoretical plate counts exceeding 100,000, enabling the resolution of complex mixtures containing hundreds of components. Advanced temperature programming allows for the separation of compounds with boiling points ranging from 30°C to over 450°C, covering most drug candidates and their metabolites.
Data processing capabilities have evolved significantly, with current software platforms offering automated deconvolution of overlapping peaks, library searching against databases containing over 1 million compounds, and sophisticated statistical tools for metabolomics and impurity profiling. These capabilities have dramatically reduced analysis time from days to hours or even minutes.
Despite these impressive capabilities, GC-MS faces several technical limitations that impact its application in pharmaceutical analysis. The most significant constraint remains the requirement for analyte volatility and thermal stability. Many modern drug candidates are large molecules (>500 Da), polar, or thermally labile, making them unsuitable for direct GC-MS analysis without derivatization.
Derivatization procedures, while expanding the range of analyzable compounds, introduce additional sample preparation steps, potential for error, and may alter the original chemical structure, complicating interpretation. Current derivatization reagents often fail to effectively modify all functional groups in complex drug molecules, leading to incomplete analysis.
Column bleed and matrix effects continue to challenge ultra-trace analysis, particularly when working with complex biological samples. Even with modern stationary phases, background interference can mask signals from minor components or metabolites critical to understanding drug behavior in vivo.
The coupling of GC with high-resolution mass spectrometry (HRMS) has improved compound identification, but computational challenges persist in interpreting the vast datasets generated. Current algorithms struggle with reliable identification of unknown compounds not present in spectral libraries, a significant limitation when analyzing novel drug candidates and their metabolites.
Miniaturization efforts have produced portable GC-MS systems, but these typically sacrifice performance for size, offering lower sensitivity and resolution than laboratory instruments. This limits their application in pharmaceutical development where analytical precision is paramount.
The separation capabilities of contemporary GC-MS systems are remarkable, with high-efficiency capillary columns providing theoretical plate counts exceeding 100,000, enabling the resolution of complex mixtures containing hundreds of components. Advanced temperature programming allows for the separation of compounds with boiling points ranging from 30°C to over 450°C, covering most drug candidates and their metabolites.
Data processing capabilities have evolved significantly, with current software platforms offering automated deconvolution of overlapping peaks, library searching against databases containing over 1 million compounds, and sophisticated statistical tools for metabolomics and impurity profiling. These capabilities have dramatically reduced analysis time from days to hours or even minutes.
Despite these impressive capabilities, GC-MS faces several technical limitations that impact its application in pharmaceutical analysis. The most significant constraint remains the requirement for analyte volatility and thermal stability. Many modern drug candidates are large molecules (>500 Da), polar, or thermally labile, making them unsuitable for direct GC-MS analysis without derivatization.
Derivatization procedures, while expanding the range of analyzable compounds, introduce additional sample preparation steps, potential for error, and may alter the original chemical structure, complicating interpretation. Current derivatization reagents often fail to effectively modify all functional groups in complex drug molecules, leading to incomplete analysis.
Column bleed and matrix effects continue to challenge ultra-trace analysis, particularly when working with complex biological samples. Even with modern stationary phases, background interference can mask signals from minor components or metabolites critical to understanding drug behavior in vivo.
The coupling of GC with high-resolution mass spectrometry (HRMS) has improved compound identification, but computational challenges persist in interpreting the vast datasets generated. Current algorithms struggle with reliable identification of unknown compounds not present in spectral libraries, a significant limitation when analyzing novel drug candidates and their metabolites.
Miniaturization efforts have produced portable GC-MS systems, but these typically sacrifice performance for size, offering lower sensitivity and resolution than laboratory instruments. This limits their application in pharmaceutical development where analytical precision is paramount.
Contemporary GC-MS Methodologies for Drug Discovery
01 GC-MS instrumentation and system design
Gas Chromatography-Mass Spectrometry (GC-MS) systems consist of specialized hardware components designed for efficient separation and detection of compounds. These systems include innovative column designs, ionization sources, detectors, and integrated data processing units. Advanced instrumentation features automated sample handling, temperature control mechanisms, and vacuum systems that enhance analytical precision and throughput. Modern GC-MS equipment incorporates miniaturized components and modular designs to improve portability and application flexibility.- GC-MS instrumentation and apparatus design: Various designs and improvements in GC-MS instrumentation focus on enhancing analytical capabilities. These include specialized sample introduction systems, detector configurations, and integrated hardware solutions that improve sensitivity, resolution, and reliability. Innovations in this area address challenges such as miniaturization, automation, and the development of portable or field-deployable systems for on-site analysis.
