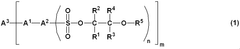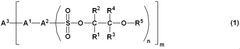Developing Electrochromic Devices with Sulphanilic Acid
JUL 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrochromic Tech Evolution and Objectives
Electrochromic devices have undergone significant evolution since their inception in the 1960s. Initially developed as a curiosity in research laboratories, these devices have now become a promising technology for various applications, including smart windows, displays, and energy-efficient buildings. The journey of electrochromic technology has been marked by continuous improvements in materials, device structures, and manufacturing processes.
The early stages of electrochromic research focused primarily on inorganic materials, such as tungsten oxide (WO3), which exhibited color changes when subjected to an electric field. However, these materials often suffered from slow switching speeds and limited color range. As the field progressed, researchers began exploring organic and polymer-based electrochromic materials, which offered advantages in terms of flexibility, cost-effectiveness, and a broader spectrum of colors.
The introduction of sulphanilic acid as a potential component in electrochromic devices represents a new frontier in this technological evolution. Sulphanilic acid, an aromatic compound with unique electrochemical properties, has shown promise in enhancing the performance of electrochromic devices. Its incorporation aims to address some of the persistent challenges in the field, such as improving switching speed, enhancing color contrast, and increasing the overall efficiency of the devices.
The primary objectives in developing electrochromic devices with sulphanilic acid are multifaceted. Firstly, researchers aim to leverage the compound's electrochemical properties to achieve faster color-switching times, a critical factor for many applications. Secondly, there is a focus on expanding the color palette available in electrochromic devices, potentially offering a wider range of aesthetic options for end-users.
Another key objective is to improve the durability and longevity of electrochromic devices. By incorporating sulphanilic acid, scientists hope to create more stable electrochromic layers that can withstand repeated cycling without significant degradation. This would address one of the major limitations of current electrochromic technologies, particularly in applications requiring frequent color changes or long-term deployment.
Energy efficiency is also a central goal in this research. The development of electrochromic devices with sulphanilic acid aims to reduce the power consumption required for color switching and maintenance, making these devices more attractive for large-scale applications in smart buildings and automotive industries.
As the research progresses, there is also an emphasis on scalability and cost-effectiveness. The ultimate aim is to develop manufacturing processes that can produce these advanced electrochromic devices at a scale and cost that makes them commercially viable across various sectors.
The early stages of electrochromic research focused primarily on inorganic materials, such as tungsten oxide (WO3), which exhibited color changes when subjected to an electric field. However, these materials often suffered from slow switching speeds and limited color range. As the field progressed, researchers began exploring organic and polymer-based electrochromic materials, which offered advantages in terms of flexibility, cost-effectiveness, and a broader spectrum of colors.
The introduction of sulphanilic acid as a potential component in electrochromic devices represents a new frontier in this technological evolution. Sulphanilic acid, an aromatic compound with unique electrochemical properties, has shown promise in enhancing the performance of electrochromic devices. Its incorporation aims to address some of the persistent challenges in the field, such as improving switching speed, enhancing color contrast, and increasing the overall efficiency of the devices.
The primary objectives in developing electrochromic devices with sulphanilic acid are multifaceted. Firstly, researchers aim to leverage the compound's electrochemical properties to achieve faster color-switching times, a critical factor for many applications. Secondly, there is a focus on expanding the color palette available in electrochromic devices, potentially offering a wider range of aesthetic options for end-users.
Another key objective is to improve the durability and longevity of electrochromic devices. By incorporating sulphanilic acid, scientists hope to create more stable electrochromic layers that can withstand repeated cycling without significant degradation. This would address one of the major limitations of current electrochromic technologies, particularly in applications requiring frequent color changes or long-term deployment.
Energy efficiency is also a central goal in this research. The development of electrochromic devices with sulphanilic acid aims to reduce the power consumption required for color switching and maintenance, making these devices more attractive for large-scale applications in smart buildings and automotive industries.
As the research progresses, there is also an emphasis on scalability and cost-effectiveness. The ultimate aim is to develop manufacturing processes that can produce these advanced electrochromic devices at a scale and cost that makes them commercially viable across various sectors.
Market Demand for Electrochromic Devices
The market demand for electrochromic devices has been steadily growing in recent years, driven by the increasing need for energy-efficient solutions in various industries. These smart devices, which can change their optical properties in response to electrical stimuli, have found applications in multiple sectors, including architecture, automotive, aerospace, and consumer electronics.
