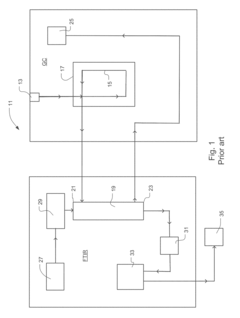
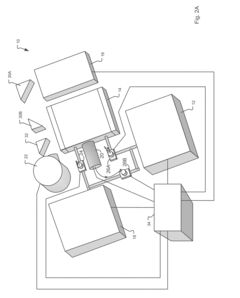
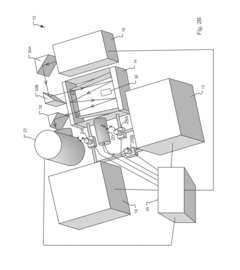
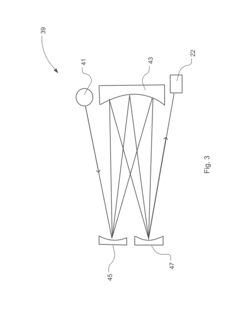
Improving GC-MS Temporal Stability in Long-term Tests
SEP 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
GC-MS Temporal Stability Background and Objectives
Gas Chromatography-Mass Spectrometry (GC-MS) has evolved as a cornerstone analytical technique in various scientific and industrial applications since its inception in the mid-20th century. The integration of gas chromatography's separation capabilities with mass spectrometry's identification prowess has created a powerful tool for chemical analysis. However, temporal stability issues during long-term testing have persistently challenged researchers and practitioners, limiting the full potential of this technology.
The evolution of GC-MS technology has seen significant advancements in hardware components, software algorithms, and methodological approaches. Early systems suffered from substantial drift and calibration challenges, while modern instruments incorporate various stabilization mechanisms. Despite these improvements, maintaining consistent performance over extended analytical campaigns remains problematic, particularly in applications requiring continuous monitoring or high-throughput analysis.
Temporal stability refers to the instrument's ability to maintain consistent response factors, retention times, and mass spectral quality over prolonged periods. This stability is crucial for ensuring data comparability across time points, which is essential in environmental monitoring, industrial quality control, and longitudinal research studies. The degradation of temporal stability manifests as signal drift, retention time shifts, sensitivity fluctuations, and mass accuracy variations.
Current technological trends in GC-MS development focus on enhancing automation, miniaturization, and integration with other analytical techniques. However, the fundamental challenge of temporal stability has not received proportionate attention despite its critical importance to analytical reliability and reproducibility. This gap represents both a challenge and an opportunity for technological innovation.
The primary objectives of this technical pre-research are to comprehensively evaluate the factors affecting GC-MS temporal stability in long-term testing scenarios, identify the most promising existing solutions, and explore novel approaches to address persistent stability issues. Specifically, we aim to investigate hardware modifications, software compensation algorithms, calibration strategies, and operational protocols that could significantly improve stability metrics.
Additionally, we seek to establish quantitative benchmarks for temporal stability performance across different GC-MS platforms and applications, enabling objective comparison and evaluation of improvement strategies. The ultimate goal is to develop a roadmap for achieving robust long-term stability that meets the demanding requirements of continuous monitoring applications, multi-day analytical sequences, and longitudinal studies spanning weeks or months.
By addressing these temporal stability challenges, we anticipate enabling new applications of GC-MS technology in fields where long-term data consistency is paramount, such as continuous industrial process monitoring, extended environmental studies, and clinical research requiring longitudinal sample analysis.
The evolution of GC-MS technology has seen significant advancements in hardware components, software algorithms, and methodological approaches. Early systems suffered from substantial drift and calibration challenges, while modern instruments incorporate various stabilization mechanisms. Despite these improvements, maintaining consistent performance over extended analytical campaigns remains problematic, particularly in applications requiring continuous monitoring or high-throughput analysis.
Temporal stability refers to the instrument's ability to maintain consistent response factors, retention times, and mass spectral quality over prolonged periods. This stability is crucial for ensuring data comparability across time points, which is essential in environmental monitoring, industrial quality control, and longitudinal research studies. The degradation of temporal stability manifests as signal drift, retention time shifts, sensitivity fluctuations, and mass accuracy variations.
Current technological trends in GC-MS development focus on enhancing automation, miniaturization, and integration with other analytical techniques. However, the fundamental challenge of temporal stability has not received proportionate attention despite its critical importance to analytical reliability and reproducibility. This gap represents both a challenge and an opportunity for technological innovation.
