Developing GC-MS Capability in Natural Compound Protection
SEP 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
GC-MS Technology Background and Objectives
Gas Chromatography-Mass Spectrometry (GC-MS) represents one of the most powerful analytical techniques in modern chemistry, combining the separation capabilities of gas chromatography with the detection specificity of mass spectrometry. The technology emerged in the 1950s when the first successful coupling of these two methods was achieved, revolutionizing analytical chemistry. Over subsequent decades, GC-MS has evolved from bulky, complex laboratory equipment to more compact, automated, and user-friendly systems with enhanced sensitivity and resolution.
The evolution of GC-MS technology has been marked by significant improvements in ionization techniques, mass analyzers, and data processing capabilities. Traditional electron impact (EI) ionization has been supplemented by chemical ionization (CI), negative chemical ionization (NCI), and other soft ionization techniques that provide complementary molecular information. Mass analyzers have progressed from simple quadrupole systems to more sophisticated time-of-flight (TOF), ion trap, and hybrid configurations, dramatically improving resolution and detection limits.
In the context of natural compound protection, GC-MS serves as a critical tool for identification, authentication, and quality control of valuable natural products. The technology enables precise chemical fingerprinting of complex natural matrices, detection of adulterants, and verification of geographical origin—all essential aspects of protecting intellectual property related to natural compounds.
The primary objective of developing GC-MS capability in natural compound protection is to establish robust analytical methodologies that can reliably characterize and authenticate natural products with legal defensibility. This includes creating comprehensive chemical profiles or "fingerprints" that uniquely identify specific natural products, developing databases of reference standards, and implementing statistical models for pattern recognition and classification.
Another key goal is to enhance detection sensitivity and specificity for trace compounds that may serve as distinctive markers for particular natural products. This requires optimization of sample preparation techniques, chromatographic separation parameters, and mass spectrometric detection methods tailored to the complex matrices typical of natural products.
Furthermore, the development aims to integrate GC-MS technology with complementary analytical approaches such as liquid chromatography-mass spectrometry (LC-MS) and nuclear magnetic resonance (NMR) spectroscopy to provide multi-dimensional characterization of natural products. This holistic analytical strategy strengthens the scientific foundation for natural compound protection by offering multiple lines of evidence for product authenticity and uniqueness.
The technological trajectory points toward more automated, high-throughput systems with enhanced data processing capabilities, including artificial intelligence and machine learning algorithms for pattern recognition and compound identification. These advancements will further solidify GC-MS as an indispensable tool in the protection of valuable natural compounds in an increasingly competitive global marketplace.
The evolution of GC-MS technology has been marked by significant improvements in ionization techniques, mass analyzers, and data processing capabilities. Traditional electron impact (EI) ionization has been supplemented by chemical ionization (CI), negative chemical ionization (NCI), and other soft ionization techniques that provide complementary molecular information. Mass analyzers have progressed from simple quadrupole systems to more sophisticated time-of-flight (TOF), ion trap, and hybrid configurations, dramatically improving resolution and detection limits.
In the context of natural compound protection, GC-MS serves as a critical tool for identification, authentication, and quality control of valuable natural products. The technology enables precise chemical fingerprinting of complex natural matrices, detection of adulterants, and verification of geographical origin—all essential aspects of protecting intellectual property related to natural compounds.
The primary objective of developing GC-MS capability in natural compound protection is to establish robust analytical methodologies that can reliably characterize and authenticate natural products with legal defensibility. This includes creating comprehensive chemical profiles or "fingerprints" that uniquely identify specific natural products, developing databases of reference standards, and implementing statistical models for pattern recognition and classification.
Another key goal is to enhance detection sensitivity and specificity for trace compounds that may serve as distinctive markers for particular natural products. This requires optimization of sample preparation techniques, chromatographic separation parameters, and mass spectrometric detection methods tailored to the complex matrices typical of natural products.
Furthermore, the development aims to integrate GC-MS technology with complementary analytical approaches such as liquid chromatography-mass spectrometry (LC-MS) and nuclear magnetic resonance (NMR) spectroscopy to provide multi-dimensional characterization of natural products. This holistic analytical strategy strengthens the scientific foundation for natural compound protection by offering multiple lines of evidence for product authenticity and uniqueness.