In the construction industry, electrochromic windows have gained significant traction due to their ability to regulate heat and light transmission, thereby reducing energy consumption in buildings. The global smart glass market, which includes electrochromic devices, is expected to experience substantial growth in the coming years. This growth is primarily attributed to the rising demand for green buildings and the implementation of stringent energy efficiency regulations worldwide.
The automotive sector has also shown a keen interest in electrochromic technology, particularly for smart mirrors and sunroofs. Major automobile manufacturers are incorporating these devices into their high-end models to enhance user comfort and vehicle aesthetics. As the automotive industry continues to evolve towards more connected and autonomous vehicles, the demand for advanced electrochromic solutions is likely to increase further.
In the aerospace industry, electrochromic devices are being explored for use in aircraft windows to improve passenger comfort and reduce cabin temperature fluctuations. This application has the potential to significantly reduce the overall weight of aircraft, leading to improved fuel efficiency and reduced operating costs for airlines.
The consumer electronics market has also begun to adopt electrochromic technology, particularly in wearable devices and smart displays. The ability to dynamically control the transparency and color of screens offers new possibilities for user interface design and energy management in portable devices.
Despite the growing demand, there are still challenges that need to be addressed to fully realize the potential of electrochromic devices. These include improving durability, reducing production costs, and enhancing switching speeds. The development of new materials, such as sulphanilic acid-based compounds, could potentially overcome some of these limitations and further drive market growth.
As environmental concerns continue to shape consumer preferences and government policies, the demand for energy-efficient technologies like electrochromic devices is expected to rise. This trend is likely to create new opportunities for innovation and market expansion in the coming years, making electrochromic technology an increasingly important area for research and development.
In the construction industry, electrochromic windows have gained significant traction due to their ability to regulate heat and light transmission, thereby reducing energy consumption in buildings. The global smart glass market, which includes electrochromic devices, is expected to experience substantial growth in the coming years. This growth is primarily attributed to the rising demand for green buildings and the implementation of stringent energy efficiency regulations worldwide.
The automotive sector has also shown a keen interest in electrochromic technology, particularly for smart mirrors and sunroofs. Major automobile manufacturers are incorporating these devices into their high-end models to enhance user comfort and vehicle aesthetics. As the automotive industry continues to evolve towards more connected and autonomous vehicles, the demand for advanced electrochromic solutions is likely to increase further.
In the aerospace industry, electrochromic devices are being explored for use in aircraft windows to improve passenger comfort and reduce cabin temperature fluctuations. This application has the potential to significantly reduce the overall weight of aircraft, leading to improved fuel efficiency and reduced operating costs for airlines.
The consumer electronics market has also begun to adopt electrochromic technology, particularly in wearable devices and smart displays. The ability to dynamically control the transparency and color of screens offers new possibilities for user interface design and energy management in portable devices.
Despite the growing demand, there are still challenges that need to be addressed to fully realize the potential of electrochromic devices. These include improving durability, reducing production costs, and enhancing switching speeds. The development of new materials, such as sulphanilic acid-based compounds, could potentially overcome some of these limitations and further drive market growth.
As environmental concerns continue to shape consumer preferences and government policies, the demand for energy-efficient technologies like electrochromic devices is expected to rise. This trend is likely to create new opportunities for innovation and market expansion in the coming years, making electrochromic technology an increasingly important area for research and development.
Sulphanilic Acid Integration Challenges
The integration of sulphanilic acid into electrochromic devices presents several significant challenges that researchers and engineers must overcome. One of the primary obstacles is the solubility of sulphanilic acid in common solvents used in electrochromic device fabrication. The acid's limited solubility can lead to difficulties in achieving uniform dispersion within the electrochromic layer, potentially resulting in inconsistent optical performance across the device.
Another critical challenge lies in the chemical stability of sulphanilic acid when incorporated into the electrochromic matrix. The acidic nature of the compound may lead to undesired reactions with other components of the device, such as the electrolyte or electrode materials. This can potentially compromise the long-term durability and performance of the electrochromic device, necessitating careful material selection and interface engineering.
The electronic properties of sulphanilic acid also pose integration challenges. While the compound exhibits promising electrochromic behavior, optimizing its electron transfer characteristics within the device structure is crucial. Researchers must develop strategies to enhance the conductivity and charge transport properties of sulphanilic acid-based layers to achieve rapid and efficient color-switching capabilities.
Furthermore, the pH sensitivity of sulphanilic acid introduces complexities in maintaining consistent electrochromic performance across various operating conditions. Fluctuations in local pH within the device can affect the protonation state of sulphanilic acid, potentially altering its optical and electrochemical properties. This necessitates the development of robust buffering systems or encapsulation techniques to stabilize the acid's environment.