- Sample preparation and introduction techniques: Advanced sample preparation methods for GC-MS analysis include extraction, concentration, and derivatization techniques that enhance detection capabilities. These methods focus on improving the efficiency of volatile compound isolation from complex matrices, reducing interference, and increasing sensitivity. Specialized introduction systems such as headspace sampling, solid-phase microextraction, and thermal desorption techniques enable analysis of challenging sample types.
- Data processing and analytical methods: Software solutions and analytical methodologies for GC-MS data interpretation focus on compound identification, quantification, and pattern recognition. These include advanced algorithms for spectral deconvolution, automated peak identification, and statistical analysis tools that enable processing of complex chromatographic data. Machine learning approaches and database integration enhance the accuracy of compound identification and facilitate the analysis of large datasets.
- Application-specific GC-MS methods: Specialized GC-MS methods have been developed for specific applications across various fields including environmental monitoring, food safety, pharmaceutical analysis, and forensic science. These methods involve optimized separation conditions, detection parameters, and analytical workflows tailored to specific compound classes or sample types. Application-specific approaches address challenges such as trace analysis, complex matrix effects, and the need for high-throughput screening.
- Hyphenated and multi-dimensional GC-MS techniques: Advanced analytical approaches combine GC-MS with additional separation or detection techniques to enhance analytical capabilities. These include two-dimensional gas chromatography (GC×GC-MS), GC-MS/MS, and hybrid systems that integrate complementary analytical methods. Such hyphenated techniques provide improved separation of complex mixtures, enhanced structural information, and increased sensitivity for challenging analytes, enabling more comprehensive chemical characterization.
02 Sample preparation techniques for GC-MS analysis
Effective sample preparation is crucial for accurate GC-MS analysis results. Methods include extraction procedures such as solid-phase extraction, liquid-liquid extraction, and headspace sampling that isolate target analytes from complex matrices. Sample derivatization techniques improve the volatility and thermal stability of compounds that are difficult to analyze directly. Concentration and purification steps remove interfering substances and enhance detection sensitivity. These preparation techniques are optimized for specific sample types including environmental samples, biological specimens, and industrial products.Expand Specific Solutions03 GC-MS method development and optimization
Method development for GC-MS analysis involves optimizing various parameters to achieve efficient separation and detection of target compounds. This includes selection of appropriate column types, temperature programming, carrier gas flow rates, and MS detection parameters. Optimization strategies focus on improving resolution between closely eluting compounds, enhancing sensitivity for trace analysis, and reducing analysis time. Advanced methods incorporate multidimensional separation techniques and specialized ionization modes to address complex analytical challenges. Validation protocols ensure method reliability, accuracy, and reproducibility across different sample types.Expand Specific Solutions04 Data processing and interpretation in GC-MS
Data processing in GC-MS analysis involves sophisticated algorithms for peak detection, integration, and compound identification. Software systems perform automated spectral deconvolution to resolve co-eluting compounds and extract clean mass spectra. Compound identification utilizes mass spectral libraries, retention indices, and fragmentation pattern analysis. Advanced chemometric approaches including principal component analysis and multivariate statistical methods help identify patterns and relationships in complex datasets. Machine learning algorithms enhance the accuracy of compound identification and quantification, particularly for complex mixtures with overlapping signals.Expand Specific Solutions05 Specialized GC-MS applications
GC-MS technology has been adapted for specialized applications across various fields. Environmental monitoring applications include analysis of pollutants, pesticides, and volatile organic compounds in air, water, and soil samples. In pharmaceutical and clinical settings, GC-MS enables drug discovery, metabolite identification, and therapeutic drug monitoring. Food safety applications involve detection of contaminants, additives, and flavor compounds. Forensic applications include analysis of controlled substances, fire debris, and biological specimens. Industrial quality control utilizes GC-MS for process monitoring and product authentication, ensuring consistency and compliance with regulations.Expand Specific Solutions
Leading Manufacturers and Research Institutions
The GC-MS organic chemistry market for drug analysis is in a growth phase, with increasing demand driven by pharmaceutical R&D expansion. The global market size is substantial, projected to grow significantly as drug discovery accelerates. Technologically, the field shows moderate maturity with ongoing innovation. Key players represent diverse segments: established analytical instrument manufacturers (Shimadzu, Resonac Holdings), pharmaceutical research entities (ScinoPharm Taiwan), tobacco research institutes (China National Tobacco Corp., Japan Tobacco), and academic institutions (Zhejiang University, California Institute of Technology). The competitive landscape features both specialized analytical instrument providers like Spectra Analysis Instruments and diversified corporations such as 3M and Philips, indicating a market that balances established technologies with emerging applications for novel drug development.
