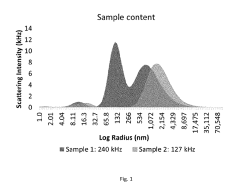
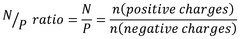Dynamic Light Scattering for Efficient Drug Delivery Systems
SEP 5, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
DLS Technology Background and Objectives
Dynamic Light Scattering (DLS) has evolved significantly since its theoretical foundations were established in the early 20th century. Initially developed as a technique to study Brownian motion, DLS has transformed into a powerful analytical method for characterizing nanoparticles and colloidal systems. The technology measures the time-dependent fluctuations in scattered light intensity caused by particles in suspension, providing critical information about particle size distribution, stability, and surface properties.
In the pharmaceutical domain, DLS has become increasingly vital for developing efficient drug delivery systems (DDS). The ability to precisely characterize nanocarriers at the submicron level has positioned DLS as an indispensable tool in modern pharmaceutical research and development. The technique offers non-invasive, rapid analysis with minimal sample preparation, making it particularly valuable for characterizing delicate biological formulations.
The evolution of DLS technology has been marked by significant improvements in laser technology, detector sensitivity, and data processing algorithms. Modern DLS systems can detect particles ranging from less than 1 nm to several microns, with enhanced resolution and reproducibility compared to earlier generations. The integration of multi-angle detection systems and advanced correlation techniques has further expanded the capabilities of DLS for complex formulations.
The primary objective of DLS application in drug delivery systems is to enable precise control over nanocarrier properties, ensuring optimal drug encapsulation, stability, release kinetics, and targeted delivery. By providing real-time monitoring of particle size and distribution, DLS facilitates the development of more effective and safer drug delivery platforms with enhanced bioavailability and reduced side effects.
Current technological trends in DLS include the development of high-throughput systems for pharmaceutical screening, miniaturization for point-of-care applications, and integration with complementary techniques such as zeta potential measurement and Raman spectroscopy. These advancements aim to provide more comprehensive characterization of drug delivery systems in a single analytical platform.
Looking forward, the trajectory of DLS technology is moving toward automated, AI-enhanced systems capable of more sophisticated data interpretation and prediction of nanocarrier behavior in biological environments. The integration of DLS with in silico modeling approaches represents a promising frontier for accelerating the development cycle of novel drug delivery systems and enabling personalized medicine applications.
The ultimate goal of DLS technology advancement in this field is to establish standardized, reliable methods for characterizing nanomedicines throughout their development lifecycle, from initial formulation to quality control in manufacturing and post-market surveillance.
In the pharmaceutical domain, DLS has become increasingly vital for developing efficient drug delivery systems (DDS). The ability to precisely characterize nanocarriers at the submicron level has positioned DLS as an indispensable tool in modern pharmaceutical research and development. The technique offers non-invasive, rapid analysis with minimal sample preparation, making it particularly valuable for characterizing delicate biological formulations.
The evolution of DLS technology has been marked by significant improvements in laser technology, detector sensitivity, and data processing algorithms. Modern DLS systems can detect particles ranging from less than 1 nm to several microns, with enhanced resolution and reproducibility compared to earlier generations. The integration of multi-angle detection systems and advanced correlation techniques has further expanded the capabilities of DLS for complex formulations.
The primary objective of DLS application in drug delivery systems is to enable precise control over nanocarrier properties, ensuring optimal drug encapsulation, stability, release kinetics, and targeted delivery. By providing real-time monitoring of particle size and distribution, DLS facilitates the development of more effective and safer drug delivery platforms with enhanced bioavailability and reduced side effects.
Current technological trends in DLS include the development of high-throughput systems for pharmaceutical screening, miniaturization for point-of-care applications, and integration with complementary techniques such as zeta potential measurement and Raman spectroscopy. These advancements aim to provide more comprehensive characterization of drug delivery systems in a single analytical platform.
Looking forward, the trajectory of DLS technology is moving toward automated, AI-enhanced systems capable of more sophisticated data interpretation and prediction of nanocarrier behavior in biological environments. The integration of DLS with in silico modeling approaches represents a promising frontier for accelerating the development cycle of novel drug delivery systems and enabling personalized medicine applications.
