GC-MS in Food Safety: Critical Compounds Delivery Rate
SEP 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
GC-MS Technology Evolution in Food Safety Analysis
Gas Chromatography-Mass Spectrometry (GC-MS) has undergone significant evolution in food safety analysis since its inception. The technology emerged in the 1950s when the first commercial GC-MS instruments were developed, primarily for petroleum analysis. By the 1970s, GC-MS began to find applications in food safety, initially focusing on detecting pesticide residues in agricultural products.
The 1980s marked a pivotal shift with the introduction of capillary columns, replacing packed columns and dramatically improving separation efficiency. This advancement enabled food safety analysts to detect contaminants at much lower concentrations, expanding the scope of detectable compounds in complex food matrices.
The 1990s witnessed the integration of computerized data systems and automated sample preparation techniques, significantly enhancing throughput capabilities. This period also saw the development of more sensitive mass analyzers, such as quadrupole and ion trap technologies, which improved detection limits for critical food contaminants.
The early 2000s brought about the miniaturization of GC-MS systems, making the technology more accessible to routine food testing laboratories. Simultaneously, tandem mass spectrometry (GC-MS/MS) emerged as a powerful tool for confirmatory analysis, providing enhanced selectivity and sensitivity for complex food matrices.
Between 2010 and 2015, high-resolution mass spectrometry (HRMS) technologies began to be coupled with GC, enabling non-targeted screening approaches for food contaminant detection. This paradigm shift allowed analysts to identify unknown compounds and emerging contaminants without prior knowledge of their presence.
Recent developments (2015-present) have focused on improving the delivery rate of critical compounds through advanced inlet systems and thermal desorption techniques. These innovations have addressed historical challenges related to thermal degradation of labile compounds during analysis, particularly important for detecting heat-sensitive food contaminants.
The evolution of data processing capabilities has paralleled hardware advancements, with machine learning algorithms now enabling automated identification of compounds and prediction of retention behaviors. Cloud-based reference libraries have expanded exponentially, facilitating rapid identification of food contaminants across global laboratory networks.
Current research frontiers include real-time monitoring systems capable of continuous sampling and analysis, portable GC-MS devices for field testing, and novel ionization techniques that enhance the detection of previously challenging compound classes in food matrices. These developments are collectively addressing the critical need for faster delivery rates of analytical results in food safety monitoring programs.
The 1980s marked a pivotal shift with the introduction of capillary columns, replacing packed columns and dramatically improving separation efficiency. This advancement enabled food safety analysts to detect contaminants at much lower concentrations, expanding the scope of detectable compounds in complex food matrices.
The 1990s witnessed the integration of computerized data systems and automated sample preparation techniques, significantly enhancing throughput capabilities. This period also saw the development of more sensitive mass analyzers, such as quadrupole and ion trap technologies, which improved detection limits for critical food contaminants.
The early 2000s brought about the miniaturization of GC-MS systems, making the technology more accessible to routine food testing laboratories. Simultaneously, tandem mass spectrometry (GC-MS/MS) emerged as a powerful tool for confirmatory analysis, providing enhanced selectivity and sensitivity for complex food matrices.
Between 2010 and 2015, high-resolution mass spectrometry (HRMS) technologies began to be coupled with GC, enabling non-targeted screening approaches for food contaminant detection. This paradigm shift allowed analysts to identify unknown compounds and emerging contaminants without prior knowledge of their presence.
Recent developments (2015-present) have focused on improving the delivery rate of critical compounds through advanced inlet systems and thermal desorption techniques. These innovations have addressed historical challenges related to thermal degradation of labile compounds during analysis, particularly important for detecting heat-sensitive food contaminants.
The evolution of data processing capabilities has paralleled hardware advancements, with machine learning algorithms now enabling automated identification of compounds and prediction of retention behaviors. Cloud-based reference libraries have expanded exponentially, facilitating rapid identification of food contaminants across global laboratory networks.
Current research frontiers include real-time monitoring systems capable of continuous sampling and analysis, portable GC-MS devices for field testing, and novel ionization techniques that enhance the detection of previously challenging compound classes in food matrices. These developments are collectively addressing the critical need for faster delivery rates of analytical results in food safety monitoring programs.