The technological trajectory points toward more automated, high-throughput systems with enhanced data processing capabilities, including artificial intelligence and machine learning algorithms for pattern recognition and compound identification. These advancements will further solidify GC-MS as an indispensable tool in the protection of valuable natural compounds in an increasingly competitive global marketplace.
Market Analysis for Natural Compound Protection
The natural compound protection market has experienced significant growth over the past decade, driven primarily by increasing consumer preference for natural ingredients in pharmaceuticals, cosmetics, food products, and agricultural applications. The global market for natural compounds was valued at approximately 5.8 billion USD in 2022 and is projected to reach 9.4 billion USD by 2028, representing a compound annual growth rate of 8.3% during the forecast period.
Consumer awareness regarding the harmful effects of synthetic chemicals has substantially increased demand for natural alternatives. This shift is particularly evident in developed regions such as North America and Europe, where regulatory bodies have implemented stricter guidelines for synthetic compounds. The Asia-Pacific region, especially China and India, is emerging as a significant market due to their rich biodiversity and traditional knowledge of natural compounds.
The pharmaceutical sector constitutes the largest application segment, accounting for roughly 38% of the market share. Natural compounds serve as essential precursors for drug development, with approximately 60% of approved drugs between 1981 and 2022 being derived from or inspired by natural sources. The cosmetic industry follows closely, with natural ingredients becoming standard in premium skincare and personal care products.
Market segmentation reveals that plant-derived compounds dominate with 65% market share, followed by marine-derived (18%), microbial-derived (12%), and other sources (5%). Among these, flavonoids, alkaloids, and terpenoids represent the most commercially valuable compound classes due to their diverse biological activities and applications.
Key market drivers include increasing research funding for natural product discovery, technological advancements in analytical techniques, growing patent expirations of blockbuster drugs prompting pharmaceutical companies to explore natural alternatives, and rising consumer demand for sustainable and eco-friendly products. The COVID-19 pandemic further accelerated interest in natural compounds with immune-boosting properties.
Challenges facing the market include supply chain inconsistencies, standardization difficulties, extraction efficiency limitations, and sustainability concerns. The development of advanced GC-MS capabilities specifically tailored for natural compound analysis represents a critical technological need, as current analytical methods often struggle with the complex matrices and structural diversity inherent in natural extracts.
Regional analysis indicates North America leads the market with 35% share, followed by Europe (28%), Asia-Pacific (25%), and rest of the world (12%). However, the highest growth rates are projected in emerging economies where traditional medicine practices are being integrated with modern scientific approaches.
Consumer awareness regarding the harmful effects of synthetic chemicals has substantially increased demand for natural alternatives. This shift is particularly evident in developed regions such as North America and Europe, where regulatory bodies have implemented stricter guidelines for synthetic compounds. The Asia-Pacific region, especially China and India, is emerging as a significant market due to their rich biodiversity and traditional knowledge of natural compounds.
The pharmaceutical sector constitutes the largest application segment, accounting for roughly 38% of the market share. Natural compounds serve as essential precursors for drug development, with approximately 60% of approved drugs between 1981 and 2022 being derived from or inspired by natural sources. The cosmetic industry follows closely, with natural ingredients becoming standard in premium skincare and personal care products.
Market segmentation reveals that plant-derived compounds dominate with 65% market share, followed by marine-derived (18%), microbial-derived (12%), and other sources (5%). Among these, flavonoids, alkaloids, and terpenoids represent the most commercially valuable compound classes due to their diverse biological activities and applications.
Key market drivers include increasing research funding for natural product discovery, technological advancements in analytical techniques, growing patent expirations of blockbuster drugs prompting pharmaceutical companies to explore natural alternatives, and rising consumer demand for sustainable and eco-friendly products. The COVID-19 pandemic further accelerated interest in natural compounds with immune-boosting properties.
Challenges facing the market include supply chain inconsistencies, standardization difficulties, extraction efficiency limitations, and sustainability concerns. The development of advanced GC-MS capabilities specifically tailored for natural compound analysis represents a critical technological need, as current analytical methods often struggle with the complex matrices and structural diversity inherent in natural extracts.