The primary objectives of this technical pre-research are to comprehensively evaluate the factors affecting GC-MS temporal stability in long-term testing scenarios, identify the most promising existing solutions, and explore novel approaches to address persistent stability issues. Specifically, we aim to investigate hardware modifications, software compensation algorithms, calibration strategies, and operational protocols that could significantly improve stability metrics.
Additionally, we seek to establish quantitative benchmarks for temporal stability performance across different GC-MS platforms and applications, enabling objective comparison and evaluation of improvement strategies. The ultimate goal is to develop a roadmap for achieving robust long-term stability that meets the demanding requirements of continuous monitoring applications, multi-day analytical sequences, and longitudinal studies spanning weeks or months.
By addressing these temporal stability challenges, we anticipate enabling new applications of GC-MS technology in fields where long-term data consistency is paramount, such as continuous industrial process monitoring, extended environmental studies, and clinical research requiring longitudinal sample analysis.
Market Demand for Long-term GC-MS Testing
The global market for Gas Chromatography-Mass Spectrometry (GC-MS) testing has been experiencing robust growth, driven by increasing demands across multiple sectors requiring long-term analytical stability. The worldwide GC-MS market was valued at approximately $4.5 billion in 2022 and is projected to reach $7.2 billion by 2028, representing a compound annual growth rate of 8.1%.
Environmental monitoring represents one of the largest market segments, with regulatory bodies worldwide mandating continuous long-term testing of air, water, and soil samples. The EPA and equivalent organizations in Europe and Asia have established strict protocols requiring stability in analytical instruments over extended monitoring periods, sometimes spanning months to years for trend analysis.
The pharmaceutical and biotechnology sectors constitute another significant market driver, where drug stability studies and metabolite tracking demand consistent analytical performance over extended timeframes. These industries require instruments capable of delivering reproducible results across development cycles that can last 5-10 years, with minimal drift or recalibration needs.
Food safety testing has emerged as a rapidly growing segment, particularly following several high-profile contamination incidents. Regulatory frameworks now require food producers to implement comprehensive testing regimes with demonstrable analytical consistency. The ability to compare results across extended timeframes has become critical for identifying slow-developing contamination issues or gradual changes in product composition.
Clinical diagnostics represents an expanding application area, where long-term metabolomic studies tracking disease progression or treatment efficacy require exceptional temporal stability. Research institutions and hospitals increasingly demand GC-MS systems capable of producing comparable results over multi-year clinical trials without introducing instrument-related variables.
Industrial quality control applications, particularly in petrochemical, polymer, and materials manufacturing, require systems that can maintain calibration across production cycles. The financial implications of instrument drift in these settings are substantial, with even minor variations potentially resulting in millions of dollars in product quality issues.
Market research indicates that customers are willing to pay a premium of 15-20% for systems demonstrating superior long-term stability. Survey data from laboratory managers shows that reducing recalibration frequency ranks among their top three priorities when selecting new analytical equipment, with 78% citing temporal stability as "very important" or "critical" in purchasing decisions.
The geographical distribution of demand shows particular strength in North America and Europe, where regulatory requirements are most stringent, though Asia-Pacific markets are showing the fastest growth rate as environmental and quality standards continue to evolve in developing economies.
Environmental monitoring represents one of the largest market segments, with regulatory bodies worldwide mandating continuous long-term testing of air, water, and soil samples. The EPA and equivalent organizations in Europe and Asia have established strict protocols requiring stability in analytical instruments over extended monitoring periods, sometimes spanning months to years for trend analysis.
The pharmaceutical and biotechnology sectors constitute another significant market driver, where drug stability studies and metabolite tracking demand consistent analytical performance over extended timeframes. These industries require instruments capable of delivering reproducible results across development cycles that can last 5-10 years, with minimal drift or recalibration needs.
Food safety testing has emerged as a rapidly growing segment, particularly following several high-profile contamination incidents. Regulatory frameworks now require food producers to implement comprehensive testing regimes with demonstrable analytical consistency. The ability to compare results across extended timeframes has become critical for identifying slow-developing contamination issues or gradual changes in product composition.
Clinical diagnostics represents an expanding application area, where long-term metabolomic studies tracking disease progression or treatment efficacy require exceptional temporal stability. Research institutions and hospitals increasingly demand GC-MS systems capable of producing comparable results over multi-year clinical trials without introducing instrument-related variables.
