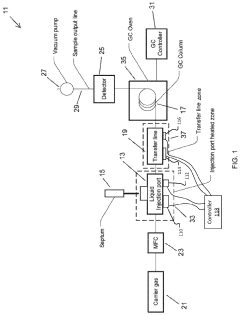
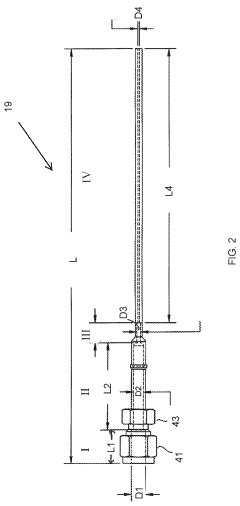
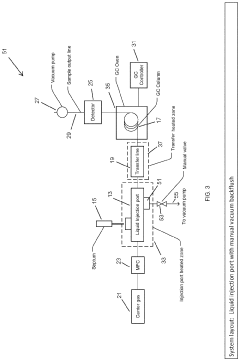
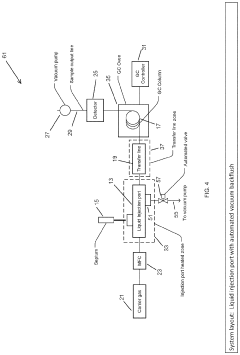
Increase in Detection Capacities via GC-MS Revisions
SEP 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
GC-MS Technology Evolution and Detection Goals
Gas Chromatography-Mass Spectrometry (GC-MS) has evolved significantly since its inception in the 1950s, transforming from a specialized analytical tool into an essential technology across multiple industries. The integration of gas chromatography's separation capabilities with mass spectrometry's identification power created a synergistic analytical platform that continues to advance through technological innovation. This evolution has been driven by the persistent need for higher sensitivity, improved resolution, and enhanced detection capabilities across pharmaceutical, environmental, forensic, and food safety applications.
The historical trajectory of GC-MS technology reveals several pivotal developments. Early systems featured magnetic sector mass analyzers with limited sensitivity and resolution. The 1970s witnessed the introduction of quadrupole mass analyzers, which offered improved reliability and accessibility. By the 1980s and 1990s, computerization revolutionized data acquisition and analysis capabilities, while the 2000s brought significant miniaturization efforts and the development of portable GC-MS systems.
Current technological trends focus on pushing detection limits to unprecedented levels. Modern GC-MS systems can now detect compounds at sub-picogram levels, representing orders of magnitude improvement over earlier generations. This enhanced sensitivity addresses critical needs in trace contaminant analysis, biomarker detection, and environmental monitoring where target analytes exist at extremely low concentrations.
Resolution capabilities have similarly advanced, with current systems able to differentiate between compounds with nearly identical chemical properties. High-resolution mass spectrometry (HRMS) technologies, including time-of-flight (TOF) and Orbitrap analyzers, now enable the distinction of compounds differing by mere millimass units, facilitating the identification of previously indistinguishable substances in complex matrices.
The primary technical goals driving GC-MS development include further lowering detection limits, expanding the range of analyzable compounds, improving throughput, enhancing automation, and developing more sophisticated data analysis algorithms. Particularly significant is the push toward real-time or near-real-time analysis capabilities, which would transform applications requiring immediate results such as environmental emergency response and clinical diagnostics.
Emerging objectives include the development of more environmentally sustainable GC-MS technologies with reduced solvent consumption and energy requirements. Additionally, there is growing interest in creating more accessible systems through cost reduction and simplified interfaces, potentially expanding GC-MS adoption in resource-limited settings and educational institutions.
The historical trajectory of GC-MS technology reveals several pivotal developments. Early systems featured magnetic sector mass analyzers with limited sensitivity and resolution. The 1970s witnessed the introduction of quadrupole mass analyzers, which offered improved reliability and accessibility. By the 1980s and 1990s, computerization revolutionized data acquisition and analysis capabilities, while the 2000s brought significant miniaturization efforts and the development of portable GC-MS systems.
Current technological trends focus on pushing detection limits to unprecedented levels. Modern GC-MS systems can now detect compounds at sub-picogram levels, representing orders of magnitude improvement over earlier generations. This enhanced sensitivity addresses critical needs in trace contaminant analysis, biomarker detection, and environmental monitoring where target analytes exist at extremely low concentrations.