Regional analysis indicates North America leads the market with 35% share, followed by Europe (28%), Asia-Pacific (25%), and rest of the world (12%). However, the highest growth rates are projected in emerging economies where traditional medicine practices are being integrated with modern scientific approaches.
Current GC-MS Capabilities and Technical Barriers
Gas Chromatography-Mass Spectrometry (GC-MS) technology has evolved significantly over the past decades, establishing itself as a cornerstone analytical method for natural compound identification and protection. Current GC-MS systems offer resolution capabilities down to parts per billion (ppb) levels, enabling the detection of trace compounds in complex natural matrices. Modern instruments typically feature mass resolution of 1,000-10,000 FWHM (Full Width at Half Maximum), with high-end systems reaching up to 50,000 FWHM, allowing for precise compound differentiation.
The sensitivity of contemporary GC-MS systems has reached impressive thresholds, with detection limits as low as 10^-12 to 10^-15 grams for many compounds. This exceptional sensitivity is crucial when analyzing natural products where active compounds may be present in minute quantities. Additionally, current systems can process samples in 15-30 minutes, with advanced fast-GC technologies reducing this time to under 5 minutes for certain applications.
Despite these advancements, significant technical barriers persist in applying GC-MS to natural compound protection. Sample preparation remains a critical challenge, as natural matrices often contain interfering substances that can mask compounds of interest or damage sensitive instrument components. Current extraction and clean-up protocols are often time-consuming and may result in the loss of volatile or unstable compounds.
Thermal degradation presents another substantial barrier, particularly for heat-sensitive natural compounds. The high temperatures (typically 250-350°C) required for GC volatilization can alter molecular structures, leading to misidentification or complete loss of certain bioactive components. This limitation is especially problematic for complex natural products containing diverse chemical classes with varying thermal stabilities.
Derivatization techniques, while helpful for improving volatility of polar compounds, introduce additional complexity and potential for error. The derivatization process itself may alter compound structures or create artifacts that complicate accurate identification and quantification of natural compounds.
Mass spectral library limitations constitute a significant technical barrier. Existing libraries predominantly contain data for synthetic compounds, with natural product representation being comparatively sparse. This gap is particularly pronounced for rare or newly discovered natural compounds, making definitive identification challenging without complementary analytical techniques.
The complexity of natural product mixtures often exceeds the peak capacity of standard GC columns, resulting in co-elution issues that compromise accurate identification. Even with high-resolution systems, the separation of structurally similar natural compounds remains problematic, especially for isomers and stereoisomers that may possess different biological activities despite their chemical similarity.
The sensitivity of contemporary GC-MS systems has reached impressive thresholds, with detection limits as low as 10^-12 to 10^-15 grams for many compounds. This exceptional sensitivity is crucial when analyzing natural products where active compounds may be present in minute quantities. Additionally, current systems can process samples in 15-30 minutes, with advanced fast-GC technologies reducing this time to under 5 minutes for certain applications.
Despite these advancements, significant technical barriers persist in applying GC-MS to natural compound protection. Sample preparation remains a critical challenge, as natural matrices often contain interfering substances that can mask compounds of interest or damage sensitive instrument components. Current extraction and clean-up protocols are often time-consuming and may result in the loss of volatile or unstable compounds.
Thermal degradation presents another substantial barrier, particularly for heat-sensitive natural compounds. The high temperatures (typically 250-350°C) required for GC volatilization can alter molecular structures, leading to misidentification or complete loss of certain bioactive components. This limitation is especially problematic for complex natural products containing diverse chemical classes with varying thermal stabilities.
Derivatization techniques, while helpful for improving volatility of polar compounds, introduce additional complexity and potential for error. The derivatization process itself may alter compound structures or create artifacts that complicate accurate identification and quantification of natural compounds.
Mass spectral library limitations constitute a significant technical barrier. Existing libraries predominantly contain data for synthetic compounds, with natural product representation being comparatively sparse. This gap is particularly pronounced for rare or newly discovered natural compounds, making definitive identification challenging without complementary analytical techniques.