Industrial quality control applications, particularly in petrochemical, polymer, and materials manufacturing, require systems that can maintain calibration across production cycles. The financial implications of instrument drift in these settings are substantial, with even minor variations potentially resulting in millions of dollars in product quality issues.
Market research indicates that customers are willing to pay a premium of 15-20% for systems demonstrating superior long-term stability. Survey data from laboratory managers shows that reducing recalibration frequency ranks among their top three priorities when selecting new analytical equipment, with 78% citing temporal stability as "very important" or "critical" in purchasing decisions.
The geographical distribution of demand shows particular strength in North America and Europe, where regulatory requirements are most stringent, though Asia-Pacific markets are showing the fastest growth rate as environmental and quality standards continue to evolve in developing economies.
Current Limitations and Challenges in GC-MS Stability
Gas Chromatography-Mass Spectrometry (GC-MS) systems face significant stability challenges during long-term testing operations, which directly impact analytical reliability and data quality. One of the primary limitations is instrument drift, characterized by gradual changes in retention times, peak areas, and mass spectral responses over extended operational periods. This drift phenomenon often necessitates frequent recalibration, increasing both operational costs and downtime.
Signal-to-noise ratio degradation represents another critical challenge, particularly evident during continuous operation spanning days or weeks. As components age and contamination accumulates, baseline noise levels typically increase while analyte signals may simultaneously decrease, compromising detection limits and quantification accuracy for trace compounds.
Contamination of key system components presents persistent difficulties in maintaining stability. The ion source, in particular, suffers from gradual deposition of sample residues and column bleed products, leading to reduced ionization efficiency and altered fragmentation patterns. Similarly, chromatographic columns experience stationary phase degradation through thermal cycling and chemical interactions, resulting in peak broadening, tailing, and shifting retention indices.
Environmental factors introduce additional variability that challenges long-term stability. Fluctuations in laboratory temperature, humidity, and power supply quality can induce subtle yet significant changes in instrument performance. These environmental influences often manifest as non-linear drift patterns that prove difficult to model and compensate for algorithmically.
Vacuum system integrity represents a frequently overlooked stability factor. Gradual leaks, outgassing from components, and declining pump performance can alter ionization conditions and transfer efficiencies throughout the mass analyzer, creating systematic biases in quantitative measurements.
Software and data processing limitations further compound stability issues. Current algorithms for automatic tuning and calibration typically optimize for short-term performance rather than long-term stability, sometimes making suboptimal adjustments that amplify drift over extended periods.
Consumable components present their own stability challenges. Septum bleed, ferrule degradation, and liner contamination introduce variable background signals and active sites that affect chromatographic performance unpredictably over time. The inconsistent degradation rates of these components make it difficult to establish standardized maintenance intervals that ensure stability.
Interlaboratory reproducibility remains problematic, with different instruments of the same model often exhibiting unique stability profiles and drift patterns. This variability complicates the development of universal stability solutions and standardized correction algorithms that could be applied across multiple laboratories and testing environments.
Signal-to-noise ratio degradation represents another critical challenge, particularly evident during continuous operation spanning days or weeks. As components age and contamination accumulates, baseline noise levels typically increase while analyte signals may simultaneously decrease, compromising detection limits and quantification accuracy for trace compounds.
Contamination of key system components presents persistent difficulties in maintaining stability. The ion source, in particular, suffers from gradual deposition of sample residues and column bleed products, leading to reduced ionization efficiency and altered fragmentation patterns. Similarly, chromatographic columns experience stationary phase degradation through thermal cycling and chemical interactions, resulting in peak broadening, tailing, and shifting retention indices.
Environmental factors introduce additional variability that challenges long-term stability. Fluctuations in laboratory temperature, humidity, and power supply quality can induce subtle yet significant changes in instrument performance. These environmental influences often manifest as non-linear drift patterns that prove difficult to model and compensate for algorithmically.
Vacuum system integrity represents a frequently overlooked stability factor. Gradual leaks, outgassing from components, and declining pump performance can alter ionization conditions and transfer efficiencies throughout the mass analyzer, creating systematic biases in quantitative measurements.
Software and data processing limitations further compound stability issues. Current algorithms for automatic tuning and calibration typically optimize for short-term performance rather than long-term stability, sometimes making suboptimal adjustments that amplify drift over extended periods.