Shimadzu Corp.
Technical Solution: Shimadzu has developed advanced GC-MS systems specifically optimized for pharmaceutical drug discovery and development. Their GCMS-TQ8050 NX triple quadrupole system offers exceptional sensitivity with detection limits in the femtogram range, crucial for identifying trace compounds in complex biological matrices. Shimadzu's Smart Compounds Database technology contains over 13,000 compounds relevant to drug development, enabling rapid identification of unknown substances and metabolites. Their proprietary Smart MRM technology automatically optimizes multiple reaction monitoring parameters, significantly reducing method development time for new drug candidates. Shimadzu has also pioneered the integration of artificial intelligence with their GC-MS systems through the LabSolutions Insight software, which employs machine learning algorithms to improve peak identification accuracy and reduce false positives in complex pharmaceutical samples[1][3]. Their systems feature specialized ionization techniques that preserve molecular integrity while maximizing sensitivity for fragile pharmaceutical compounds.
Strengths: Industry-leading sensitivity allows detection of compounds at ultra-trace levels; comprehensive databases specifically tailored for pharmaceutical applications; automated method development reduces time-to-results. Weaknesses: Higher initial investment cost compared to some competitors; proprietary software ecosystems may require significant training; complex systems may have steeper learning curves for new users.
ScinoPharm Taiwan Ltd.
Technical Solution: ScinoPharm has developed a comprehensive GC-MS analytical platform specifically for active pharmaceutical ingredient (API) characterization and impurity profiling. Their system employs multi-dimensional GC-MS/MS technology that provides enhanced separation capabilities for complex pharmaceutical mixtures, enabling identification of closely eluting impurities that might co-elute in conventional systems. ScinoPharm's proprietary derivatization protocols optimize detection of polar functional groups common in pharmaceutical compounds, improving chromatographic behavior and enhancing mass spectral information. They have implemented specialized headspace sampling techniques for volatile impurity analysis in accordance with ICH Q3C guidelines, achieving detection limits well below regulatory requirements. Their analytical methods incorporate isotopically labeled internal standards for precise quantification of genotoxic impurities at ppm levels, critical for ensuring drug safety. ScinoPharm has developed extensive spectral libraries containing over 5,000 pharmaceutical-related compounds, including starting materials, intermediates, and potential degradation products[5]. Their integrated data processing system automates impurity identification and quantification, streamlining regulatory documentation for new drug applications.
Strengths: Specialized expertise in pharmaceutical impurity analysis; validated methods compliant with regulatory requirements; comprehensive spectral libraries specific to pharmaceutical manufacturing. Weaknesses: Focus primarily on small molecule APIs rather than biologics; services may be less accessible to smaller research organizations; proprietary methods may limit technology transfer to other laboratories.