The ultimate goal of DLS technology advancement in this field is to establish standardized, reliable methods for characterizing nanomedicines throughout their development lifecycle, from initial formulation to quality control in manufacturing and post-market surveillance.
Market Analysis for DLS-Based Drug Delivery
The global market for Dynamic Light Scattering (DLS) technology in drug delivery systems has experienced significant growth over the past decade, driven by increasing demand for advanced drug formulation techniques and personalized medicine approaches. Current market valuations indicate that the DLS-based pharmaceutical analysis segment reached approximately 320 million USD in 2022, with projections suggesting a compound annual growth rate of 7.8% through 2028.
The pharmaceutical industry represents the largest end-user segment for DLS technology, accounting for nearly 45% of the total market share. This dominance stems from the critical role DLS plays in characterizing nanoparticle-based drug delivery systems, liposomes, and other colloidal formulations. Academic research institutions constitute the second-largest market segment at 30%, followed by contract research organizations at 15%.
Geographically, North America leads the market with approximately 38% share, attributed to substantial R&D investments and the presence of major pharmaceutical companies. Europe follows closely at 32%, with particular strength in countries like Germany, Switzerland, and the UK where precision medicine initiatives have gained significant traction. The Asia-Pacific region represents the fastest-growing market at a 9.2% CAGR, driven by expanding healthcare infrastructure in China and India, coupled with increasing government funding for nanotechnology research.
Key market drivers include the rising prevalence of chronic diseases requiring targeted drug delivery solutions, growing adoption of nanomedicine approaches, and increasing regulatory emphasis on thorough characterization of drug delivery systems. The trend toward personalized medicine has further accelerated demand for precise particle characterization technologies like DLS.
Market challenges include the high cost of advanced DLS instrumentation, technical complexity requiring specialized training, and competition from alternative particle characterization methods such as nanoparticle tracking analysis and electron microscopy. Additionally, regulatory uncertainties regarding nanomedicine approval pathways in emerging markets pose adoption barriers.
Future market opportunities lie in the integration of artificial intelligence with DLS for automated data interpretation, development of portable DLS devices for point-of-care applications, and expansion into biologics characterization as the biopharmaceutical sector continues its rapid growth. The increasing focus on lipid nanoparticle formulations for mRNA therapeutics, catalyzed by COVID-19 vaccine development, represents a particularly promising growth segment for DLS technology applications.
The pharmaceutical industry represents the largest end-user segment for DLS technology, accounting for nearly 45% of the total market share. This dominance stems from the critical role DLS plays in characterizing nanoparticle-based drug delivery systems, liposomes, and other colloidal formulations. Academic research institutions constitute the second-largest market segment at 30%, followed by contract research organizations at 15%.
Geographically, North America leads the market with approximately 38% share, attributed to substantial R&D investments and the presence of major pharmaceutical companies. Europe follows closely at 32%, with particular strength in countries like Germany, Switzerland, and the UK where precision medicine initiatives have gained significant traction. The Asia-Pacific region represents the fastest-growing market at a 9.2% CAGR, driven by expanding healthcare infrastructure in China and India, coupled with increasing government funding for nanotechnology research.
Key market drivers include the rising prevalence of chronic diseases requiring targeted drug delivery solutions, growing adoption of nanomedicine approaches, and increasing regulatory emphasis on thorough characterization of drug delivery systems. The trend toward personalized medicine has further accelerated demand for precise particle characterization technologies like DLS.
Market challenges include the high cost of advanced DLS instrumentation, technical complexity requiring specialized training, and competition from alternative particle characterization methods such as nanoparticle tracking analysis and electron microscopy. Additionally, regulatory uncertainties regarding nanomedicine approval pathways in emerging markets pose adoption barriers.
Future market opportunities lie in the integration of artificial intelligence with DLS for automated data interpretation, development of portable DLS devices for point-of-care applications, and expansion into biologics characterization as the biopharmaceutical sector continues its rapid growth. The increasing focus on lipid nanoparticle formulations for mRNA therapeutics, catalyzed by COVID-19 vaccine development, represents a particularly promising growth segment for DLS technology applications.