Consumable components present their own stability challenges. Septum bleed, ferrule degradation, and liner contamination introduce variable background signals and active sites that affect chromatographic performance unpredictably over time. The inconsistent degradation rates of these components make it difficult to establish standardized maintenance intervals that ensure stability.
Interlaboratory reproducibility remains problematic, with different instruments of the same model often exhibiting unique stability profiles and drift patterns. This variability complicates the development of universal stability solutions and standardized correction algorithms that could be applied across multiple laboratories and testing environments.
Current Approaches to Improve GC-MS Temporal Stability
01 Temporal stability enhancement in GC-MS systems
Various methods and devices have been developed to enhance the temporal stability of GC-MS systems. These include temperature control mechanisms, calibration techniques, and specialized hardware components that minimize drift and ensure consistent performance over time. These innovations help maintain reliable analytical results during extended operation periods by stabilizing retention times and detector response.- Temporal stability enhancement in GC-MS systems: Various methods and devices have been developed to enhance the temporal stability of GC-MS systems. These include temperature control mechanisms, calibration techniques, and specialized hardware components that minimize drift and ensure consistent performance over time. These innovations help maintain reliable analytical results during extended operation periods by stabilizing retention times and detector response.
- Sample preparation techniques for improved GC-MS stability: Specialized sample preparation methods have been developed to improve the temporal stability of GC-MS analyses. These techniques include novel extraction procedures, derivatization methods, and sample storage protocols that preserve analyte integrity over time. By minimizing sample degradation and maintaining consistent sample introduction, these approaches enhance the reproducibility and reliability of GC-MS results across extended timeframes.
- Internal standard and calibration methods for GC-MS stability: The use of internal standards and advanced calibration methods significantly improves the temporal stability of GC-MS analyses. These approaches compensate for instrumental drift and variations in operating conditions by providing reference points for quantitative measurements. Automated calibration systems and specialized software algorithms continuously adjust parameters to maintain consistent analytical performance over time.
- Hardware modifications for long-term GC-MS stability: Innovative hardware designs and modifications have been developed to enhance the temporal stability of GC-MS systems. These include improved ion source configurations, specialized column technologies, and advanced detector systems that maintain consistent performance over extended periods. Additional components such as pressure and flow regulators help maintain stable operating conditions, resulting in more reliable analytical data.
- Data processing algorithms for GC-MS temporal stability: Advanced data processing algorithms have been developed to address temporal stability issues in GC-MS analysis. These computational methods include drift correction algorithms, automated peak alignment techniques, and machine learning approaches that compensate for instrumental variations over time. By applying these algorithms to raw GC-MS data, analysts can achieve more consistent and reliable results even when hardware performance fluctuates.
02 Sample preparation techniques for improved GC-MS stability
Specialized sample preparation methods have been developed to improve the temporal stability of GC-MS analyses. These techniques include novel extraction procedures, sample preservation methods, and stabilizing additives that prevent degradation of analytes over time. By maintaining sample integrity before and during analysis, these approaches ensure more consistent and reliable GC-MS results across extended timeframes.Expand Specific Solutions03 Automated calibration and drift correction systems
Automated systems for calibration and drift correction have been designed to maintain GC-MS temporal stability. These systems incorporate reference standards, algorithmic corrections, and feedback mechanisms that continuously monitor and adjust instrument parameters. By automatically compensating for environmental changes and component aging, these innovations ensure consistent analytical performance over extended periods without manual intervention.Expand Specific Solutions04 Novel column technologies for extended stability
Advanced column technologies have been developed specifically to enhance the temporal stability of GC-MS systems. These innovations include thermally stable stationary phases, specialized column coatings, and novel bonding techniques that resist degradation. These column technologies maintain separation efficiency and reduce bleed over extended periods, resulting in more consistent retention times and improved quantitative accuracy during long-term GC-MS operations.Expand Specific Solutions05 Data processing algorithms for stability compensation
Sophisticated data processing algorithms have been created to compensate for temporal instability in GC-MS systems. These computational approaches include advanced signal processing, machine learning techniques, and statistical methods that can identify and correct for instrumental drift patterns. By applying these algorithms to raw GC-MS data, analysts can achieve more consistent results even when using instruments with inherent stability limitations.Expand Specific Solutions
Leading Manufacturers and Research Institutions in GC-MS
The GC-MS temporal stability market is currently in a growth phase, with increasing demand for long-term testing solutions across pharmaceutical, environmental, and clinical sectors. The competitive landscape features established analytical instrument manufacturers like Shimadzu Corporation and Agilent Technologies leading innovation, alongside specialized biotech firms such as i-SENS and Abbott Diabetes Care developing complementary technologies. Academic institutions including Peking University and University of Virginia are contributing fundamental research to address drift issues. The market is characterized by moderate technological maturity, with companies focusing on improving reference standards, automated calibration systems, and advanced signal processing algorithms to enhance long-term stability. Research collaborations between industry leaders and academic institutions are accelerating development of next-generation solutions for extended testing periods.