Resolution capabilities have similarly advanced, with current systems able to differentiate between compounds with nearly identical chemical properties. High-resolution mass spectrometry (HRMS) technologies, including time-of-flight (TOF) and Orbitrap analyzers, now enable the distinction of compounds differing by mere millimass units, facilitating the identification of previously indistinguishable substances in complex matrices.
The primary technical goals driving GC-MS development include further lowering detection limits, expanding the range of analyzable compounds, improving throughput, enhancing automation, and developing more sophisticated data analysis algorithms. Particularly significant is the push toward real-time or near-real-time analysis capabilities, which would transform applications requiring immediate results such as environmental emergency response and clinical diagnostics.
Emerging objectives include the development of more environmentally sustainable GC-MS technologies with reduced solvent consumption and energy requirements. Additionally, there is growing interest in creating more accessible systems through cost reduction and simplified interfaces, potentially expanding GC-MS adoption in resource-limited settings and educational institutions.
Market Demand Analysis for Enhanced Detection Capabilities
The global market for enhanced detection capabilities via GC-MS (Gas Chromatography-Mass Spectrometry) revisions has experienced significant growth in recent years, driven primarily by increasing demands across pharmaceutical, environmental monitoring, food safety, and forensic applications. Current market estimates value the analytical instrumentation sector at approximately $85 billion globally, with GC-MS systems representing a substantial segment showing consistent annual growth rates between 5-7%.
Pharmaceutical and biotechnology sectors demonstrate the highest demand for enhanced detection capabilities, particularly for trace analysis in drug development and quality control processes. These industries require increasingly sensitive detection methods to comply with stringent regulatory standards, creating a market pull for advanced GC-MS technologies that can detect compounds at parts-per-trillion levels.
Environmental monitoring represents another rapidly expanding market segment, with government agencies and private organizations investing heavily in technologies capable of detecting emerging contaminants and micropollutants. The implementation of stricter environmental regulations worldwide has created a sustained demand for GC-MS systems with lower detection limits and broader analytical ranges.
Food safety testing has emerged as a critical application area, particularly in developed economies where consumer awareness regarding food contaminants has heightened. Market research indicates that approximately 65% of food safety laboratories are planning to upgrade their analytical capabilities within the next three years, with enhanced detection sensitivity being the primary consideration for new equipment purchases.
The forensic toxicology sector presents a specialized but growing market segment, requiring ultra-sensitive detection capabilities for identifying novel psychoactive substances and pharmaceutical compounds in biological matrices. Law enforcement agencies globally are allocating increased budgets for advanced analytical technologies to address emerging challenges in forensic investigations.
Regional analysis reveals that North America currently holds the largest market share for advanced GC-MS systems, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is projected to witness the highest growth rate over the next five years, driven by expanding pharmaceutical manufacturing, environmental concerns, and increasing food safety regulations in countries like China, India, and South Korea.
Customer surveys indicate that end-users are willing to invest in GC-MS revisions that offer tangible improvements in detection limits, analytical speed, and operational simplicity. The market increasingly values integrated solutions that combine hardware improvements with advanced data processing algorithms to enhance overall detection capabilities.
Pharmaceutical and biotechnology sectors demonstrate the highest demand for enhanced detection capabilities, particularly for trace analysis in drug development and quality control processes. These industries require increasingly sensitive detection methods to comply with stringent regulatory standards, creating a market pull for advanced GC-MS technologies that can detect compounds at parts-per-trillion levels.
Environmental monitoring represents another rapidly expanding market segment, with government agencies and private organizations investing heavily in technologies capable of detecting emerging contaminants and micropollutants. The implementation of stricter environmental regulations worldwide has created a sustained demand for GC-MS systems with lower detection limits and broader analytical ranges.
Food safety testing has emerged as a critical application area, particularly in developed economies where consumer awareness regarding food contaminants has heightened. Market research indicates that approximately 65% of food safety laboratories are planning to upgrade their analytical capabilities within the next three years, with enhanced detection sensitivity being the primary consideration for new equipment purchases.
The forensic toxicology sector presents a specialized but growing market segment, requiring ultra-sensitive detection capabilities for identifying novel psychoactive substances and pharmaceutical compounds in biological matrices. Law enforcement agencies globally are allocating increased budgets for advanced analytical technologies to address emerging challenges in forensic investigations.