Market Demand for Rapid Food Contaminant Detection
The global market for rapid food contaminant detection technologies has witnessed substantial growth in recent years, driven primarily by increasing consumer awareness about food safety and stringent regulatory frameworks. The demand for Gas Chromatography-Mass Spectrometry (GC-MS) systems specifically designed for food safety applications has expanded at a compound annual growth rate of 7.2% between 2018 and 2022, with projections indicating continued strong growth through 2028.
Food safety incidents across major markets have heightened consumer vigilance and regulatory scrutiny. Notable cases include pesticide contamination in fruits and vegetables, mycotoxin presence in grains, and chemical adulterants in processed foods. These incidents have catalyzed market demand for more sensitive, accurate, and rapid detection technologies, with particular emphasis on systems capable of detecting contaminants at increasingly lower concentrations.
Regulatory bodies worldwide have responded by implementing more stringent food safety standards. The European Food Safety Authority (EFSA) has reduced acceptable limits for several pesticide residues, while the FDA has expanded testing requirements under the Food Safety Modernization Act. These regulatory developments have directly translated into market demand for advanced analytical instruments with improved detection capabilities and faster processing times.
The food export market represents a significant driver for GC-MS technology adoption. Countries exporting to regions with strict regulatory requirements must demonstrate compliance through comprehensive testing. This has created substantial demand in emerging economies seeking to access premium export markets, with particular growth observed in Southeast Asia and Latin America, where investment in food testing infrastructure has increased by over 15% annually.
Commercial food producers and processors constitute the largest market segment, accounting for approximately 45% of GC-MS system purchases for food safety applications. These organizations require high-throughput systems capable of screening large sample volumes efficiently. The contract testing laboratory segment follows at 30%, with government regulatory agencies and research institutions comprising the remainder of the market.
Market research indicates growing demand for integrated systems that combine sample preparation automation with advanced GC-MS analysis. End users increasingly prioritize solutions that minimize manual handling while maximizing analytical throughput. The critical compounds delivery rate has emerged as a key performance metric, with users seeking systems capable of processing multiple samples with consistent results in shorter timeframes.
Consumer willingness to pay premium prices for food products with verified safety credentials has created additional market incentives for producers to invest in comprehensive testing programs. This trend is particularly pronounced in infant foods, organic products, and premium food categories, where perceived safety directly influences consumer purchasing decisions.
Food safety incidents across major markets have heightened consumer vigilance and regulatory scrutiny. Notable cases include pesticide contamination in fruits and vegetables, mycotoxin presence in grains, and chemical adulterants in processed foods. These incidents have catalyzed market demand for more sensitive, accurate, and rapid detection technologies, with particular emphasis on systems capable of detecting contaminants at increasingly lower concentrations.
Regulatory bodies worldwide have responded by implementing more stringent food safety standards. The European Food Safety Authority (EFSA) has reduced acceptable limits for several pesticide residues, while the FDA has expanded testing requirements under the Food Safety Modernization Act. These regulatory developments have directly translated into market demand for advanced analytical instruments with improved detection capabilities and faster processing times.
The food export market represents a significant driver for GC-MS technology adoption. Countries exporting to regions with strict regulatory requirements must demonstrate compliance through comprehensive testing. This has created substantial demand in emerging economies seeking to access premium export markets, with particular growth observed in Southeast Asia and Latin America, where investment in food testing infrastructure has increased by over 15% annually.
Commercial food producers and processors constitute the largest market segment, accounting for approximately 45% of GC-MS system purchases for food safety applications. These organizations require high-throughput systems capable of screening large sample volumes efficiently. The contract testing laboratory segment follows at 30%, with government regulatory agencies and research institutions comprising the remainder of the market.
Market research indicates growing demand for integrated systems that combine sample preparation automation with advanced GC-MS analysis. End users increasingly prioritize solutions that minimize manual handling while maximizing analytical throughput. The critical compounds delivery rate has emerged as a key performance metric, with users seeking systems capable of processing multiple samples with consistent results in shorter timeframes.
Consumer willingness to pay premium prices for food products with verified safety credentials has created additional market incentives for producers to invest in comprehensive testing programs. This trend is particularly pronounced in infant foods, organic products, and premium food categories, where perceived safety directly influences consumer purchasing decisions.