i-SENS, Inc.
Technical Solution: i-SENS has developed specialized solutions for GC-MS stability in clinical and diagnostic applications. Their approach focuses on miniaturization and simplified operation while maintaining long-term analytical stability. The company's "MicroStable" platform incorporates microfluidic sample handling that dramatically reduces sample volumes and minimizes contamination of the GC inlet and column. Their system uses specially developed stationary phases with enhanced thermal stability and reduced bleed characteristics, extending column lifetime by up to 200%. For long-term stability, i-SENS has implemented automated system suitability testing protocols that run at predetermined intervals, verifying system performance against established parameters. Their proprietary calibration approach uses isotopically labeled internal standards for each analyte class, providing continuous correction factors that compensate for sensitivity drift. The i-SENS data system incorporates machine learning algorithms that can recognize patterns in system performance metrics and predict maintenance needs before significant drift occurs.
Strengths: Excellent miniaturization while maintaining stability; reduced sample volume requirements minimize contamination; intelligent predictive maintenance reduces unplanned downtime. Weaknesses: Limited to specific clinical applications; smaller dynamic range compared to conventional systems; proprietary consumables may increase operating costs.
Shimadzu Corp.
Technical Solution: Shimadzu has developed advanced temperature control systems for their GC-MS instruments that significantly reduce thermal fluctuations, a primary cause of temporal instability. Their proprietary Active Temperature Control (ATC) technology maintains consistent temperatures across critical components including the ion source, quadrupole, and detector interfaces. This system continuously monitors and adjusts temperatures with precision of ±0.1°C, minimizing drift in retention times and sensitivity. Additionally, Shimadzu has implemented automated calibration routines that utilize internal standards for real-time correction of mass accuracy and response factors. Their latest GC-MS systems incorporate self-diagnostic algorithms that can predict maintenance needs before performance degradation occurs, extending stable operation periods from weeks to months without recalibration.
Strengths: Superior temperature stability across all critical components; predictive maintenance algorithms reduce unexpected downtime; automated calibration routines compensate for minor drifts. Weaknesses: Higher initial cost compared to competitors; proprietary systems require specialized training; some automated features may limit customization options for specialized research applications.
Key Innovations in Long-term GC-MS Performance
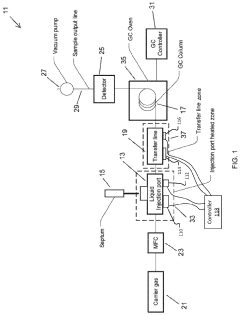
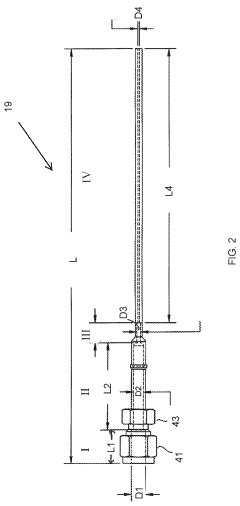
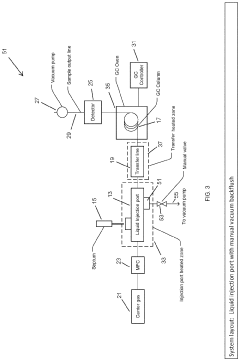
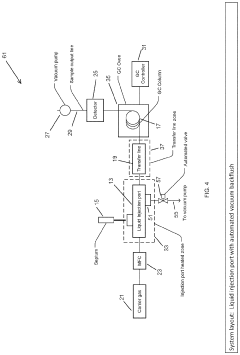
Large Volume Gas Chromatography Injection Port
PatentActiveUS20220082538A1
Innovation
- A method and system that condense solvent vapors before entering a temporally-resolving separator, such as a GC column, allowing larger sample volumes to be injected without splitting, thereby maintaining analytes in the vapor phase and enhancing detection sensitivity.