Current Challenges in DLS Technology
Despite significant advancements in Dynamic Light Scattering (DLS) technology for drug delivery systems, several critical challenges continue to impede its widespread application and reliability. One fundamental limitation is the inherent bias towards larger particles in polydisperse samples. When analyzing mixtures containing particles of varying sizes, DLS algorithms tend to overrepresent larger particles due to their stronger scattering intensity, potentially masking the presence of smaller components that may be crucial for drug delivery efficacy.
Resolution constraints present another significant hurdle. Current DLS systems struggle to differentiate between particles with size differences less than a factor of 3-5, making it difficult to accurately characterize complex drug delivery vehicles with multiple components or layers. This limitation becomes particularly problematic when analyzing lipid nanoparticles or polymer-based delivery systems with intricate structures.
Sample preparation inconsistencies further complicate DLS measurements. Factors such as concentration effects, buffer composition, and temperature fluctuations can significantly alter scattering patterns, leading to poor reproducibility between laboratories. The lack of standardized protocols for sample preparation specifically tailored to drug delivery applications exacerbates this issue.
Data interpretation challenges also persist in the field. Converting autocorrelation functions to size distributions involves complex mathematical models that rely on assumptions about particle shape and homogeneity. These assumptions often fail to account for the complex morphologies of modern drug delivery systems, such as non-spherical particles or aggregation phenomena, resulting in misleading size distributions.
Environmental sensitivity represents another technical barrier. DLS measurements are highly susceptible to dust contamination, temperature gradients, and mechanical vibrations. This sensitivity necessitates specialized laboratory conditions that may not be available in all research or quality control settings, limiting the technology's accessibility and routine implementation.
The integration of DLS with complementary techniques remains underdeveloped. While DLS provides valuable information about particle size and distribution, it lacks the ability to characterize other critical parameters for drug delivery systems, such as surface charge, drug loading capacity, and release kinetics. Current systems rarely offer seamless integration with techniques like zeta potential measurement or spectroscopic methods that could provide more comprehensive characterization.
Lastly, real-time monitoring capabilities are still limited. The ability to track dynamic changes in drug delivery systems under physiological conditions or during drug release processes would significantly enhance the technology's utility but remains challenging due to instrumental and methodological constraints.
Resolution constraints present another significant hurdle. Current DLS systems struggle to differentiate between particles with size differences less than a factor of 3-5, making it difficult to accurately characterize complex drug delivery vehicles with multiple components or layers. This limitation becomes particularly problematic when analyzing lipid nanoparticles or polymer-based delivery systems with intricate structures.
Sample preparation inconsistencies further complicate DLS measurements. Factors such as concentration effects, buffer composition, and temperature fluctuations can significantly alter scattering patterns, leading to poor reproducibility between laboratories. The lack of standardized protocols for sample preparation specifically tailored to drug delivery applications exacerbates this issue.
Data interpretation challenges also persist in the field. Converting autocorrelation functions to size distributions involves complex mathematical models that rely on assumptions about particle shape and homogeneity. These assumptions often fail to account for the complex morphologies of modern drug delivery systems, such as non-spherical particles or aggregation phenomena, resulting in misleading size distributions.
Environmental sensitivity represents another technical barrier. DLS measurements are highly susceptible to dust contamination, temperature gradients, and mechanical vibrations. This sensitivity necessitates specialized laboratory conditions that may not be available in all research or quality control settings, limiting the technology's accessibility and routine implementation.
The integration of DLS with complementary techniques remains underdeveloped. While DLS provides valuable information about particle size and distribution, it lacks the ability to characterize other critical parameters for drug delivery systems, such as surface charge, drug loading capacity, and release kinetics. Current systems rarely offer seamless integration with techniques like zeta potential measurement or spectroscopic methods that could provide more comprehensive characterization.
Lastly, real-time monitoring capabilities are still limited. The ability to track dynamic changes in drug delivery systems under physiological conditions or during drug release processes would significantly enhance the technology's utility but remains challenging due to instrumental and methodological constraints.