Current Challenges in Compound Delivery Rate Optimization
Despite significant advancements in GC-MS technology for food safety applications, compound delivery rate optimization remains a critical challenge that impacts analytical accuracy, sensitivity, and reliability. Current systems struggle with maintaining consistent delivery rates across diverse compound types, particularly when analyzing complex food matrices containing both volatile and semi-volatile compounds with varying physicochemical properties.
Temperature programming inconsistencies represent a major obstacle, as thermal gradients within the GC system can lead to unpredictable compound volatilization and irregular delivery to the mass spectrometer. This is especially problematic when analyzing thermally labile compounds in food samples, where even minor temperature fluctuations can cause degradation or transformation of target analytes before detection.
Sample introduction mechanisms present another significant challenge, with current autosampler technologies exhibiting variability in injection volumes and speeds. This inconsistency directly impacts quantitative analysis, particularly when detecting contaminants at trace levels where precision is paramount. The industry still lacks standardized approaches for optimizing injection parameters across different food matrices.
Matrix effects continue to interfere with compound delivery rates, as food samples contain numerous components that can interact with target analytes during the separation process. These interactions may cause adsorption, degradation, or competitive binding within the column, resulting in unpredictable elution patterns and compromised delivery rates to the detector.
Carrier gas flow stability issues further complicate delivery rate optimization. Pressure fluctuations, even minor ones, can significantly alter compound migration through the column, affecting retention times and peak shapes. Current pressure regulation systems have not fully resolved these challenges, particularly when analyzing complex food samples requiring extended run times.
Detector response linearity problems emerge when compounds arrive at inconsistent rates, leading to saturation effects or signal suppression. This is particularly problematic when analyzing food samples with compounds present at vastly different concentration levels, from major constituents to trace contaminants.
Interface design limitations between the GC and MS components create additional bottlenecks in compound delivery. Current transfer line technologies may introduce cold spots or adsorption sites that delay or prevent efficient compound transfer, particularly for polar compounds commonly found in food matrices.
Calibration and standardization protocols remain inadequate for addressing these delivery rate challenges across different laboratory settings and instrument configurations, hampering reproducibility efforts in food safety testing programs globally.
Temperature programming inconsistencies represent a major obstacle, as thermal gradients within the GC system can lead to unpredictable compound volatilization and irregular delivery to the mass spectrometer. This is especially problematic when analyzing thermally labile compounds in food samples, where even minor temperature fluctuations can cause degradation or transformation of target analytes before detection.
Sample introduction mechanisms present another significant challenge, with current autosampler technologies exhibiting variability in injection volumes and speeds. This inconsistency directly impacts quantitative analysis, particularly when detecting contaminants at trace levels where precision is paramount. The industry still lacks standardized approaches for optimizing injection parameters across different food matrices.
Matrix effects continue to interfere with compound delivery rates, as food samples contain numerous components that can interact with target analytes during the separation process. These interactions may cause adsorption, degradation, or competitive binding within the column, resulting in unpredictable elution patterns and compromised delivery rates to the detector.
Carrier gas flow stability issues further complicate delivery rate optimization. Pressure fluctuations, even minor ones, can significantly alter compound migration through the column, affecting retention times and peak shapes. Current pressure regulation systems have not fully resolved these challenges, particularly when analyzing complex food samples requiring extended run times.
Detector response linearity problems emerge when compounds arrive at inconsistent rates, leading to saturation effects or signal suppression. This is particularly problematic when analyzing food samples with compounds present at vastly different concentration levels, from major constituents to trace contaminants.
Interface design limitations between the GC and MS components create additional bottlenecks in compound delivery. Current transfer line technologies may introduce cold spots or adsorption sites that delay or prevent efficient compound transfer, particularly for polar compounds commonly found in food matrices.
Calibration and standardization protocols remain inadequate for addressing these delivery rate challenges across different laboratory settings and instrument configurations, hampering reproducibility efforts in food safety testing programs globally.