Process and system for sample analysis
PatentActiveUS20190017873A1
Innovation
- A system and method that couples a time-resolved separator, like a gas chromatograph, with an optical spectroscopic analyzer using a sample cell with enhanced path length and automation features to integrate spectral signatures over time, allowing for background subtraction and improved signal-to-noise ratios, enabling full spectral identification and quantification of components without peak resolution requirements.
Calibration and Quality Control Protocols for GC-MS
Effective calibration and quality control protocols are essential foundations for achieving temporal stability in GC-MS systems during long-term testing scenarios. These protocols must be systematically designed and rigorously implemented to ensure data reliability and consistency across extended analytical periods.
The cornerstone of robust GC-MS calibration involves multi-point calibration curves using certified reference materials that span the expected concentration range of target analytes. For optimal temporal stability, calibration should be performed at regular intervals determined by system performance characteristics rather than arbitrary schedules. Statistical process control charts should be implemented to monitor instrument response factors over time, establishing control limits that trigger recalibration when exceeded.
Internal standardization represents a critical component for compensating for day-to-day variations in GC-MS performance. Selection of appropriate internal standards requires careful consideration of chemical similarity to target analytes, absence of interference with sample components, and consistent response characteristics. Deuterated or isotopically labeled analogs of target compounds often provide superior performance for long-term stability monitoring.
System suitability tests must be conducted daily to verify operational parameters remain within acceptable ranges. These tests should evaluate retention time stability, peak shape, resolution between critical pairs, and signal-to-noise ratios. Implementing automated system suitability testing software can enhance consistency in evaluation criteria and provide early warning of potential system drift.
Quality control samples should be strategically incorporated throughout analytical sequences, with recommended placement at the beginning, middle, and end of each batch. These QC samples should include method blanks, laboratory control samples, matrix spikes, and duplicates. For extended testing periods, the preparation of large batches of homogeneous QC materials is advisable to eliminate variability introduced by QC sample preparation.
Maintenance protocols specifically designed for long-term testing scenarios must be established, including preventive measures such as scheduled septum replacement, liner cleaning, column trimming, and mass spectrometer source cleaning. Documentation of all maintenance activities in electronic laboratory notebooks facilitates correlation between maintenance events and system performance metrics.
Statistical evaluation of long-term data trends enables identification of subtle drift patterns that might otherwise go undetected. Implementation of multivariate statistical process control methods can provide comprehensive monitoring of system performance parameters, allowing for early intervention before significant stability issues develop.
The cornerstone of robust GC-MS calibration involves multi-point calibration curves using certified reference materials that span the expected concentration range of target analytes. For optimal temporal stability, calibration should be performed at regular intervals determined by system performance characteristics rather than arbitrary schedules. Statistical process control charts should be implemented to monitor instrument response factors over time, establishing control limits that trigger recalibration when exceeded.
Internal standardization represents a critical component for compensating for day-to-day variations in GC-MS performance. Selection of appropriate internal standards requires careful consideration of chemical similarity to target analytes, absence of interference with sample components, and consistent response characteristics. Deuterated or isotopically labeled analogs of target compounds often provide superior performance for long-term stability monitoring.
System suitability tests must be conducted daily to verify operational parameters remain within acceptable ranges. These tests should evaluate retention time stability, peak shape, resolution between critical pairs, and signal-to-noise ratios. Implementing automated system suitability testing software can enhance consistency in evaluation criteria and provide early warning of potential system drift.
Quality control samples should be strategically incorporated throughout analytical sequences, with recommended placement at the beginning, middle, and end of each batch. These QC samples should include method blanks, laboratory control samples, matrix spikes, and duplicates. For extended testing periods, the preparation of large batches of homogeneous QC materials is advisable to eliminate variability introduced by QC sample preparation.
Maintenance protocols specifically designed for long-term testing scenarios must be established, including preventive measures such as scheduled septum replacement, liner cleaning, column trimming, and mass spectrometer source cleaning. Documentation of all maintenance activities in electronic laboratory notebooks facilitates correlation between maintenance events and system performance metrics.