Regional analysis reveals that North America currently holds the largest market share for advanced GC-MS systems, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is projected to witness the highest growth rate over the next five years, driven by expanding pharmaceutical manufacturing, environmental concerns, and increasing food safety regulations in countries like China, India, and South Korea.
Customer surveys indicate that end-users are willing to invest in GC-MS revisions that offer tangible improvements in detection limits, analytical speed, and operational simplicity. The market increasingly values integrated solutions that combine hardware improvements with advanced data processing algorithms to enhance overall detection capabilities.
Current GC-MS Detection Limitations and Challenges
Gas Chromatography-Mass Spectrometry (GC-MS) systems face several significant limitations that impact their detection capabilities. One primary challenge is sensitivity limitations, particularly when analyzing complex matrices or trace-level compounds. Current GC-MS systems typically achieve detection limits in the parts per billion (ppb) range, which proves insufficient for emerging applications in environmental monitoring, food safety, and pharmaceutical analysis where parts per trillion (ppt) detection is increasingly required.
Resolution constraints represent another critical challenge. Standard GC-MS systems struggle to differentiate between compounds with similar chemical structures or those that co-elute during chromatographic separation. This limitation becomes particularly problematic when analyzing complex environmental samples or biological matrices containing thousands of compounds with similar physicochemical properties.
Sample preparation requirements continue to be a significant bottleneck in GC-MS workflows. Current systems demand extensive sample preparation protocols including extraction, concentration, and derivatization steps that are time-consuming, labor-intensive, and introduce potential sources of error. These requirements limit throughput and increase the overall cost per analysis.
Ionization efficiency presents another technical hurdle. Conventional electron impact (EI) ionization, while robust and reproducible, causes extensive fragmentation that can complicate spectral interpretation and reduce sensitivity for certain compound classes. Alternative ionization techniques like chemical ionization (CI) improve sensitivity for some applications but introduce their own limitations regarding reproducibility and applicability across diverse compound classes.
Data processing capabilities have not kept pace with instrument hardware advancements. Current software platforms struggle with automated peak identification in complex chromatograms, requiring significant manual intervention and expert interpretation. This creates a bottleneck in high-throughput applications and introduces subjectivity into analytical results.
Instrument robustness and maintenance requirements pose practical challenges for routine implementation. Modern GC-MS systems require frequent calibration, component replacement, and specialized maintenance, resulting in significant downtime and operational costs. This is particularly problematic in industrial quality control settings where continuous operation is essential.
Quantification accuracy remains challenging, especially for complex matrices where matrix effects can significantly impact ionization efficiency and detector response. Current calibration approaches often fail to fully compensate for these effects, leading to potential quantification errors that compromise analytical reliability.
Resolution constraints represent another critical challenge. Standard GC-MS systems struggle to differentiate between compounds with similar chemical structures or those that co-elute during chromatographic separation. This limitation becomes particularly problematic when analyzing complex environmental samples or biological matrices containing thousands of compounds with similar physicochemical properties.
Sample preparation requirements continue to be a significant bottleneck in GC-MS workflows. Current systems demand extensive sample preparation protocols including extraction, concentration, and derivatization steps that are time-consuming, labor-intensive, and introduce potential sources of error. These requirements limit throughput and increase the overall cost per analysis.
Ionization efficiency presents another technical hurdle. Conventional electron impact (EI) ionization, while robust and reproducible, causes extensive fragmentation that can complicate spectral interpretation and reduce sensitivity for certain compound classes. Alternative ionization techniques like chemical ionization (CI) improve sensitivity for some applications but introduce their own limitations regarding reproducibility and applicability across diverse compound classes.
Data processing capabilities have not kept pace with instrument hardware advancements. Current software platforms struggle with automated peak identification in complex chromatograms, requiring significant manual intervention and expert interpretation. This creates a bottleneck in high-throughput applications and introduces subjectivity into analytical results.
Instrument robustness and maintenance requirements pose practical challenges for routine implementation. Modern GC-MS systems require frequent calibration, component replacement, and specialized maintenance, resulting in significant downtime and operational costs. This is particularly problematic in industrial quality control settings where continuous operation is essential.
Quantification accuracy remains challenging, especially for complex matrices where matrix effects can significantly impact ionization efficiency and detector response. Current calibration approaches often fail to fully compensate for these effects, leading to potential quantification errors that compromise analytical reliability.