Current DLS Solutions for Drug Delivery
01 Improved DLS measurement techniques
Various techniques have been developed to improve the efficiency and accuracy of dynamic light scattering measurements. These include optimized optical configurations, advanced signal processing algorithms, and innovative detection methods that enhance the sensitivity and resolution of DLS systems. These improvements allow for more precise particle size analysis, better detection of polydisperse samples, and reduced measurement time while maintaining high data quality.- Improved DLS measurement techniques: Advanced techniques for enhancing dynamic light scattering measurements focus on improving signal quality and data acquisition. These innovations include optimized optical configurations, enhanced detection systems, and refined algorithms for signal processing. Such improvements allow for more accurate particle size determination, better resolution of polydisperse samples, and increased sensitivity for detecting small particles in solution.
- DLS instrumentation enhancements: Innovations in DLS instrumentation focus on hardware components that improve measurement efficiency and accuracy. These include advanced laser sources with improved stability, high-sensitivity detectors, temperature control systems, and automated sample handling mechanisms. Such enhancements reduce measurement time, minimize sample volume requirements, and enable more reliable measurements across diverse sample types.
- DLS applications in specific fields: Dynamic light scattering technology has been adapted for specialized applications across various fields. These include pharmaceutical formulation analysis, nanoparticle characterization, protein aggregation studies, and quality control in industrial processes. Specialized DLS systems have been developed to address field-specific challenges such as high-throughput screening, in-line process monitoring, and analysis of complex biological samples.
- Multi-angle and multi-wavelength DLS systems: Advanced DLS systems utilize measurements at multiple angles and/or multiple wavelengths to extract more comprehensive information about particle characteristics. These approaches provide enhanced resolution for polydisperse samples, improved accuracy for non-spherical particles, and better discrimination between different particle populations. The combination of data from various angles and wavelengths enables more robust analysis of complex colloidal systems.
- Data analysis and computational methods for DLS: Sophisticated computational approaches have been developed to enhance the analysis of DLS data. These include advanced correlation algorithms, machine learning techniques for data interpretation, and statistical methods for improving measurement reliability. Such computational methods enable more accurate particle size distribution analysis, better handling of background noise, and improved detection of multimodal distributions in complex samples.
02 Enhanced DLS instrumentation design
Advancements in DLS instrumentation design focus on improving the efficiency of light scattering detection systems. These innovations include specialized optical components, improved laser sources, and optimized detector arrangements that maximize signal collection while minimizing noise. Novel instrument configurations enable more efficient analysis of challenging samples, such as highly concentrated suspensions or samples with low scattering intensity.Expand Specific Solutions03 DLS data analysis and processing methods
Advanced data analysis and processing methods significantly enhance the efficiency of dynamic light scattering measurements. These include sophisticated algorithms for correlation function analysis, machine learning approaches for data interpretation, and automated processing workflows that reduce analysis time. These methods improve the extraction of meaningful information from raw scattering data, enabling more accurate particle characterization and increased throughput.Expand Specific Solutions04 Application-specific DLS optimization
Specialized DLS systems and methodologies have been developed for specific applications to maximize efficiency in particular use cases. These include adaptations for biological samples, nanoparticle characterization, pharmaceutical formulations, and industrial quality control. By optimizing parameters such as scattering angle, wavelength selection, and sample handling for specific applications, these systems achieve higher efficiency and more relevant results for targeted analyses.Expand Specific Solutions05 Integration of DLS with complementary techniques
Combining dynamic light scattering with complementary analytical techniques creates more efficient characterization workflows. These integrated approaches pair DLS with techniques such as static light scattering, microscopy, spectroscopy, or chromatography to provide comprehensive sample analysis. Such combinations enable simultaneous measurement of multiple parameters, validation of results through orthogonal methods, and more complete characterization of complex samples while improving overall analytical efficiency.Expand Specific Solutions
Key Industry Players in DLS Drug Delivery
Dynamic Light Scattering (DLS) technology for drug delivery systems is in a growth phase, with an expanding market driven by increasing demand for targeted therapeutics. The global market is projected to reach significant scale as pharmaceutical companies invest in advanced drug delivery technologies. Companies like Sanofi, Eli Lilly, and Novo Nordisk are leading commercial applications, while Malvern Panalytical and Agilent Technologies provide specialized instrumentation. Research institutions such as California Institute of Technology and Duke University contribute fundamental advancements. The technology has reached moderate maturity in pharmaceutical applications but continues to evolve with innovations from companies like Nanoco Technologies and National Institute for Materials Science focusing on nanoparticle characterization for next-generation drug delivery systems.