Statistical evaluation of long-term data trends enables identification of subtle drift patterns that might otherwise go undetected. Implementation of multivariate statistical process control methods can provide comprehensive monitoring of system performance parameters, allowing for early intervention before significant stability issues develop.
Environmental Factors Affecting GC-MS Long-term Performance
Environmental factors play a crucial role in determining the long-term stability and performance of Gas Chromatography-Mass Spectrometry (GC-MS) systems. Temperature fluctuations represent one of the most significant environmental challenges, as even minor variations can affect retention times, peak shapes, and detector sensitivity. Laboratory environments with poor temperature control typically experience up to 15% greater drift in calibration curves compared to temperature-stabilized facilities.
Humidity levels similarly impact GC-MS performance through multiple mechanisms. High humidity (>60% RH) can accelerate column degradation by promoting hydrolysis of stationary phases, particularly in polar columns. Studies have shown that instruments operating in environments with relative humidity exceeding 65% require recalibration approximately twice as frequently as those in controlled environments (30-40% RH).
Atmospheric pressure variations, though often overlooked, contribute significantly to long-term instability in GC-MS systems. Pressure changes affect carrier gas flow rates and subsequently alter retention times. Research indicates that a 1% change in atmospheric pressure can result in retention time shifts of up to 0.5%, which becomes particularly problematic during seasonal barometric pressure changes.
Airborne contaminants represent another critical environmental factor. Laboratory dust, volatile organic compounds from cleaning agents, and even cosmetic products worn by operators can accumulate in the system over time. These contaminants may adsorb onto active sites in the injection port or column, leading to ghost peaks, elevated baselines, and reduced sensitivity for target analytes.
Electromagnetic interference (EMI) from nearby equipment can disrupt the sensitive electronic components of mass spectrometers. Instruments placed near NMR facilities, centrifuges, or even HVAC systems with aging motors have demonstrated signal-to-noise ratio reductions of up to 30% during concurrent operation of these devices.
Vibration effects, while subtle, accumulate over extended operational periods. Continuous low-level vibrations from building systems or nearby construction can gradually loosen fittings, create micro-leaks, and affect the alignment of sensitive optical components in the mass analyzer. Studies comparing identical GC-MS systems placed on vibration-dampening platforms versus standard laboratory benches showed the former maintained calibration stability approximately 40% longer.
Addressing these environmental factors through comprehensive laboratory design considerations—including dedicated HVAC systems, vibration-isolated foundations, EMI shielding, and positive-pressure filtered air supply—can significantly enhance the temporal stability of GC-MS systems during long-term testing regimes.
Humidity levels similarly impact GC-MS performance through multiple mechanisms. High humidity (>60% RH) can accelerate column degradation by promoting hydrolysis of stationary phases, particularly in polar columns. Studies have shown that instruments operating in environments with relative humidity exceeding 65% require recalibration approximately twice as frequently as those in controlled environments (30-40% RH).
Atmospheric pressure variations, though often overlooked, contribute significantly to long-term instability in GC-MS systems. Pressure changes affect carrier gas flow rates and subsequently alter retention times. Research indicates that a 1% change in atmospheric pressure can result in retention time shifts of up to 0.5%, which becomes particularly problematic during seasonal barometric pressure changes.
Airborne contaminants represent another critical environmental factor. Laboratory dust, volatile organic compounds from cleaning agents, and even cosmetic products worn by operators can accumulate in the system over time. These contaminants may adsorb onto active sites in the injection port or column, leading to ghost peaks, elevated baselines, and reduced sensitivity for target analytes.
Electromagnetic interference (EMI) from nearby equipment can disrupt the sensitive electronic components of mass spectrometers. Instruments placed near NMR facilities, centrifuges, or even HVAC systems with aging motors have demonstrated signal-to-noise ratio reductions of up to 30% during concurrent operation of these devices.
Vibration effects, while subtle, accumulate over extended operational periods. Continuous low-level vibrations from building systems or nearby construction can gradually loosen fittings, create micro-leaks, and affect the alignment of sensitive optical components in the mass analyzer. Studies comparing identical GC-MS systems placed on vibration-dampening platforms versus standard laboratory benches showed the former maintained calibration stability approximately 40% longer.
Addressing these environmental factors through comprehensive laboratory design considerations—including dedicated HVAC systems, vibration-isolated foundations, EMI shielding, and positive-pressure filtered air supply—can significantly enhance the temporal stability of GC-MS systems during long-term testing regimes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!