Breakthrough Patents and Literature in GC-MS Technology
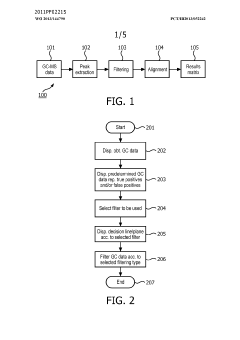
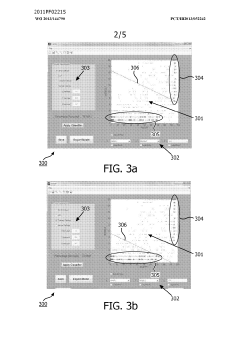
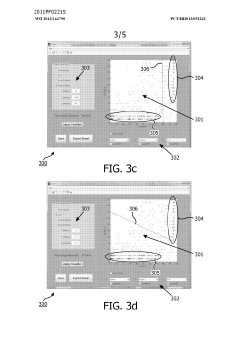
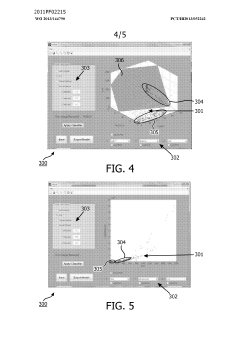
Method and system for filtering gas chromatography-mass spectrometry data
PatentWO2013144790A1
Innovation
- A method and system for filtering GC-MS data that distinguishes between true and false positives, allowing users to visually select filtering methods based on predetermined data structures and decision lines or planes, reducing data noise and improving processing efficiency.
Regulatory Compliance in GC-MS Drug Analysis
Regulatory compliance represents a critical framework governing the application of GC-MS technology in pharmaceutical drug development and analysis. The FDA, EMA, and other global regulatory bodies have established stringent guidelines that manufacturers must adhere to when utilizing GC-MS for drug analysis. These regulations ensure that analytical methods are validated, reproducible, and capable of delivering accurate results that support patient safety and drug efficacy.
The ICH (International Council for Harmonisation) guidelines, particularly Q2(R1) for analytical validation, provide specific parameters that GC-MS methods must satisfy, including accuracy, precision, specificity, detection limit, quantitation limit, linearity, and range. Compliance with these standards requires comprehensive method validation protocols and robust quality control systems that can withstand regulatory scrutiny.
GC-MS laboratories involved in drug development must implement Good Laboratory Practices (GLP) and Good Manufacturing Practices (GMP) to maintain regulatory compliance. This includes detailed documentation of instrument calibration, maintenance records, analyst training, and standard operating procedures (SOPs). The 21 CFR Part 11 regulations in the United States further mandate specific requirements for electronic records and signatures, affecting how GC-MS data is stored, processed, and protected against unauthorized manipulation.
Method transfer represents another significant regulatory challenge, as analytical procedures developed in research settings must be successfully transferred to quality control laboratories. This process requires demonstration of equivalent performance across different instruments, operators, and laboratory environments, necessitating robust validation protocols and statistical analysis of comparative data.
Regulatory bodies increasingly emphasize risk-based approaches to compliance, focusing on critical quality attributes that directly impact patient safety. For GC-MS analysis, this includes particular attention to impurity profiling, residual solvent analysis, and degradation product identification. The ICH Q3 guidelines specifically address impurities in new drug substances and products, establishing thresholds for reporting, identification, and qualification.
Emerging regulations are also addressing the application of GC-MS in specialized areas such as biomarker discovery, metabolomics, and personalized medicine. These evolving frameworks recognize the expanding role of GC-MS beyond traditional quality control applications, creating new compliance challenges as the technology interfaces with clinical diagnostics and therapeutic decision-making processes.
The ICH (International Council for Harmonisation) guidelines, particularly Q2(R1) for analytical validation, provide specific parameters that GC-MS methods must satisfy, including accuracy, precision, specificity, detection limit, quantitation limit, linearity, and range. Compliance with these standards requires comprehensive method validation protocols and robust quality control systems that can withstand regulatory scrutiny.
GC-MS laboratories involved in drug development must implement Good Laboratory Practices (GLP) and Good Manufacturing Practices (GMP) to maintain regulatory compliance. This includes detailed documentation of instrument calibration, maintenance records, analyst training, and standard operating procedures (SOPs). The 21 CFR Part 11 regulations in the United States further mandate specific requirements for electronic records and signatures, affecting how GC-MS data is stored, processed, and protected against unauthorized manipulation.
Method transfer represents another significant regulatory challenge, as analytical procedures developed in research settings must be successfully transferred to quality control laboratories. This process requires demonstration of equivalent performance across different instruments, operators, and laboratory environments, necessitating robust validation protocols and statistical analysis of comparative data.
Regulatory bodies increasingly emphasize risk-based approaches to compliance, focusing on critical quality attributes that directly impact patient safety. For GC-MS analysis, this includes particular attention to impurity profiling, residual solvent analysis, and degradation product identification. The ICH Q3 guidelines specifically address impurities in new drug substances and products, establishing thresholds for reporting, identification, and qualification.
