Dynamic Light Scattering for Enhancing Bioreactor Designs
SEP 5, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
DLS Technology Background and Objectives
Dynamic Light Scattering (DLS) technology has evolved significantly since its inception in the 1960s, transitioning from a purely academic research tool to an essential analytical technique in various industries. The fundamental principle of DLS involves measuring the Brownian motion of particles in suspension and correlating this to particle size through the Stokes-Einstein relationship. This non-invasive technique has gained prominence for its ability to analyze particles in the nanometer to micrometer range without sample disruption.
The evolution of DLS technology has been marked by significant improvements in laser technology, detection systems, and data processing algorithms. Early DLS systems utilized basic photon correlation spectroscopy with limited resolution, while modern systems incorporate advanced multi-angle detection, machine learning algorithms, and real-time analysis capabilities that substantially enhance measurement accuracy and application scope.
In the context of bioreactor design, DLS technology presents a transformative opportunity to monitor critical parameters in real-time during bioprocessing operations. Traditional bioreactor monitoring relies heavily on offline sampling and analysis, creating significant time delays between measurement and process adjustment. The integration of DLS offers the potential for continuous, in-situ monitoring of crucial parameters such as protein aggregation, cell density, and biomolecule interactions.
The primary technical objectives for implementing DLS in bioreactor designs include developing robust in-line measurement capabilities that can withstand the challenging environment of industrial bioreactors, including high cell densities, temperature variations, and mechanical agitation. Additionally, there is a need to establish correlations between DLS measurements and critical quality attributes of biopharmaceutical products to enable real-time quality control.
Current research trends focus on miniaturization of DLS components for seamless integration into existing bioreactor systems, development of specialized algorithms for interpreting DLS data in complex biological media, and creation of standardized protocols for DLS implementation across different bioprocessing platforms. The technology aims to address the growing industry demand for Process Analytical Technology (PAT) tools that support Quality by Design (QbD) approaches in biopharmaceutical manufacturing.
The convergence of DLS technology with artificial intelligence and machine learning represents the next frontier, potentially enabling predictive capabilities that could transform bioreactor operation from reactive to proactive process control. This evolution aligns with the broader industry trend toward smart manufacturing and increased process automation in biopharmaceutical production.
The evolution of DLS technology has been marked by significant improvements in laser technology, detection systems, and data processing algorithms. Early DLS systems utilized basic photon correlation spectroscopy with limited resolution, while modern systems incorporate advanced multi-angle detection, machine learning algorithms, and real-time analysis capabilities that substantially enhance measurement accuracy and application scope.
In the context of bioreactor design, DLS technology presents a transformative opportunity to monitor critical parameters in real-time during bioprocessing operations. Traditional bioreactor monitoring relies heavily on offline sampling and analysis, creating significant time delays between measurement and process adjustment. The integration of DLS offers the potential for continuous, in-situ monitoring of crucial parameters such as protein aggregation, cell density, and biomolecule interactions.
The primary technical objectives for implementing DLS in bioreactor designs include developing robust in-line measurement capabilities that can withstand the challenging environment of industrial bioreactors, including high cell densities, temperature variations, and mechanical agitation. Additionally, there is a need to establish correlations between DLS measurements and critical quality attributes of biopharmaceutical products to enable real-time quality control.
Current research trends focus on miniaturization of DLS components for seamless integration into existing bioreactor systems, development of specialized algorithms for interpreting DLS data in complex biological media, and creation of standardized protocols for DLS implementation across different bioprocessing platforms. The technology aims to address the growing industry demand for Process Analytical Technology (PAT) tools that support Quality by Design (QbD) approaches in biopharmaceutical manufacturing.
The convergence of DLS technology with artificial intelligence and machine learning represents the next frontier, potentially enabling predictive capabilities that could transform bioreactor operation from reactive to proactive process control. This evolution aligns with the broader industry trend toward smart manufacturing and increased process automation in biopharmaceutical production.
Bioreactor Market Demand Analysis
The global bioreactor market has been experiencing robust growth, driven primarily by increasing demand for biopharmaceuticals, growing adoption of single-use technologies, and expanding applications in regenerative medicine. As of recent market analyses, the bioreactor market is valued at approximately 3.5 billion USD with projections to reach 6.2 billion USD by 2027, representing a compound annual growth rate (CAGR) of 10.2%.