Current Technical Solutions for Improving Delivery Rates
01 GC-MS flow rate control systems
Systems for controlling and optimizing the flow rate in gas chromatography-mass spectrometry equipment. These systems include mechanisms for precise regulation of carrier gas flow, automated flow controllers, and feedback systems that maintain stable delivery rates during analysis. Such control systems are crucial for achieving consistent and reliable analytical results, especially when analyzing complex samples that require specific flow parameters.- Flow rate control in GC-MS systems: Various methods and devices are used to control the flow rate in gas chromatography-mass spectrometry systems. These include precision flow controllers, electronic flow control modules, and automated systems that maintain consistent delivery rates of carrier gas and sample. Proper flow rate control is essential for achieving reproducible retention times, optimal separation efficiency, and accurate mass spectral data in GC-MS analysis.
- Sample introduction and delivery systems: Specialized sample introduction systems are designed to deliver precise amounts of analytes to GC-MS instruments at controlled rates. These include autosampler technologies, thermal desorption units, headspace samplers, and direct injection systems. These delivery systems ensure consistent sample introduction rates, which is critical for quantitative analysis and method reproducibility in GC-MS applications.
- Carrier gas optimization techniques: Optimization of carrier gas parameters significantly affects GC-MS delivery rates. This includes selection of appropriate carrier gases (hydrogen, helium, nitrogen), pressure/flow programming, and split/splitless injection ratios. Advanced techniques involve electronic pressure control systems and software algorithms that automatically adjust carrier gas delivery rates based on column dimensions, temperature programs, and desired chromatographic performance.
- Interface technologies for improved mass transfer: Specialized interface technologies enhance the delivery rate of analytes from the GC column to the MS detector. These include heated transfer lines, direct column interfaces, and molecular separators. Advanced interfaces minimize band broadening and optimize the transfer efficiency of compounds, ensuring that analytes reach the mass spectrometer at optimal rates for detection and identification.
- Calibration and standardization methods for delivery rate: Various calibration and standardization methods are employed to ensure accurate and consistent delivery rates in GC-MS systems. These include the use of internal standards, calibration compounds with known properties, and reference materials. Regular system calibration, along with quality control procedures, helps maintain consistent delivery rates across different analyses and ensures reliable quantitative results in GC-MS applications.
02 Sample introduction and delivery mechanisms
Specialized mechanisms for introducing and delivering samples to GC-MS systems at controlled rates. These include automated sample injectors, thermal desorption units, and headspace samplers that ensure precise and reproducible sample delivery. The technologies focus on minimizing sample loss, preventing contamination, and maintaining sample integrity throughout the delivery process to enhance analytical accuracy.Expand Specific Solutions03 Microfluidic and miniaturized GC-MS delivery systems
Miniaturized and microfluidic technologies for sample delivery in GC-MS applications. These systems utilize micro-channels, nano-flow controllers, and integrated chip-based solutions to achieve precise delivery rates at extremely low volumes. The miniaturized designs offer advantages in terms of reduced sample consumption, faster analysis times, and potential for portable or field-deployable GC-MS instrumentation.Expand Specific Solutions04 Interface optimization for improved delivery rate
Technological improvements in the interface between gas chromatography and mass spectrometry components to optimize sample delivery rates. These innovations include enhanced transfer line designs, improved ionization source configurations, and specialized interface materials that minimize dead volume and sample loss. The optimized interfaces ensure efficient transfer of analytes from the GC column to the MS detector, maintaining chromatographic resolution while improving sensitivity.Expand Specific Solutions05 Software and algorithms for delivery rate monitoring
Advanced software solutions and algorithms specifically designed for monitoring and adjusting GC-MS delivery rates in real-time. These systems incorporate predictive modeling, machine learning approaches, and automated calibration routines to maintain optimal delivery parameters throughout analytical runs. The software can compensate for environmental variations, column aging, and other factors that might affect delivery rates, ensuring consistent analytical performance over time.Expand Specific Solutions
Leading Manufacturers and Research Institutions in GC-MS
The GC-MS food safety market is in a growth phase, with increasing regulatory demands driving adoption. The global market size is expanding rapidly due to heightened consumer awareness and stringent safety standards. Technologically, the field shows varying maturity levels across applications. Leading players include Agilent Technologies, which dominates with comprehensive analytical solutions, and Shanghai Biotree, specializing in metabolomics platforms. Government institutions like the Chinese Academy of Inspection & Quarantine and China Institute of Metrology are advancing standardization efforts. Pharmaceutical giants AstraZeneca and Bayer contribute significant R&D in compound detection methodologies. The competitive landscape reveals a mix of established instrumentation providers and specialized testing service companies like Shandong Jienuo and Nanjing Mingjie, focusing on rapid compound delivery rate optimization for food contaminant detection.