Emerging regulations are also addressing the application of GC-MS in specialized areas such as biomarker discovery, metabolomics, and personalized medicine. These evolving frameworks recognize the expanding role of GC-MS beyond traditional quality control applications, creating new compliance challenges as the technology interfaces with clinical diagnostics and therapeutic decision-making processes.
Data Processing Advancements for Complex Sample Analysis
The evolution of data processing techniques for GC-MS analysis has revolutionized complex sample analysis in drug discovery. Traditional data processing methods often struggled with the massive datasets generated during pharmaceutical compound screening, resulting in lengthy analysis times and potential oversight of critical molecular markers. Recent advancements have introduced sophisticated algorithms capable of processing complex chromatographic and mass spectral data with unprecedented efficiency and accuracy.
Machine learning integration represents a significant breakthrough in GC-MS data processing. Neural networks and deep learning models now enable automated peak identification and quantification, reducing analysis time from days to hours. These systems can recognize patterns in complex biological matrices that would be imperceptible to conventional processing methods, facilitating the identification of novel drug candidates and their metabolites even at trace concentrations.
Deconvolution algorithms have dramatically improved the analysis of co-eluting compounds, a persistent challenge in complex pharmaceutical samples. Modern software can now separate overlapping peaks with minimal user intervention, providing clearer spectral data for compound identification. This capability is particularly valuable when analyzing biological samples containing thousands of compounds with similar chemical properties, as is common in early-stage drug discovery.
Cloud-based processing platforms have transformed collaborative research in pharmaceutical development. These systems allow simultaneous access to GC-MS data by multiple researchers across different geographical locations, enabling real-time analysis and decision-making. The scalable computing resources available through cloud infrastructure accommodate the processing of increasingly large datasets generated by high-throughput screening methods, eliminating computational bottlenecks that previously hindered rapid drug development.
Chemometric approaches have enhanced the extraction of meaningful information from complex GC-MS data. Principal component analysis, partial least squares, and other multivariate statistical methods now enable researchers to identify subtle relationships between chemical structures and biological activities. These techniques facilitate the prediction of drug efficacy and potential side effects based on molecular fingerprints, accelerating the drug candidate selection process.
Database integration has streamlined compound identification through automated comparison with extensive spectral libraries. Modern systems can rapidly match unknown compounds against millions of reference spectra, providing instant identification of known substances and structural insights for novel compounds. This capability significantly reduces the time required for characterizing new drug candidates and their metabolic products in complex biological samples.
Machine learning integration represents a significant breakthrough in GC-MS data processing. Neural networks and deep learning models now enable automated peak identification and quantification, reducing analysis time from days to hours. These systems can recognize patterns in complex biological matrices that would be imperceptible to conventional processing methods, facilitating the identification of novel drug candidates and their metabolites even at trace concentrations.
Deconvolution algorithms have dramatically improved the analysis of co-eluting compounds, a persistent challenge in complex pharmaceutical samples. Modern software can now separate overlapping peaks with minimal user intervention, providing clearer spectral data for compound identification. This capability is particularly valuable when analyzing biological samples containing thousands of compounds with similar chemical properties, as is common in early-stage drug discovery.
Cloud-based processing platforms have transformed collaborative research in pharmaceutical development. These systems allow simultaneous access to GC-MS data by multiple researchers across different geographical locations, enabling real-time analysis and decision-making. The scalable computing resources available through cloud infrastructure accommodate the processing of increasingly large datasets generated by high-throughput screening methods, eliminating computational bottlenecks that previously hindered rapid drug development.
Chemometric approaches have enhanced the extraction of meaningful information from complex GC-MS data. Principal component analysis, partial least squares, and other multivariate statistical methods now enable researchers to identify subtle relationships between chemical structures and biological activities. These techniques facilitate the prediction of drug efficacy and potential side effects based on molecular fingerprints, accelerating the drug candidate selection process.
Database integration has streamlined compound identification through automated comparison with extensive spectral libraries. Modern systems can rapidly match unknown compounds against millions of reference spectra, providing instant identification of known substances and structural insights for novel compounds. This capability significantly reduces the time required for characterizing new drug candidates and their metabolic products in complex biological samples.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!