The complexity of natural product mixtures often exceeds the peak capacity of standard GC columns, resulting in co-elution issues that compromise accurate identification. Even with high-resolution systems, the separation of structurally similar natural compounds remains problematic, especially for isomers and stereoisomers that may possess different biological activities despite their chemical similarity.
Existing GC-MS Methodologies for Natural Compounds
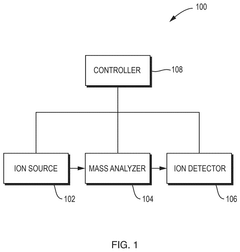
01 GC-MS instrumentation and system design
Gas Chromatography-Mass Spectrometry (GC-MS) systems consist of specialized hardware components designed for efficient separation and detection of compounds. These systems include innovative column designs, ionization sources, detectors, and integrated data processing units. Advanced system designs focus on improving sensitivity, resolution, and throughput while reducing analysis time and sample requirements.- GC-MS instrumentation and apparatus design: Various designs and improvements in GC-MS instrumentation focus on enhancing analytical capabilities through specialized apparatus configurations. These include innovative ion source designs, detector arrangements, and integrated systems that optimize the separation and identification of compounds. Advanced hardware configurations enable better sensitivity, resolution, and reliability in analytical measurements across different applications.
- Sample preparation and introduction methods: Specialized techniques for sample preparation and introduction into GC-MS systems improve analysis efficiency and accuracy. These methods include automated sample handling, novel extraction procedures, and specialized injection techniques that enhance the detection of target compounds. Proper sample preparation is crucial for reducing interference and improving the quality of analytical results in complex matrices.
- Data processing and analytical methods: Advanced data processing algorithms and analytical methodologies enhance the interpretation of GC-MS results. These include specialized software for peak identification, quantification techniques, and statistical analysis methods that improve the accuracy of compound identification. Machine learning and artificial intelligence approaches are increasingly being integrated to handle complex data sets and automate analytical workflows.
- Application-specific GC-MS techniques: Tailored GC-MS methodologies for specific applications across various fields including environmental monitoring, food safety, pharmaceutical analysis, and forensic science. These specialized techniques involve optimized separation parameters, detection methods, and analytical protocols designed for particular compound classes or sample types. Application-specific approaches enable more sensitive and selective analysis in complex matrices.
- Hyphenated and hybrid GC-MS technologies: Integration of GC-MS with complementary analytical techniques creates powerful hybrid systems for comprehensive analysis. These include GC-MS/MS configurations, GC-MS coupled with other separation techniques, and multi-dimensional systems that provide enhanced separation power and structural information. Hyphenated technologies overcome limitations of traditional GC-MS by improving compound identification capabilities and reducing analytical interferences.
02 Sample preparation and injection techniques
Effective sample preparation methods are crucial for GC-MS analysis, including extraction, concentration, and derivatization techniques to enhance volatility and detection of target compounds. Advanced injection systems allow for precise delivery of samples into the GC column, with technologies such as split/splitless injection, programmed temperature vaporization, and cold on-column injection improving analytical performance and reducing sample degradation.Expand Specific Solutions03 Method development and optimization
Development of specialized GC-MS methods involves optimizing separation parameters, temperature programs, carrier gas flow rates, and mass spectrometric detection settings. These methods are tailored for specific applications, ensuring reliable identification and quantification of target compounds in complex matrices. Optimization strategies focus on improving chromatographic resolution, reducing analysis time, and enhancing detection sensitivity.Expand Specific Solutions04 Data analysis and interpretation software
Advanced software solutions for GC-MS data processing enable automated peak detection, spectral deconvolution, compound identification, and quantification. These systems incorporate spectral libraries, statistical analysis tools, and machine learning algorithms to process complex chromatographic and mass spectral data. Modern software platforms improve workflow efficiency, data accuracy, and facilitate the identification of unknown compounds in complex mixtures.Expand Specific Solutions05 Application-specific GC-MS technologies
Specialized GC-MS technologies have been developed for specific applications including environmental monitoring, food safety testing, pharmaceutical analysis, forensic investigations, and metabolomics research. These technologies incorporate modifications to standard GC-MS systems, such as specialized columns, detection methods, and sample introduction techniques tailored to the unique requirements of each application field, enabling more sensitive and selective analysis of target compounds.Expand Specific Solutions
Leading Manufacturers and Research Institutions in GC-MS Field
The GC-MS capability development for natural compound protection is currently in a growth phase, with an estimated market size of $3-4 billion annually and expanding at 5-7% CAGR. The competitive landscape features established analytical instrumentation leaders like Shimadzu, Agilent Technologies, and Revvity Health Sciences dominating with mature technology offerings, alongside specialized players such as Metir and Spectra Analysis Instruments focusing on niche applications. Research institutions including Zhengzhou Tobacco Research Institute, Chinese Academy of Inspection & Quarantine, and Southwest University are advancing application-specific innovations. The technology demonstrates high maturity in standard applications, while specialized natural compound protection applications are still evolving, with companies like NUCTECH and Beijing Uni Star developing region-specific solutions tailored to regulatory frameworks.