The integration of sulphanilic acid also raises concerns regarding the overall device architecture. Traditional electrochromic device designs may require modification to accommodate the unique properties of sulphanilic acid. This could involve redesigning electrode structures, adjusting layer thicknesses, or incorporating additional functional layers to optimize device performance and stability.
Scalability and manufacturing challenges must also be addressed when integrating sulphanilic acid into electrochromic devices. The acid's sensitivity to environmental factors, such as moisture and temperature, may necessitate stringent control measures during the production process. Developing cost-effective and reliable manufacturing techniques that maintain the integrity of sulphanilic acid-based components is crucial for commercial viability.
Lastly, the potential environmental and health impacts of sulphanilic acid must be carefully evaluated. While the compound offers promising electrochromic properties, its use in large-scale device production requires thorough assessment of any potential risks associated with handling, disposal, and long-term exposure. Developing environmentally friendly synthesis routes and exploring safer alternatives or derivatives of sulphanilic acid may be necessary to address these concerns.
Another critical challenge lies in the chemical stability of sulphanilic acid when incorporated into the electrochromic matrix. The acidic nature of the compound may lead to undesired reactions with other components of the device, such as the electrolyte or electrode materials. This can potentially compromise the long-term durability and performance of the electrochromic device, necessitating careful material selection and interface engineering.
The electronic properties of sulphanilic acid also pose integration challenges. While the compound exhibits promising electrochromic behavior, optimizing its electron transfer characteristics within the device structure is crucial. Researchers must develop strategies to enhance the conductivity and charge transport properties of sulphanilic acid-based layers to achieve rapid and efficient color-switching capabilities.
Furthermore, the pH sensitivity of sulphanilic acid introduces complexities in maintaining consistent electrochromic performance across various operating conditions. Fluctuations in local pH within the device can affect the protonation state of sulphanilic acid, potentially altering its optical and electrochemical properties. This necessitates the development of robust buffering systems or encapsulation techniques to stabilize the acid's environment.
The integration of sulphanilic acid also raises concerns regarding the overall device architecture. Traditional electrochromic device designs may require modification to accommodate the unique properties of sulphanilic acid. This could involve redesigning electrode structures, adjusting layer thicknesses, or incorporating additional functional layers to optimize device performance and stability.
Scalability and manufacturing challenges must also be addressed when integrating sulphanilic acid into electrochromic devices. The acid's sensitivity to environmental factors, such as moisture and temperature, may necessitate stringent control measures during the production process. Developing cost-effective and reliable manufacturing techniques that maintain the integrity of sulphanilic acid-based components is crucial for commercial viability.
Lastly, the potential environmental and health impacts of sulphanilic acid must be carefully evaluated. While the compound offers promising electrochromic properties, its use in large-scale device production requires thorough assessment of any potential risks associated with handling, disposal, and long-term exposure. Developing environmentally friendly synthesis routes and exploring safer alternatives or derivatives of sulphanilic acid may be necessary to address these concerns.
Current Sulphanilic Acid-based Solutions
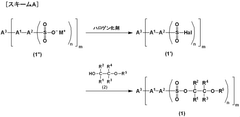
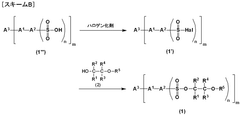
01 Electrochromic materials and compositions
Various materials and compositions are used in electrochromic devices to achieve color-changing properties. These can include transition metal oxides, conducting polymers, and organic compounds. The choice of materials affects the device's switching speed, color range, and durability.- Electrochromic materials and compositions: Various materials and compositions are used in electrochromic devices to achieve color-changing properties. These can include transition metal oxides, conducting polymers, and organic compounds. The choice of materials affects the device's optical properties, switching speed, and durability.
- Device structure and fabrication: The structure of electrochromic devices typically involves multiple layers, including transparent conductors, ion storage layers, and the electrochromic layer itself. Fabrication techniques such as sputtering, sol-gel processes, and electrodeposition are used to create these layered structures for optimal performance.
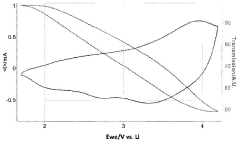
- Optical and electrical characteristics: Electrochromic devices exhibit specific optical and electrical properties, including transmittance range, coloration efficiency, and response time. These characteristics are influenced by factors such as applied voltage, ion conductivity, and the nature of the electrochromic materials used.