Chinese Academy of Inspection & Quarantine
Technical Solution: The Chinese Academy of Inspection & Quarantine (CAIQ) has developed comprehensive GC-MS methodologies specifically tailored for food safety applications with emphasis on critical compounds delivery rate optimization. Their approach integrates multi-dimensional GC-MS techniques with specialized sample preparation protocols designed to maximize extraction efficiency while minimizing matrix effects that can impact compound delivery rates. CAIQ has pioneered temperature-programmed desorption techniques coupled with cold-trap focusing systems that significantly enhance volatile compound recovery and transfer efficiency to the analytical column. Their research has established optimized parameters for carrier gas velocity, injection port design, and column dimensions that maximize delivery rates for different classes of food contaminants. CAIQ has also developed specialized internal standard mixtures containing isotopically-labeled compounds that allow precise quantification of delivery rate variations across different food matrices. Additionally, they have created a comprehensive database of matrix-specific correction factors to standardize delivery rates across diverse food types, enabling more accurate risk assessment of contaminant exposure.
Strengths: Extensive experience with diverse Asian food matrices that present unique analytical challenges; strong integration with regulatory frameworks; comprehensive validation protocols for method reliability. Weaknesses: Some technologies may be more focused on regulatory compliance than commercial throughput; international collaboration and technology sharing may be limited by institutional constraints.
China Institute of Metrology
Technical Solution: The China Institute of Metrology has developed sophisticated GC-MS methodologies focused on standardizing critical compounds delivery rate measurements for food safety applications. Their approach centers on metrological traceability in GC-MS analysis, establishing reference materials and calibration standards specifically designed to normalize compound delivery rates across different instrumental platforms. They have pioneered flow-controlled temperature ramping techniques that maintain consistent compound volatilization and transfer rates regardless of matrix complexity. Their research has established standardized protocols for determining method uncertainty in delivery rate measurements, incorporating factors such as matrix effects, instrument variability, and environmental conditions. The Institute has developed specialized headspace sampling techniques with precisely controlled equilibration parameters that optimize volatile compound transfer efficiency from complex food matrices. Additionally, they have created reference methods for determining recovery correction factors specific to different food contaminant classes, enabling accurate comparison of delivery rates across different laboratories and instrumental configurations. Their work includes development of certified reference materials specifically designed for validating delivery rate consistency in routine food safety testing.
Strengths: Exceptional expertise in measurement science and standardization; strong focus on method validation and uncertainty analysis; development of internationally recognized reference materials. Weaknesses: Technologies may prioritize metrological precision over commercial throughput requirements; less focus on user-friendly interfaces and workflow automation compared to commercial vendors.
Key Patents and Innovations in Sample Introduction Systems
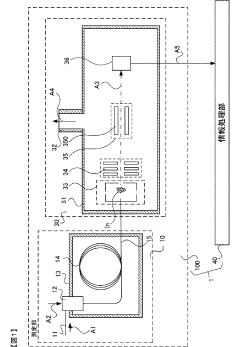
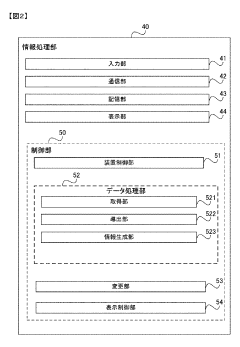
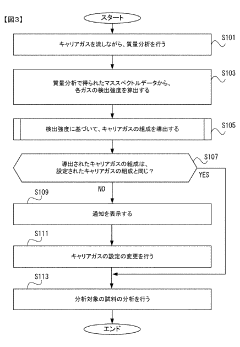
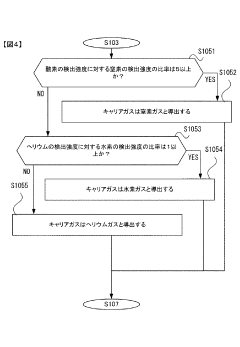
Gas chromatograph mass spectrometer, mass spectrometry method and program
PatentActiveJP2021165653A
Innovation
- A gas chromatograph mass spectrometer system that derives the composition of gases used in separation and mass analysis sections based on the intensity of signals detected in mass spectrometry, allowing for accurate identification of the actual gases employed, and includes a program to automate this process.