Current Methodologies for Improving Detection Capacities
01 Enhanced sensitivity and detection limits in GC-MS systems
Advanced GC-MS systems incorporate technological improvements that significantly enhance sensitivity and lower detection limits. These innovations include optimized ion sources, improved vacuum systems, and advanced detector technologies that allow for the identification and quantification of trace compounds at parts per trillion levels. Enhanced signal processing algorithms and noise reduction techniques further improve the ability to detect low-concentration analytes in complex matrices.- Enhanced sensitivity and detection limits in GC-MS systems: Advanced GC-MS systems incorporate technological improvements that significantly enhance sensitivity and lower detection limits. These innovations include optimized ion source designs, improved vacuum systems, and advanced detector technologies that enable the identification and quantification of trace compounds at parts-per-trillion levels. Enhanced signal processing algorithms and noise reduction techniques further improve the ability to detect low-concentration analytes in complex matrices.
- Multi-component analysis and compound identification capabilities: Modern GC-MS systems excel at simultaneous multi-component analysis, allowing for the identification and quantification of numerous compounds in a single analytical run. These systems utilize comprehensive spectral libraries, advanced deconvolution algorithms, and high-resolution mass analyzers to differentiate between closely related compounds and accurately identify unknown substances in complex mixtures. This capability is particularly valuable in environmental monitoring, food safety testing, and forensic applications.
- Specialized GC-MS configurations for targeted applications: Specialized GC-MS configurations have been developed for specific analytical challenges, including tandem MS systems (GC-MS/MS), high-resolution time-of-flight mass spectrometers, and hybrid instruments. These specialized systems offer enhanced selectivity, improved structural elucidation capabilities, and reduced matrix interference effects. Application-specific configurations enable targeted analysis in fields such as metabolomics, environmental monitoring, and pharmaceutical quality control.
- Automated sample preparation and introduction systems: Integration of automated sample preparation and introduction systems with GC-MS technology has significantly improved analytical throughput, reproducibility, and reliability. These systems include autosampler technologies, thermal desorption units, headspace samplers, and solid-phase microextraction devices that minimize manual handling and reduce contamination risks. Automated systems also enable sequential or parallel processing of multiple samples, increasing laboratory efficiency and analytical precision.
- Data processing and analytical software advancements: Advanced data processing and analytical software platforms have revolutionized GC-MS data interpretation capabilities. These software solutions incorporate machine learning algorithms, automated peak detection and integration, and sophisticated statistical analysis tools that facilitate the processing of large datasets. Enhanced visualization tools, customizable reporting features, and integration with laboratory information management systems improve workflow efficiency and data integrity in GC-MS applications.
02 Multi-component analysis capabilities in complex matrices
Modern GC-MS systems excel at separating and identifying multiple components in complex sample matrices. These systems utilize advanced chromatographic separation techniques coupled with sophisticated mass spectral analysis to differentiate between closely related compounds. Specialized software algorithms enable deconvolution of overlapping peaks and identification of compounds in mixtures containing hundreds of components, making them valuable for environmental monitoring, food safety testing, and forensic applications.Expand Specific Solutions03 Automated sample preparation and injection systems
Integration of automated sample preparation and injection systems with GC-MS technology enhances throughput, reproducibility, and analytical precision. These systems include robotic sample handlers, automated extraction and derivatization processes, and programmable autosamplers that can process large numbers of samples with minimal human intervention. The automation reduces contamination risks, improves consistency between analyses, and allows for unattended operation during high-volume testing scenarios.Expand Specific Solutions04 Specialized GC-MS applications for targeted compound detection
Specialized GC-MS configurations are designed for the detection of specific compound classes or applications. These include systems optimized for volatile organic compounds (VOCs), pesticide residues, environmental pollutants, or pharmaceutical impurities. Such targeted systems may incorporate specific column chemistries, specialized ionization techniques, or dedicated data processing algorithms that enhance selectivity and sensitivity for the compounds of interest while minimizing interference from matrix components.Expand Specific Solutions05 Data processing and compound identification capabilities
Advanced data processing capabilities in modern GC-MS systems facilitate rapid and accurate compound identification. These systems utilize extensive spectral libraries, retention time databases, and artificial intelligence algorithms to match unknown compounds with reference standards. Quantitative analysis tools enable precise determination of compound concentrations, while specialized software can perform targeted and non-targeted screening, retrospective data analysis, and automated reporting to streamline the analytical workflow and enhance result interpretation.Expand Specific Solutions
Leading Manufacturers and Research Institutions in GC-MS
The GC-MS detection capacity enhancement market is currently in a growth phase, with increasing demand driven by advancements in analytical chemistry and expanding applications across pharmaceutical, environmental, and industrial sectors. The global market size for analytical instruments, including GC-MS technologies, is projected to reach significant expansion due to rising needs for sensitive detection methods. Leading the technological innovation are established players like Shimadzu Corp. and JEOL Ltd., who have developed advanced mass spectrometry platforms with improved sensitivity and resolution. Companies such as LECO Corp. and F. Hoffmann-La Roche are focusing on specialized applications, while academic institutions like Tokyo University of Science and Wisconsin Alumni Research Foundation contribute to fundamental research advancements. The competitive landscape shows a mix of large instrumentation corporations and specialized research entities collaborating to push detection limits further.