- Control systems and driving methods: Advanced control systems and driving methods are employed to manage the operation of electrochromic devices. These can include pulse-width modulation, voltage ramping, and feedback loops to optimize switching speed, color uniformity, and energy efficiency.
- Applications and integration: Electrochromic devices find applications in various fields, including smart windows, automotive mirrors, and display technologies. Integration challenges involve addressing issues such as large-area scaling, environmental stability, and interfacing with other electronic systems.
02 Device structure and fabrication
The structure of electrochromic devices typically involves multiple layers, including transparent conductors, ion storage layers, and the active electrochromic layer. Fabrication techniques such as sputtering, sol-gel processes, and electrodeposition are used to create these layers and optimize device performance.Expand Specific Solutions03 Switching mechanisms and control
Electrochromic devices change color in response to an applied voltage. The switching mechanism involves ion insertion and extraction, which can be controlled through various electrical circuits and driving schemes. Improving switching speed and uniformity is a key focus of research.Expand Specific Solutions04 Applications and integration
Electrochromic devices find applications in smart windows, displays, and automotive mirrors. Integration challenges include scaling up for large area applications, interfacing with other electronic systems, and ensuring long-term stability in various environmental conditions.Expand Specific Solutions05 Performance enhancement and characterization
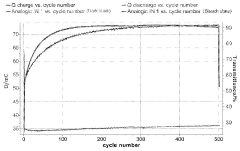
Improving electrochromic device performance involves enhancing contrast ratio, cycling stability, and energy efficiency. Various characterization techniques, such as spectroelectrochemistry and in-situ measurements, are used to analyze and optimize device properties.Expand Specific Solutions
Key Players in Electrochromic Industry
The development of electrochromic devices using sulphanilic acid is in an emerging stage, with a growing market driven by increasing demand for energy-efficient smart windows and displays. The technology's maturity is still evolving, with key players like Gentex Corp., SAGE Electrochromics, and Samsung Electronics leading research and development efforts. Other companies such as LG Chem and Nissan Chemical are also contributing to advancements in this field. The market size is expanding, particularly in automotive, architectural, and consumer electronics sectors, as the technology offers improved control over light transmission and energy efficiency. However, challenges in scalability and cost-effectiveness remain, indicating potential for further innovation and market growth.
Gentex Corp.
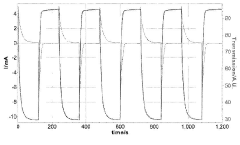
Technical Solution: Gentex Corp. has developed advanced electrochromic devices incorporating sulphanilic acid as a key component. Their approach involves using sulphanilic acid as a dopant in the electrochromic layer to enhance color-changing properties and durability. The company has implemented a multi-layer structure with a sulphanilic acid-doped tungsten oxide layer sandwiched between transparent conductive electrodes[1]. This configuration allows for rapid and reversible color changes when an electric field is applied. Gentex has also developed proprietary ion-conducting electrolytes that work synergistically with the sulphanilic acid-doped electrochromic layer to improve switching speed and uniformity[2]. Their devices demonstrate excellent long-term stability, with over 100,000 color-changing cycles reported in laboratory tests[3].
Strengths: Industry leader in automotive electrochromic mirrors, established manufacturing capabilities, and extensive R&D resources. Weaknesses: Primarily focused on automotive applications, potentially limiting broader market penetration.
SAGE Electrochromics, Inc.
Technical Solution: SAGE Electrochromics has pioneered the use of sulphanilic acid in large-scale electrochromic glazing for architectural applications. Their innovative approach involves incorporating sulphanilic acid into a nanostructured nickel oxide counter electrode, which complements their proprietary tungsten oxide-based primary electrochromic layer[4]. This dual-layer system allows for enhanced color neutrality and improved solar heat gain control. SAGE's electrochromic devices utilize a unique sol-gel deposition process that enables uniform distribution of sulphanilic acid throughout the counter electrode, resulting in superior optical performance and durability[5]. The company has also developed advanced control algorithms that optimize the use of sulphanilic acid-enhanced layers to achieve rapid tinting response times, typically under 5 minutes for large-scale windows[6].
Strengths: Leader in electrochromic building materials, scalable manufacturing processes for large area devices. Weaknesses: Higher cost compared to traditional glazing, limited to new construction or major renovations.
Core Innovations in Electrochromic Materials
Electrochromic device and method of preparing the same
PatentInactiveIN202018033193A
Innovation
- Development of electrochromic devices using ternary metal oxides, specifically lithium nickel metal oxides with dopant oxides like vanadium, tantalum, and niobium, which form a nanocomposite material with enhanced ionic and electronic conductivity, allowing for efficient switching between low and high optical transmission states and improved durability.