Eli Lilly & Co.
Technical Solution: Eli Lilly has pioneered a DLS-based quality control system specifically designed for protein and peptide-based therapeutics delivery systems. Their approach combines traditional DLS with machine learning algorithms to predict stability and bioavailability of complex biologic formulations. The technology employs temperature-controlled DLS measurements across physiologically relevant conditions to simulate in vivo behavior of their drug delivery systems. Particularly innovative is their correlation of DLS data with pharmacokinetic outcomes, creating predictive models that significantly reduce development timelines for new formulations. Lilly's platform includes specialized sample preparation protocols that maintain the integrity of delicate biological materials during measurement. Their system has been instrumental in developing several market-leading injectable formulations with extended release profiles, including their diabetes portfolio where precise particle characterization directly impacts glucose control duration.
Strengths: Exceptional integration with biologics development pipeline; strong correlation between DLS measurements and clinical outcomes; sophisticated predictive modeling capabilities. Weaknesses: Highly specialized for protein/peptide therapeutics with less application to small molecule delivery systems; requires substantial computational resources for data processing.
F. Hoffmann-La Roche Ltd.
Technical Solution: Roche has developed an advanced Dynamic Light Scattering (DLS) platform for nanomedicine characterization that integrates with their drug delivery systems. Their technology utilizes multi-angle DLS measurements to precisely characterize nanoparticle-based drug carriers in complex biological media. The system incorporates real-time monitoring capabilities that track changes in particle size distribution during drug release, allowing for optimization of controlled release kinetics. Roche's platform includes proprietary algorithms that can distinguish between drug-loaded nanoparticles and biological components in blood or tissue samples, enabling accurate in vivo tracking. Their DLS technology has been particularly successful in developing long-circulating liposomal formulations that achieve targeted delivery to tumor sites through enhanced permeability and retention effects, with demonstrated improvements in therapeutic index for several oncology compounds.
Strengths: Superior characterization capabilities in complex biological media; integrated approach combining DLS with drug delivery optimization; extensive pharmaceutical development expertise. Weaknesses: System complexity requires specialized training; higher implementation costs compared to standard DLS systems; primarily optimized for their own pharmaceutical pipeline rather than as a general platform.
Core Patents and Literature in DLS Technology
A drug delivery system for increased endosomal escape
PatentWO2025021286A1
Innovation
- A liposome formulation with a lipid bilayer comprising an encapsulating agent, a fusogenic agent, and an acid-cleavable PEGylated lipid, which enhances endosomal escape by combining the fusogenic properties of lipids like LBPA with the acid-cleavable PEG lipid, allowing for increased cytosolic delivery of therapeutic agents.
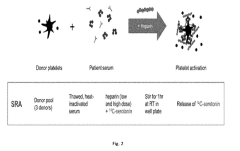
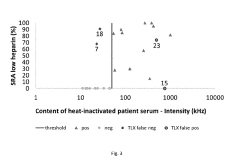
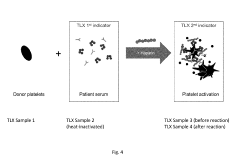
Methods and Apparatus for Predicting and Confirming Drug-Induced Thrombocytopenia Through Particle Detection with Dynamic Light Scattering
PatentInactiveUS20190250088A1
Innovation
- Dynamic Light Scattering (DLS) techniques are used to measure all particles in a patient sample, including platelets, microparticles, and aggregates, to predict and confirm DIT by analyzing changes in particle size distribution before and after exposure to suspected drugs, providing a more comprehensive assessment than existing methods.
Regulatory Framework for DLS Drug Delivery Systems
The regulatory landscape for Dynamic Light Scattering (DLS) in drug delivery systems presents a complex framework that varies significantly across global jurisdictions. In the United States, the FDA has established specific guidelines under 21 CFR Part 11 for analytical methods used in pharmaceutical development, including DLS characterization of nanoparticle-based drug delivery systems. These regulations mandate validation protocols for DLS instruments, requiring demonstration of accuracy, precision, specificity, linearity, and robustness in size and polydispersity measurements.