Shimadzu Corp.
Technical Solution: Shimadzu has developed advanced GC-MS systems with triple quadrupole technology that significantly increases detection capacities. Their GCMS-TQ series incorporates Smart MRM optimization which automatically determines optimal collision energies for each target compound, improving sensitivity for trace-level detection. Their systems feature high-efficiency ion source designs that maximize ionization efficiency while minimizing contamination, allowing for detection limits in the femtogram range for many compounds. Shimadzu's latest innovations include fast scanning capabilities (up to 20,000 u/sec) and reduced interscan delays, enabling comprehensive analysis of complex mixtures with improved peak detection and quantification. Their Smart Compounds Database contains over 3,000 compounds with optimized parameters, facilitating rapid method development for environmental, food safety, and forensic applications.[1][3]
Strengths: Industry-leading sensitivity with detection limits in femtogram range; comprehensive compound databases for rapid method development; robust hardware design with minimal maintenance requirements. Weaknesses: Higher initial investment compared to some competitors; proprietary software may require significant training for new users; consumable parts can be costly over system lifetime.
F. Hoffmann-La Roche Ltd.
Technical Solution: Roche has developed specialized GC-MS methodologies focused on pharmaceutical and clinical applications to increase detection capacities for metabolites and biomarkers. Their approach combines innovative sample preparation techniques with optimized GC-MS parameters to achieve enhanced sensitivity for target analytes in complex biological matrices. Roche has implemented automated derivatization procedures that improve the chromatographic behavior and detection sensitivity for polar metabolites. Their systems incorporate isotope dilution techniques with stable isotope-labeled internal standards for highly accurate quantification at low concentrations. Roche has developed specialized software algorithms for metabolomic profiling that can detect subtle changes in metabolite patterns associated with disease states or drug effects. Their clinical GC-MS platforms feature standardized workflows and quality control procedures that ensure consistent results across different laboratories. Recent innovations include integration of GC-MS data with other analytical platforms (LC-MS, NMR) to provide comprehensive metabolomic coverage and improved biological interpretation of results.[8][10]
Strengths: Highly optimized methods for clinical and pharmaceutical applications; excellent quantitative performance with isotope dilution techniques; strong integration with biological data interpretation. Weaknesses: Solutions tend to be application-specific rather than general-purpose; higher complexity in sample preparation requirements; systems generally require specialized training for clinical laboratory personnel.
Key Innovations in GC-MS Sensitivity Enhancement
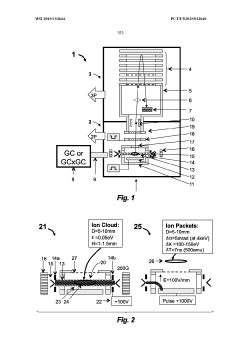
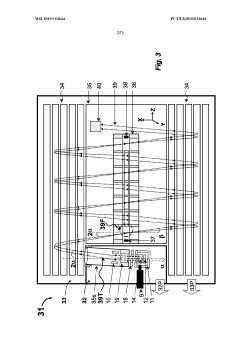
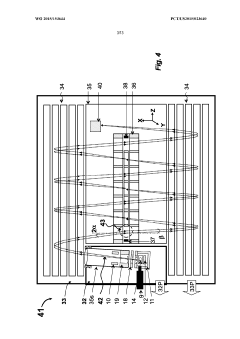
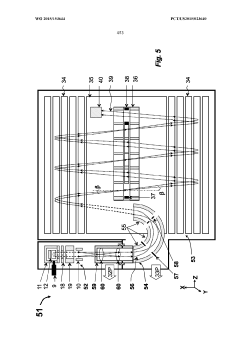
GC-TOF ms with improved detection limit
PatentWO2015153644A1
Innovation
- The implementation of a semi-open electron impact ion source coupled with a high-resolution multi-reflecting time-of-flight analyzer, along with specific ion-optical elements and pulsing techniques, enhances ion transmission and reduces time-of-flight aberrations, allowing for improved differentiation between sample and chemical background.