Pharmaceutical and biotechnology companies constitute the largest segment of bioreactor end-users, accounting for nearly 60% of the market share. This dominance stems from the rising prevalence of chronic diseases and the subsequent need for biopharmaceutical interventions. The COVID-19 pandemic has further accelerated this trend, with unprecedented demand for vaccine production capabilities worldwide.
Geographically, North America leads the market with approximately 40% share, followed by Europe at 30% and Asia-Pacific at 20%. However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years due to increasing investments in biotechnology infrastructure, particularly in China, India, and South Korea.
The market demand for advanced bioreactor technologies is increasingly focused on process optimization and real-time monitoring capabilities. According to industry surveys, 78% of biopharmaceutical manufacturers cite process optimization as their primary challenge, while 65% express interest in technologies that enable real-time monitoring without compromising sterility.
Dynamic Light Scattering (DLS) technology addresses these market needs by offering non-invasive, real-time monitoring of particle size distribution and aggregation behavior within bioreactors. This capability is particularly valuable for protein-based therapeutics production, where aggregation issues can significantly impact product quality and yield.
Market research indicates that technologies enhancing process analytical technology (PAT) capabilities, such as DLS, can reduce production costs by up to 25% through minimizing batch failures and optimizing process parameters. Furthermore, regulatory bodies including the FDA and EMA are increasingly encouraging the implementation of PAT tools, creating additional market pull for DLS integration in bioreactor designs.
The cell and gene therapy segment represents the fastest-growing application area for bioreactors, with a projected CAGR of 15.3%. This segment particularly benefits from advanced monitoring technologies like DLS due to the high value and sensitivity of these therapeutic products. Industry experts predict that by 2025, approximately 40% of new bioreactor installations will incorporate some form of advanced real-time monitoring technology.
Pharmaceutical and biotechnology companies constitute the largest segment of bioreactor end-users, accounting for nearly 60% of the market share. This dominance stems from the rising prevalence of chronic diseases and the subsequent need for biopharmaceutical interventions. The COVID-19 pandemic has further accelerated this trend, with unprecedented demand for vaccine production capabilities worldwide.
Geographically, North America leads the market with approximately 40% share, followed by Europe at 30% and Asia-Pacific at 20%. However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years due to increasing investments in biotechnology infrastructure, particularly in China, India, and South Korea.
The market demand for advanced bioreactor technologies is increasingly focused on process optimization and real-time monitoring capabilities. According to industry surveys, 78% of biopharmaceutical manufacturers cite process optimization as their primary challenge, while 65% express interest in technologies that enable real-time monitoring without compromising sterility.
Dynamic Light Scattering (DLS) technology addresses these market needs by offering non-invasive, real-time monitoring of particle size distribution and aggregation behavior within bioreactors. This capability is particularly valuable for protein-based therapeutics production, where aggregation issues can significantly impact product quality and yield.
Market research indicates that technologies enhancing process analytical technology (PAT) capabilities, such as DLS, can reduce production costs by up to 25% through minimizing batch failures and optimizing process parameters. Furthermore, regulatory bodies including the FDA and EMA are increasingly encouraging the implementation of PAT tools, creating additional market pull for DLS integration in bioreactor designs.
The cell and gene therapy segment represents the fastest-growing application area for bioreactors, with a projected CAGR of 15.3%. This segment particularly benefits from advanced monitoring technologies like DLS due to the high value and sensitivity of these therapeutic products. Industry experts predict that by 2025, approximately 40% of new bioreactor installations will incorporate some form of advanced real-time monitoring technology.
Current DLS Implementation Challenges
Despite the significant potential of Dynamic Light Scattering (DLS) in bioreactor monitoring and optimization, several critical implementation challenges currently limit its widespread adoption in industrial settings. The primary obstacle remains the integration of DLS systems with existing bioreactor infrastructure, particularly in retrofitting scenarios where space constraints and compatibility issues with established monitoring systems create significant engineering hurdles.