The European Medicines Agency (EMA) has implemented parallel but distinct requirements through ICH Q2(R1) guidelines, which specifically address the validation of analytical procedures for novel drug delivery systems. For DLS applications, the EMA emphasizes reproducibility across different laboratory settings and standardization of measurement protocols to ensure consistent particle characterization across the European market.
In emerging markets, particularly China and India, regulatory frameworks for DLS-based characterization are rapidly evolving. The Chinese National Medical Products Administration (NMPA) has recently introduced technical guidelines specifically addressing nanomedicine characterization, with dedicated sections on light scattering techniques and their validation requirements for quality control purposes.
Harmonization efforts through the International Council for Harmonisation (ICH) have attempted to standardize DLS measurement protocols globally, particularly through the Q8-Q11 guidelines on pharmaceutical development and manufacturing. These initiatives aim to establish consistent quality standards for particle characterization across international boundaries, facilitating global development and approval of advanced drug delivery systems.
Regulatory challenges specific to DLS technology include the establishment of acceptable polydispersity index thresholds for different delivery systems, validation of measurements in complex biological media, and correlation of DLS data with in vivo performance. The FDA's Nanotechnology Task Force has published guidance documents addressing these challenges, providing recommendations for appropriate characterization methodologies based on the intended clinical application.
Recent regulatory developments have focused on real-time release testing using DLS for continuous manufacturing processes, with both the FDA and EMA publishing draft guidance documents on Process Analytical Technology (PAT) implementation. These frameworks encourage the integration of DLS as an in-line monitoring tool during pharmaceutical production, requiring validation of measurement accuracy under dynamic manufacturing conditions.
Compliance with these regulatory frameworks necessitates comprehensive documentation of DLS method development, validation protocols, and quality control procedures throughout the drug development lifecycle. Manufacturers must demonstrate consistent particle size distribution across multiple batches and stability under various storage conditions to satisfy regulatory requirements for market approval.
The European Medicines Agency (EMA) has implemented parallel but distinct requirements through ICH Q2(R1) guidelines, which specifically address the validation of analytical procedures for novel drug delivery systems. For DLS applications, the EMA emphasizes reproducibility across different laboratory settings and standardization of measurement protocols to ensure consistent particle characterization across the European market.
In emerging markets, particularly China and India, regulatory frameworks for DLS-based characterization are rapidly evolving. The Chinese National Medical Products Administration (NMPA) has recently introduced technical guidelines specifically addressing nanomedicine characterization, with dedicated sections on light scattering techniques and their validation requirements for quality control purposes.
Harmonization efforts through the International Council for Harmonisation (ICH) have attempted to standardize DLS measurement protocols globally, particularly through the Q8-Q11 guidelines on pharmaceutical development and manufacturing. These initiatives aim to establish consistent quality standards for particle characterization across international boundaries, facilitating global development and approval of advanced drug delivery systems.
Regulatory challenges specific to DLS technology include the establishment of acceptable polydispersity index thresholds for different delivery systems, validation of measurements in complex biological media, and correlation of DLS data with in vivo performance. The FDA's Nanotechnology Task Force has published guidance documents addressing these challenges, providing recommendations for appropriate characterization methodologies based on the intended clinical application.
Recent regulatory developments have focused on real-time release testing using DLS for continuous manufacturing processes, with both the FDA and EMA publishing draft guidance documents on Process Analytical Technology (PAT) implementation. These frameworks encourage the integration of DLS as an in-line monitoring tool during pharmaceutical production, requiring validation of measurement accuracy under dynamic manufacturing conditions.
Compliance with these regulatory frameworks necessitates comprehensive documentation of DLS method development, validation protocols, and quality control procedures throughout the drug development lifecycle. Manufacturers must demonstrate consistent particle size distribution across multiple batches and stability under various storage conditions to satisfy regulatory requirements for market approval.