Shimadzu Corp.
Technical Solution: Shimadzu Corporation has developed advanced GC-MS systems specifically designed for natural compound protection and analysis. Their GCMS-TQ8050 NX triple quadrupole system offers exceptional sensitivity with detection limits in the femtogram range, crucial for identifying trace compounds in natural products. The system incorporates Smart Productivity features that enable automated method development and optimization, significantly reducing analysis time while maintaining high accuracy. Shimadzu's proprietary Smart MRM technology allows for the simultaneous monitoring of hundreds of natural compounds in a single run, with their database containing over 13,000 compound spectra specifically relevant to natural product research. Their systems feature specialized ion source technology that minimizes contamination and extends maintenance intervals, particularly valuable when working with complex natural matrices. Additionally, Shimadzu has developed specialized sample preparation protocols and column technologies optimized for natural compound extraction and separation, enhancing overall analytical performance.
Strengths: Industry-leading sensitivity allows detection of trace compounds that competitors might miss; extensive compound libraries specifically for natural products; robust design suitable for high-throughput environments. Weaknesses: Higher initial investment cost compared to some competitors; proprietary software systems may require specialized training; consumables can be more expensive than generic alternatives.
Agilent Technologies, Inc.
Technical Solution: Agilent Technologies has pioneered comprehensive GC-MS solutions for natural compound protection with their 8890/7000D Triple Quadrupole GC/MS system. This platform features their proprietary JetClean self-cleaning ion source technology that significantly reduces maintenance downtime when analyzing complex natural matrices. Agilent's MassHunter software incorporates specialized workflows for natural product analysis, including their Natural Products Library containing over 15,000 compounds with retention indices and mass spectral data. Their Retention Time Locking (RTL) technology ensures consistent identification across different instruments and laboratories, critical for establishing defensible protection of natural compounds. The company has developed specialized QuEChERS kits optimized for natural product extraction, coupled with their Ultra Inert flow path components that minimize compound loss or degradation during analysis. Agilent's system offers both targeted and non-targeted screening capabilities, allowing researchers to identify known compounds while simultaneously discovering novel entities that might require protection.
Strengths: Exceptional reproducibility across instruments enables consistent compound identification; comprehensive software ecosystem integrates seamlessly with other analytical techniques; extensive global support network. Weaknesses: Complex software interface can have steep learning curve for new users; premium pricing model may be prohibitive for smaller organizations; some specialized applications require additional software modules at extra cost.
Key Patents and Innovations in GC-MS Technology
Gas chromatography mass spectrometry
PatentInactiveJP1986225644A
Innovation
- Using nitrogen gas as a carrier in GC-MS, directly connecting a fused quartz capillary column to the ionization chamber without a separator, and employing a high volume pump and electron impact ionization at 200-500 eV to enhance ionization efficiency, while maintaining a suitable vacuum using differential pumping.