Large Volume Gas Chromatography Injection Port
PatentActiveUS20220082538A1
Innovation
- A method and system that condense solvent vapors before entering a temporally-resolving separator, such as a GC column, allowing larger sample volumes to be injected without splitting, thereby maintaining analytes in the vapor phase and enhancing detection sensitivity.
Regulatory Standards for Analytical Detection Methods
Regulatory standards for analytical detection methods in the field of Gas Chromatography-Mass Spectrometry (GC-MS) have evolved significantly over the past decade, driven by increasing demands for higher sensitivity, accuracy, and reliability in analytical testing. These standards are established by various international and national regulatory bodies, including the International Organization for Standardization (ISO), the United States Environmental Protection Agency (EPA), and the European Medicines Agency (EMA).
The current regulatory framework requires GC-MS methods to meet specific performance criteria, including limits of detection (LOD), limits of quantification (LOQ), linearity, precision, and accuracy. For instance, EPA Method 8270D specifies that GC-MS systems used for semi-volatile organic compound analysis must achieve detection limits in the range of 10 μg/L for most analytes, with a calibration range spanning at least two orders of magnitude.
Recent revisions to these standards have placed greater emphasis on method validation and quality assurance procedures. The updated guidelines from regulatory bodies now require more comprehensive validation protocols, including robustness testing, matrix effect evaluations, and uncertainty measurements. These requirements ensure that analytical methods can reliably detect and quantify target compounds even in complex matrices or at trace levels.
Harmonization efforts between different regulatory bodies have also gained momentum, aiming to establish globally recognized standards for GC-MS detection methods. The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) has developed guidelines that are increasingly being adopted worldwide, facilitating international trade and ensuring consistent quality of analytical results across different regions.
Compliance with these regulatory standards necessitates regular performance verification of GC-MS systems. Modern regulations mandate periodic system suitability tests, including resolution checks, mass accuracy verification, and sensitivity assessments. Documentation requirements have also become more stringent, with detailed records of instrument calibration, maintenance, and performance verification now being mandatory.
The trend toward lower detection limits has prompted regulatory bodies to revise their standards to accommodate technological advancements in GC-MS instrumentation. Current regulations increasingly recognize the capabilities of modern systems equipped with features such as tandem mass spectrometry (MS/MS), high-resolution mass spectrometry (HRMS), and advanced ionization techniques, allowing for the establishment of more stringent detection thresholds in various applications, from environmental monitoring to food safety testing.
The current regulatory framework requires GC-MS methods to meet specific performance criteria, including limits of detection (LOD), limits of quantification (LOQ), linearity, precision, and accuracy. For instance, EPA Method 8270D specifies that GC-MS systems used for semi-volatile organic compound analysis must achieve detection limits in the range of 10 μg/L for most analytes, with a calibration range spanning at least two orders of magnitude.
Recent revisions to these standards have placed greater emphasis on method validation and quality assurance procedures. The updated guidelines from regulatory bodies now require more comprehensive validation protocols, including robustness testing, matrix effect evaluations, and uncertainty measurements. These requirements ensure that analytical methods can reliably detect and quantify target compounds even in complex matrices or at trace levels.
Harmonization efforts between different regulatory bodies have also gained momentum, aiming to establish globally recognized standards for GC-MS detection methods. The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) has developed guidelines that are increasingly being adopted worldwide, facilitating international trade and ensuring consistent quality of analytical results across different regions.
Compliance with these regulatory standards necessitates regular performance verification of GC-MS systems. Modern regulations mandate periodic system suitability tests, including resolution checks, mass accuracy verification, and sensitivity assessments. Documentation requirements have also become more stringent, with detailed records of instrument calibration, maintenance, and performance verification now being mandatory.