Phospholipid containing garlic, curry leaves and turmeric extracts for treatment of adipogenesis
PatentPendingIN202141048482A
Innovation
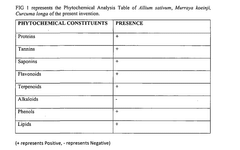
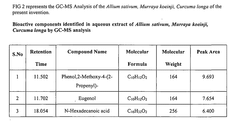
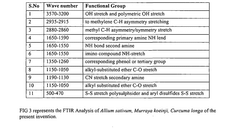
- A synergistic extract derived from Allium sativum, Murraya koenji, and Curcuma longa, combined with phospholipid as a Phytosome complex, is developed for enhanced bioavailability and therapeutic potential, involving a method of extraction, purification, and characterization using GC-MS, FTIR, and SEM, demonstrating the presence of bioactive compounds and antioxidant activity.
Regulatory Standards for Food Safety Testing Methods
Regulatory frameworks governing food safety testing methods have evolved significantly to address the challenges posed by modern analytical techniques such as GC-MS (Gas Chromatography-Mass Spectrometry). The European Union's Regulation (EC) No 396/2005 establishes maximum residue levels (MRLs) for pesticides in food products, with specific requirements for analytical methods including GC-MS. These regulations mandate that testing methods must demonstrate a delivery rate accuracy of ±20% for critical compounds, ensuring reliable detection and quantification of contaminants.
In the United States, the Food and Drug Administration (FDA) has implemented the Food Safety Modernization Act (FSMA), which emphasizes preventive controls and validation of analytical methods. For GC-MS applications, the FDA requires method validation protocols that address compound delivery rates, with particular attention to recovery rates exceeding 80% for priority contaminants. The FDA's Pesticide Analytical Manual (PAM) provides specific guidance on acceptable performance criteria for GC-MS methods used in regulatory testing.
The Codex Alimentarius Commission, established by the FAO and WHO, has developed international standards that influence global regulatory frameworks. Their Guidelines on Good Laboratory Practice in Pesticide Residue Analysis (CAC/GL 40-1993, revised 2010) specify that analytical methods must demonstrate consistent delivery rates for target compounds, with relative standard deviations not exceeding 15% for reproducibility studies.
Japan's Ministry of Health, Labour and Welfare enforces the Food Sanitation Act, which includes the Positive List System for agricultural chemicals. This system requires GC-MS methods to achieve detection limits at least one-tenth of the MRL, with compound delivery rates validated through multi-laboratory studies to ensure consistency across different testing facilities.
China's National Food Safety Standard GB 2763-2021 establishes MRLs for pesticide residues in food and specifies technical requirements for testing methods. For GC-MS applications, the standard mandates validation studies that demonstrate consistent compound delivery rates across different food matrices, with recovery rates between 70-120% for most compounds.
International Organization for Standardization (ISO) has developed ISO/IEC 17025, which provides general requirements for the competence of testing laboratories. This standard requires laboratories to validate methods and establish measurement uncertainty, including factors affecting compound delivery rates in GC-MS analysis. Accreditation bodies worldwide use this standard to assess laboratory competence in food safety testing.
In the United States, the Food and Drug Administration (FDA) has implemented the Food Safety Modernization Act (FSMA), which emphasizes preventive controls and validation of analytical methods. For GC-MS applications, the FDA requires method validation protocols that address compound delivery rates, with particular attention to recovery rates exceeding 80% for priority contaminants. The FDA's Pesticide Analytical Manual (PAM) provides specific guidance on acceptable performance criteria for GC-MS methods used in regulatory testing.
The Codex Alimentarius Commission, established by the FAO and WHO, has developed international standards that influence global regulatory frameworks. Their Guidelines on Good Laboratory Practice in Pesticide Residue Analysis (CAC/GL 40-1993, revised 2010) specify that analytical methods must demonstrate consistent delivery rates for target compounds, with relative standard deviations not exceeding 15% for reproducibility studies.