Carrier gas ion scavenger to reduce peak tailing and reactions
PatentPendingUS20250123250A1
Innovation
- A system and method for GC-MS using a carrier gas other than helium, which includes a scavenger gas with a lower ionization energy than the carrier gas, such as methane or a mixture of methane and ammonia, to reduce peak tailing and reactions by undergoing charge exchange with carrier gas ions in the ionization chamber.
Sustainability and Green Chemistry Considerations
The integration of sustainability principles into GC-MS methodologies represents a critical evolution in natural compound protection technologies. Green Chemistry approaches are increasingly becoming fundamental considerations rather than optional additions to analytical processes. Current GC-MS techniques often rely on environmentally problematic solvents and generate significant waste, creating an urgent need for more sustainable alternatives.
Solvent selection presents a primary opportunity for environmental improvement. Traditional GC-MS methods frequently utilize chlorinated solvents and other toxic chemicals that pose environmental and health risks. The transition toward bio-based solvents derived from renewable resources offers promising alternatives with reduced environmental footprints. Recent innovations include supercritical CO2 extraction methods that minimize organic solvent usage while maintaining analytical precision.
Energy efficiency considerations are equally important in sustainable GC-MS development. Modern instrumentation designs now incorporate power management systems that reduce electricity consumption during standby periods. Additionally, miniaturized GC-MS systems require less energy while maintaining analytical capabilities, representing an important advancement in sustainable laboratory practices.
Sample preparation techniques have evolved to align with green chemistry principles. Microextraction methods significantly reduce solvent volumes while maintaining extraction efficiency. Solid-phase microextraction (SPME) techniques eliminate solvents entirely in many applications, demonstrating how technological innovation can simultaneously improve both analytical performance and environmental sustainability.
Waste reduction strategies have become central to sustainable GC-MS methodologies. Automated sample handling systems minimize reagent consumption and waste generation. Reusable components and consumables extend the lifecycle of laboratory materials, reducing the environmental impact of analytical processes while potentially lowering operational costs.
The circular economy concept is increasingly being applied to analytical chemistry equipment. Manufacturers are developing take-back programs for instruments and implementing modular designs that facilitate repairs and upgrades rather than complete replacements. These approaches extend equipment lifespan and reduce electronic waste associated with laboratory operations.
Regulatory frameworks worldwide are increasingly mandating sustainable practices in analytical chemistry. Organizations developing GC-MS capabilities must consider compliance with evolving environmental regulations that restrict certain solvents and chemicals. Forward-thinking implementation of green chemistry principles not only supports environmental goals but also positions laboratories advantageously for future regulatory requirements.
Solvent selection presents a primary opportunity for environmental improvement. Traditional GC-MS methods frequently utilize chlorinated solvents and other toxic chemicals that pose environmental and health risks. The transition toward bio-based solvents derived from renewable resources offers promising alternatives with reduced environmental footprints. Recent innovations include supercritical CO2 extraction methods that minimize organic solvent usage while maintaining analytical precision.
Energy efficiency considerations are equally important in sustainable GC-MS development. Modern instrumentation designs now incorporate power management systems that reduce electricity consumption during standby periods. Additionally, miniaturized GC-MS systems require less energy while maintaining analytical capabilities, representing an important advancement in sustainable laboratory practices.
Sample preparation techniques have evolved to align with green chemistry principles. Microextraction methods significantly reduce solvent volumes while maintaining extraction efficiency. Solid-phase microextraction (SPME) techniques eliminate solvents entirely in many applications, demonstrating how technological innovation can simultaneously improve both analytical performance and environmental sustainability.
Waste reduction strategies have become central to sustainable GC-MS methodologies. Automated sample handling systems minimize reagent consumption and waste generation. Reusable components and consumables extend the lifecycle of laboratory materials, reducing the environmental impact of analytical processes while potentially lowering operational costs.
The circular economy concept is increasingly being applied to analytical chemistry equipment. Manufacturers are developing take-back programs for instruments and implementing modular designs that facilitate repairs and upgrades rather than complete replacements. These approaches extend equipment lifespan and reduce electronic waste associated with laboratory operations.
Regulatory frameworks worldwide are increasingly mandating sustainable practices in analytical chemistry. Organizations developing GC-MS capabilities must consider compliance with evolving environmental regulations that restrict certain solvents and chemicals. Forward-thinking implementation of green chemistry principles not only supports environmental goals but also positions laboratories advantageously for future regulatory requirements.