The trend toward lower detection limits has prompted regulatory bodies to revise their standards to accommodate technological advancements in GC-MS instrumentation. Current regulations increasingly recognize the capabilities of modern systems equipped with features such as tandem mass spectrometry (MS/MS), high-resolution mass spectrometry (HRMS), and advanced ionization techniques, allowing for the establishment of more stringent detection thresholds in various applications, from environmental monitoring to food safety testing.
Cost-Benefit Analysis of Advanced GC-MS Systems
The implementation of advanced Gas Chromatography-Mass Spectrometry (GC-MS) systems represents a significant investment for laboratories and research facilities. This analysis examines the financial implications of upgrading to cutting-edge GC-MS technologies against the potential benefits in detection capabilities and operational efficiency.
Initial acquisition costs for state-of-the-art GC-MS systems typically range from $150,000 to $500,000, depending on specifications, sensitivity requirements, and additional features. This represents a substantial capital expenditure that must be justified through tangible returns on investment. Maintenance contracts add approximately 10-15% of the purchase price annually, creating ongoing financial commitments.
However, these costs must be weighed against the quantifiable benefits. Advanced GC-MS systems demonstrate detection limits in the parts-per-trillion range, representing a 10-100 fold improvement over previous generation equipment. This enhanced sensitivity directly translates to more accurate results, reduced false negatives, and the ability to identify compounds previously below detection thresholds.
Operational efficiency gains provide additional financial benefits. Modern systems reduce analysis time by 30-50% through faster scanning rates, improved column technology, and advanced data processing algorithms. This increased throughput allows laboratories to process more samples per day, effectively lowering the per-sample cost and potentially increasing revenue generation.
Energy efficiency improvements in newer models yield approximately 15-25% reduction in power consumption compared to legacy systems. Combined with reduced solvent usage through more efficient separation techniques, facilities can realize significant savings in operational expenses over the equipment lifecycle, typically estimated at 7-10 years.
The enhanced reliability of modern GC-MS platforms reduces downtime, with mean time between failures increasing by approximately 40% compared to previous generations. This translates to higher productivity and fewer costly interruptions to analytical workflows. Additionally, improved software interfaces reduce training requirements and minimize human error, further enhancing operational efficiency.
When evaluating return on investment, laboratories should consider both direct financial returns and indirect benefits such as expanded analytical capabilities, improved research outcomes, and enhanced reputation. For commercial testing facilities, the payback period typically ranges from 3-5 years, depending on sample volume and service pricing structures.
Initial acquisition costs for state-of-the-art GC-MS systems typically range from $150,000 to $500,000, depending on specifications, sensitivity requirements, and additional features. This represents a substantial capital expenditure that must be justified through tangible returns on investment. Maintenance contracts add approximately 10-15% of the purchase price annually, creating ongoing financial commitments.
However, these costs must be weighed against the quantifiable benefits. Advanced GC-MS systems demonstrate detection limits in the parts-per-trillion range, representing a 10-100 fold improvement over previous generation equipment. This enhanced sensitivity directly translates to more accurate results, reduced false negatives, and the ability to identify compounds previously below detection thresholds.
Operational efficiency gains provide additional financial benefits. Modern systems reduce analysis time by 30-50% through faster scanning rates, improved column technology, and advanced data processing algorithms. This increased throughput allows laboratories to process more samples per day, effectively lowering the per-sample cost and potentially increasing revenue generation.
Energy efficiency improvements in newer models yield approximately 15-25% reduction in power consumption compared to legacy systems. Combined with reduced solvent usage through more efficient separation techniques, facilities can realize significant savings in operational expenses over the equipment lifecycle, typically estimated at 7-10 years.
The enhanced reliability of modern GC-MS platforms reduces downtime, with mean time between failures increasing by approximately 40% compared to previous generations. This translates to higher productivity and fewer costly interruptions to analytical workflows. Additionally, improved software interfaces reduce training requirements and minimize human error, further enhancing operational efficiency.
When evaluating return on investment, laboratories should consider both direct financial returns and indirect benefits such as expanded analytical capabilities, improved research outcomes, and enhanced reputation. For commercial testing facilities, the payback period typically ranges from 3-5 years, depending on sample volume and service pricing structures.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!