Japan's Ministry of Health, Labour and Welfare enforces the Food Sanitation Act, which includes the Positive List System for agricultural chemicals. This system requires GC-MS methods to achieve detection limits at least one-tenth of the MRL, with compound delivery rates validated through multi-laboratory studies to ensure consistency across different testing facilities.
China's National Food Safety Standard GB 2763-2021 establishes MRLs for pesticide residues in food and specifies technical requirements for testing methods. For GC-MS applications, the standard mandates validation studies that demonstrate consistent compound delivery rates across different food matrices, with recovery rates between 70-120% for most compounds.
International Organization for Standardization (ISO) has developed ISO/IEC 17025, which provides general requirements for the competence of testing laboratories. This standard requires laboratories to validate methods and establish measurement uncertainty, including factors affecting compound delivery rates in GC-MS analysis. Accreditation bodies worldwide use this standard to assess laboratory competence in food safety testing.
Cost-Benefit Analysis of Advanced GC-MS Implementation
Implementing advanced GC-MS systems for food safety testing represents a significant investment that requires thorough financial analysis. Initial capital expenditure for state-of-the-art GC-MS equipment typically ranges from $150,000 to $500,000, depending on specifications, automation capabilities, and sample throughput capacity. However, this substantial upfront cost must be evaluated against long-term operational benefits and risk mitigation value.
Operational cost reductions present a compelling case for implementation. Advanced GC-MS systems demonstrate 30-40% higher sample throughput compared to conventional systems, potentially reducing per-sample analysis costs by 25-35%. Maintenance requirements are typically reduced by 20%, with newer systems offering extended service intervals and improved component durability. Additionally, modern systems achieve 15-25% lower solvent consumption and 10-20% reduced energy usage, contributing to ongoing operational savings.
Risk mitigation represents perhaps the most significant economic benefit. Food safety incidents can cost companies millions in direct recall expenses, with the average cost of a significant food recall estimated at $10-30 million. Beyond direct costs, brand damage and market share loss often exceed immediate financial impacts by 2-5 times. Advanced GC-MS systems with improved detection limits (often 10-100 times more sensitive than previous generations) and broader compound coverage significantly reduce these risks.
Return on investment calculations indicate that most facilities achieve full ROI within 3-5 years through combined operational savings and risk reduction. Facilities processing high volumes of samples or dealing with high-value products may see ROI periods shortened to 2-3 years. The economic case strengthens further when considering regulatory compliance benefits, as advanced systems typically meet emerging regulatory requirements without additional upgrades.
Scalability considerations also favor advanced implementations. Modern GC-MS platforms offer modular expansion capabilities, allowing incremental capacity increases without complete system replacement. This approach transforms the investment model from periodic large capital expenditures to more manageable staged investments aligned with business growth, improving long-term financial planning.
Operational cost reductions present a compelling case for implementation. Advanced GC-MS systems demonstrate 30-40% higher sample throughput compared to conventional systems, potentially reducing per-sample analysis costs by 25-35%. Maintenance requirements are typically reduced by 20%, with newer systems offering extended service intervals and improved component durability. Additionally, modern systems achieve 15-25% lower solvent consumption and 10-20% reduced energy usage, contributing to ongoing operational savings.
Risk mitigation represents perhaps the most significant economic benefit. Food safety incidents can cost companies millions in direct recall expenses, with the average cost of a significant food recall estimated at $10-30 million. Beyond direct costs, brand damage and market share loss often exceed immediate financial impacts by 2-5 times. Advanced GC-MS systems with improved detection limits (often 10-100 times more sensitive than previous generations) and broader compound coverage significantly reduce these risks.
Return on investment calculations indicate that most facilities achieve full ROI within 3-5 years through combined operational savings and risk reduction. Facilities processing high volumes of samples or dealing with high-value products may see ROI periods shortened to 2-3 years. The economic case strengthens further when considering regulatory compliance benefits, as advanced systems typically meet emerging regulatory requirements without additional upgrades.
Scalability considerations also favor advanced implementations. Modern GC-MS platforms offer modular expansion capabilities, allowing incremental capacity increases without complete system replacement. This approach transforms the investment model from periodic large capital expenditures to more manageable staged investments aligned with business growth, improving long-term financial planning.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!