Regulatory Framework for Natural Compound Analysis
The regulatory landscape governing natural compound analysis through GC-MS technology presents a complex framework that varies significantly across global jurisdictions. In the United States, the FDA maintains stringent guidelines for analytical methods used in natural product characterization, particularly under 21 CFR Part 11 for electronic records and signatures. These regulations require validated analytical procedures with documented accuracy, precision, specificity, and robustness when GC-MS is employed for compound identification and quantification in natural products.
The European Union operates under Regulation (EC) No 1907/2006 (REACH) and Regulation (EC) No 1223/2009 for cosmetic products, both of which establish specific requirements for natural compound analysis. The European Medicines Agency (EMA) further provides guidelines on quality control methods for herbal medicinal products, where GC-MS serves as a critical analytical tool for compound fingerprinting and standardization.
In Asia, particularly China and Japan, regulatory frameworks emphasize traditional medicine authentication through chemical profiling. China's National Medical Products Administration (NMPA) has established the Chinese Pharmacopoeia Commission standards that increasingly incorporate advanced analytical techniques like GC-MS for quality assessment of natural compounds.
International harmonization efforts are led by organizations such as the International Conference on Harmonisation (ICH), which provides guidelines for analytical procedure validation (Q2(R1)) directly applicable to GC-MS methodologies. The Association of Official Analytical Chemists (AOAC) International also develops standardized methods for natural product analysis that are recognized globally.
Compliance challenges specific to GC-MS implementation include method validation requirements, which typically demand demonstration of linearity, precision, accuracy, specificity, detection limits, and robustness. Documentation requirements are particularly stringent, requiring complete audit trails and data integrity measures that satisfy regulatory scrutiny.
Emerging regulatory trends indicate a shift toward more comprehensive chemical profiling approaches, with authorities increasingly requiring untargeted analysis capabilities alongside targeted compound identification. This evolution reflects growing recognition of the complex nature of natural products and potential adulterants or contaminants that may be present.
For organizations developing GC-MS capabilities for natural compound protection, establishing a regulatory compliance strategy is essential. This includes implementing quality management systems that address method validation, instrument qualification, analyst training, and data integrity requirements across all applicable jurisdictions where the analytical results will be utilized.
The European Union operates under Regulation (EC) No 1907/2006 (REACH) and Regulation (EC) No 1223/2009 for cosmetic products, both of which establish specific requirements for natural compound analysis. The European Medicines Agency (EMA) further provides guidelines on quality control methods for herbal medicinal products, where GC-MS serves as a critical analytical tool for compound fingerprinting and standardization.
In Asia, particularly China and Japan, regulatory frameworks emphasize traditional medicine authentication through chemical profiling. China's National Medical Products Administration (NMPA) has established the Chinese Pharmacopoeia Commission standards that increasingly incorporate advanced analytical techniques like GC-MS for quality assessment of natural compounds.
International harmonization efforts are led by organizations such as the International Conference on Harmonisation (ICH), which provides guidelines for analytical procedure validation (Q2(R1)) directly applicable to GC-MS methodologies. The Association of Official Analytical Chemists (AOAC) International also develops standardized methods for natural product analysis that are recognized globally.
Compliance challenges specific to GC-MS implementation include method validation requirements, which typically demand demonstration of linearity, precision, accuracy, specificity, detection limits, and robustness. Documentation requirements are particularly stringent, requiring complete audit trails and data integrity measures that satisfy regulatory scrutiny.
Emerging regulatory trends indicate a shift toward more comprehensive chemical profiling approaches, with authorities increasingly requiring untargeted analysis capabilities alongside targeted compound identification. This evolution reflects growing recognition of the complex nature of natural products and potential adulterants or contaminants that may be present.
For organizations developing GC-MS capabilities for natural compound protection, establishing a regulatory compliance strategy is essential. This includes implementing quality management systems that address method validation, instrument qualification, analyst training, and data integrity requirements across all applicable jurisdictions where the analytical results will be utilized.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!