Scalability and Manufacturing Considerations
Scaling up Dynamic Light Scattering (DLS) technology from laboratory to industrial production presents significant challenges that must be addressed for commercial viability of advanced drug delivery systems. The transition requires careful consideration of equipment specifications, process parameters, and quality control measures to maintain consistent particle size distributions across batches.
Manufacturing DLS-based drug delivery systems at scale demands specialized equipment capable of handling larger volumes while maintaining precise control over critical parameters such as temperature, pressure, and mixing conditions. Current industrial implementations typically utilize continuous flow systems rather than batch processes to improve throughput and consistency. These systems must incorporate in-line monitoring capabilities to ensure real-time quality control during production.
Raw material sourcing becomes increasingly critical at commercial scale, as variations in lipid purity, polymer molecular weight distributions, or surfactant quality can significantly impact the final product characteristics. Establishing robust supplier qualification programs and implementing comprehensive material testing protocols are essential steps in ensuring manufacturing consistency.
Regulatory considerations present another dimension of complexity in scaling DLS processes. Manufacturers must demonstrate that the scaled-up process produces drug delivery systems with equivalent critical quality attributes to those produced during clinical development. This includes maintaining consistent particle size distributions, drug loading efficiency, and release kinetics across different production scales.
Cost efficiency represents a major consideration in commercial manufacturing. While laboratory-scale DLS equipment may utilize expensive components and labor-intensive processes, industrial implementation requires process optimization to reduce production costs. This often involves automation of key steps, reduction of energy consumption, and minimization of waste streams. Recent innovations in microfluidic-based manufacturing platforms have shown promise in addressing these challenges by offering better control over mixing parameters while reducing equipment footprint.
Environmental sustainability has emerged as an additional consideration in scaling DLS manufacturing processes. Traditional methods often require significant quantities of organic solvents, which present both environmental and worker safety concerns. Newer approaches focus on green chemistry principles, including the use of supercritical CO2 as an alternative processing medium and the development of solvent recycling systems to minimize waste generation.
The integration of Industry 4.0 principles, including advanced process analytical technology (PAT) and artificial intelligence-driven process control, represents the frontier of DLS manufacturing technology. These approaches enable adaptive manufacturing processes that can respond to variations in input materials or environmental conditions, maintaining consistent product quality despite external variables.
Manufacturing DLS-based drug delivery systems at scale demands specialized equipment capable of handling larger volumes while maintaining precise control over critical parameters such as temperature, pressure, and mixing conditions. Current industrial implementations typically utilize continuous flow systems rather than batch processes to improve throughput and consistency. These systems must incorporate in-line monitoring capabilities to ensure real-time quality control during production.
Raw material sourcing becomes increasingly critical at commercial scale, as variations in lipid purity, polymer molecular weight distributions, or surfactant quality can significantly impact the final product characteristics. Establishing robust supplier qualification programs and implementing comprehensive material testing protocols are essential steps in ensuring manufacturing consistency.
Regulatory considerations present another dimension of complexity in scaling DLS processes. Manufacturers must demonstrate that the scaled-up process produces drug delivery systems with equivalent critical quality attributes to those produced during clinical development. This includes maintaining consistent particle size distributions, drug loading efficiency, and release kinetics across different production scales.
Cost efficiency represents a major consideration in commercial manufacturing. While laboratory-scale DLS equipment may utilize expensive components and labor-intensive processes, industrial implementation requires process optimization to reduce production costs. This often involves automation of key steps, reduction of energy consumption, and minimization of waste streams. Recent innovations in microfluidic-based manufacturing platforms have shown promise in addressing these challenges by offering better control over mixing parameters while reducing equipment footprint.
Environmental sustainability has emerged as an additional consideration in scaling DLS manufacturing processes. Traditional methods often require significant quantities of organic solvents, which present both environmental and worker safety concerns. Newer approaches focus on green chemistry principles, including the use of supercritical CO2 as an alternative processing medium and the development of solvent recycling systems to minimize waste generation.
The integration of Industry 4.0 principles, including advanced process analytical technology (PAT) and artificial intelligence-driven process control, represents the frontier of DLS manufacturing technology. These approaches enable adaptive manufacturing processes that can respond to variations in input materials or environmental conditions, maintaining consistent product quality despite external variables.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!