Sulfonic acid ester compound and use therefor
PatentWO2017217455A1
Innovation
- Development of sulfonic acid ester compounds with high solubility in low polar solvents, specifically esters of sulfonic acid and glycol ethers, which form stable solutions and maintain charge-transporting properties, enabling the creation of stable varnishes for organic EL devices.
Environmental Impact of Electrochromic Devices
The environmental impact of electrochromic devices, particularly those incorporating sulphanilic acid, is a crucial consideration in their development and deployment. These devices offer significant potential for energy conservation in buildings and vehicles by dynamically controlling light transmission and heat gain. However, their production, use, and disposal also present environmental challenges that must be carefully addressed.
During the manufacturing process of electrochromic devices, the use of sulphanilic acid and other chemical components raises concerns about potential environmental contamination. Proper handling, storage, and disposal protocols are essential to prevent the release of these substances into ecosystems. Additionally, the production of these devices often involves energy-intensive processes, contributing to carbon emissions and resource depletion.
On the positive side, the operational phase of electrochromic devices can lead to substantial environmental benefits. By reducing the need for artificial lighting and air conditioning, these devices can significantly lower energy consumption in buildings. This translates to reduced greenhouse gas emissions and a decreased reliance on fossil fuels for power generation. Studies have shown that buildings equipped with electrochromic windows can achieve energy savings of up to 20-30% compared to conventional glazing systems.
The longevity of electrochromic devices is another factor influencing their environmental impact. Devices with longer lifespans reduce the frequency of replacement and associated waste generation. However, the eventual disposal of these devices presents challenges due to the presence of various chemical compounds and electronic components. Developing effective recycling and disposal methods is crucial to mitigate potential environmental hazards and recover valuable materials.
Water consumption during the production and cleaning of electrochromic devices is another environmental consideration. Implementing water-efficient manufacturing processes and exploring alternative cleaning methods can help reduce the overall water footprint of these technologies.
As the adoption of electrochromic devices increases, their impact on urban heat island effects and local microclimates should be evaluated. While these devices can help reduce heat gain in buildings, their reflective properties may affect surrounding areas, potentially altering local temperature patterns and ecosystems.
To fully assess and optimize the environmental impact of electrochromic devices, life cycle assessments (LCAs) are essential. These comprehensive analyses consider all stages of a product's life, from raw material extraction to end-of-life disposal. LCAs can identify areas for improvement and guide the development of more sustainable electrochromic technologies.
In conclusion, while electrochromic devices offer significant environmental benefits through energy conservation, careful consideration must be given to their entire life cycle impact. Balancing the positive effects of reduced energy consumption with the potential negative impacts of production and disposal is crucial for ensuring the overall sustainability of these innovative technologies.
During the manufacturing process of electrochromic devices, the use of sulphanilic acid and other chemical components raises concerns about potential environmental contamination. Proper handling, storage, and disposal protocols are essential to prevent the release of these substances into ecosystems. Additionally, the production of these devices often involves energy-intensive processes, contributing to carbon emissions and resource depletion.
On the positive side, the operational phase of electrochromic devices can lead to substantial environmental benefits. By reducing the need for artificial lighting and air conditioning, these devices can significantly lower energy consumption in buildings. This translates to reduced greenhouse gas emissions and a decreased reliance on fossil fuels for power generation. Studies have shown that buildings equipped with electrochromic windows can achieve energy savings of up to 20-30% compared to conventional glazing systems.
The longevity of electrochromic devices is another factor influencing their environmental impact. Devices with longer lifespans reduce the frequency of replacement and associated waste generation. However, the eventual disposal of these devices presents challenges due to the presence of various chemical compounds and electronic components. Developing effective recycling and disposal methods is crucial to mitigate potential environmental hazards and recover valuable materials.
Water consumption during the production and cleaning of electrochromic devices is another environmental consideration. Implementing water-efficient manufacturing processes and exploring alternative cleaning methods can help reduce the overall water footprint of these technologies.
As the adoption of electrochromic devices increases, their impact on urban heat island effects and local microclimates should be evaluated. While these devices can help reduce heat gain in buildings, their reflective properties may affect surrounding areas, potentially altering local temperature patterns and ecosystems.
To fully assess and optimize the environmental impact of electrochromic devices, life cycle assessments (LCAs) are essential. These comprehensive analyses consider all stages of a product's life, from raw material extraction to end-of-life disposal. LCAs can identify areas for improvement and guide the development of more sustainable electrochromic technologies.
In conclusion, while electrochromic devices offer significant environmental benefits through energy conservation, careful consideration must be given to their entire life cycle impact. Balancing the positive effects of reduced energy consumption with the potential negative impacts of production and disposal is crucial for ensuring the overall sustainability of these innovative technologies.
Scalability and Manufacturing Considerations
The scalability and manufacturing considerations for developing electrochromic devices with sulphanilic acid are crucial factors in determining the commercial viability and widespread adoption of this technology. One of the primary challenges lies in the large-scale production of sulphanilic acid-based electrochromic materials. While laboratory-scale synthesis may yield promising results, transitioning to industrial-scale production often presents unforeseen obstacles.
The manufacturing process for these devices requires precise control over the deposition of sulphanilic acid-based layers onto transparent conductive substrates. Techniques such as spin coating, dip coating, and spray coating have shown potential for small-scale production, but their scalability to large-area devices remains a significant hurdle. Uniformity and thickness control become increasingly challenging as the substrate size increases, potentially affecting the device's optical and electrical properties.
Another critical aspect is the integration of sulphanilic acid-based electrochromic layers with other components of the device, such as ion storage layers and electrolytes. Ensuring consistent performance across large areas and maintaining proper interfacial contact between layers are essential for device functionality. Additionally, the stability of sulphanilic acid under various environmental conditions during the manufacturing process must be carefully considered to prevent degradation or unwanted side reactions.
Cost-effectiveness is a key factor in scaling up production. The availability and price of raw materials, particularly high-purity sulphanilic acid, can significantly impact the overall manufacturing costs. Developing efficient recycling processes for unused materials and optimizing material utilization during production are essential strategies for improving cost-effectiveness.
Quality control and reproducibility present additional challenges in large-scale manufacturing. Establishing robust quality assurance protocols and implementing in-line monitoring systems are crucial for maintaining consistent device performance across production batches. This may involve developing specialized testing equipment and procedures tailored to sulphanilic acid-based electrochromic devices.
Environmental considerations and regulatory compliance must also be addressed in the manufacturing process. Sulphanilic acid and its derivatives may pose environmental risks if not handled properly. Implementing closed-loop systems, waste treatment processes, and adhering to relevant environmental regulations are essential for sustainable large-scale production.
Lastly, the development of automated manufacturing processes and specialized equipment for handling sulphanilic acid-based materials will be crucial for achieving high-volume production. This may involve collaborations with equipment manufacturers to design custom tools and machinery tailored to the unique requirements of these electrochromic devices.
The manufacturing process for these devices requires precise control over the deposition of sulphanilic acid-based layers onto transparent conductive substrates. Techniques such as spin coating, dip coating, and spray coating have shown potential for small-scale production, but their scalability to large-area devices remains a significant hurdle. Uniformity and thickness control become increasingly challenging as the substrate size increases, potentially affecting the device's optical and electrical properties.
Another critical aspect is the integration of sulphanilic acid-based electrochromic layers with other components of the device, such as ion storage layers and electrolytes. Ensuring consistent performance across large areas and maintaining proper interfacial contact between layers are essential for device functionality. Additionally, the stability of sulphanilic acid under various environmental conditions during the manufacturing process must be carefully considered to prevent degradation or unwanted side reactions.
Cost-effectiveness is a key factor in scaling up production. The availability and price of raw materials, particularly high-purity sulphanilic acid, can significantly impact the overall manufacturing costs. Developing efficient recycling processes for unused materials and optimizing material utilization during production are essential strategies for improving cost-effectiveness.
Quality control and reproducibility present additional challenges in large-scale manufacturing. Establishing robust quality assurance protocols and implementing in-line monitoring systems are crucial for maintaining consistent device performance across production batches. This may involve developing specialized testing equipment and procedures tailored to sulphanilic acid-based electrochromic devices.
Environmental considerations and regulatory compliance must also be addressed in the manufacturing process. Sulphanilic acid and its derivatives may pose environmental risks if not handled properly. Implementing closed-loop systems, waste treatment processes, and adhering to relevant environmental regulations are essential for sustainable large-scale production.
Lastly, the development of automated manufacturing processes and specialized equipment for handling sulphanilic acid-based materials will be crucial for achieving high-volume production. This may involve collaborations with equipment manufacturers to design custom tools and machinery tailored to the unique requirements of these electrochromic devices.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!