Signal interpretation complexity presents another substantial challenge. DLS data from bioreactor environments contains multiple scattering effects due to high cell densities and diverse particle populations. Current algorithms struggle to deconvolute these complex signals, often resulting in ambiguous size distribution data that requires expert interpretation, limiting the technology's accessibility to non-specialists.
Real-time monitoring capabilities are hampered by processing latency issues. While DLS measurements themselves can be rapid, the computational requirements for analyzing scattered light patterns from heterogeneous biological samples create delays that reduce the technology's effectiveness for dynamic process control applications. Most commercial systems still operate with processing delays of 30-120 seconds, insufficient for capturing rapid biological events in high-throughput production environments.
Fouling and contamination of optical components represent persistent operational challenges. Protein adsorption on measurement windows and probes can gradually alter light transmission characteristics, introducing measurement drift over extended production runs. Current solutions involving automated cleaning cycles or disposable interfaces add complexity and cost while potentially interrupting continuous monitoring.
Standardization remains inadequate across the industry. Different DLS instrument manufacturers employ proprietary algorithms and reporting methods, creating inconsistencies in particle size measurements between platforms. This lack of standardization complicates technology transfer between research and production environments and hinders regulatory compliance documentation.
Cost barriers continue to impede adoption, particularly for smaller bioprocessing operations. High-precision DLS systems with sufficient sensitivity for bioreactor applications typically require investments of $50,000-150,000, with additional expenses for specialized probes, software licenses, and technical support. The return on investment remains difficult to quantify, especially for processes where traditional monitoring methods are well-established.
Validation challenges persist in demonstrating clear correlations between DLS measurements and critical quality attributes of biopharmaceutical products. Regulatory agencies require robust evidence that novel monitoring technologies provide meaningful process understanding and control, creating a significant burden of proof for early adopters of DLS technology in GMP manufacturing environments.
Signal interpretation complexity presents another substantial challenge. DLS data from bioreactor environments contains multiple scattering effects due to high cell densities and diverse particle populations. Current algorithms struggle to deconvolute these complex signals, often resulting in ambiguous size distribution data that requires expert interpretation, limiting the technology's accessibility to non-specialists.
Real-time monitoring capabilities are hampered by processing latency issues. While DLS measurements themselves can be rapid, the computational requirements for analyzing scattered light patterns from heterogeneous biological samples create delays that reduce the technology's effectiveness for dynamic process control applications. Most commercial systems still operate with processing delays of 30-120 seconds, insufficient for capturing rapid biological events in high-throughput production environments.
Fouling and contamination of optical components represent persistent operational challenges. Protein adsorption on measurement windows and probes can gradually alter light transmission characteristics, introducing measurement drift over extended production runs. Current solutions involving automated cleaning cycles or disposable interfaces add complexity and cost while potentially interrupting continuous monitoring.
Standardization remains inadequate across the industry. Different DLS instrument manufacturers employ proprietary algorithms and reporting methods, creating inconsistencies in particle size measurements between platforms. This lack of standardization complicates technology transfer between research and production environments and hinders regulatory compliance documentation.
Cost barriers continue to impede adoption, particularly for smaller bioprocessing operations. High-precision DLS systems with sufficient sensitivity for bioreactor applications typically require investments of $50,000-150,000, with additional expenses for specialized probes, software licenses, and technical support. The return on investment remains difficult to quantify, especially for processes where traditional monitoring methods are well-established.
Validation challenges persist in demonstrating clear correlations between DLS measurements and critical quality attributes of biopharmaceutical products. Regulatory agencies require robust evidence that novel monitoring technologies provide meaningful process understanding and control, creating a significant burden of proof for early adopters of DLS technology in GMP manufacturing environments.
Current DLS Integration Solutions
01 Bioreactor designs with integrated DLS monitoring systems
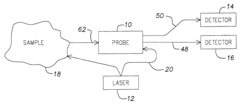
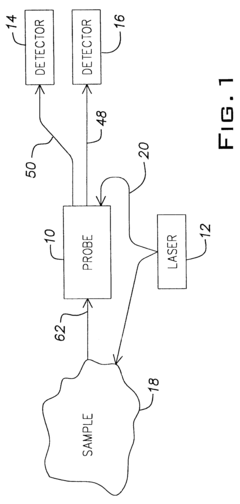
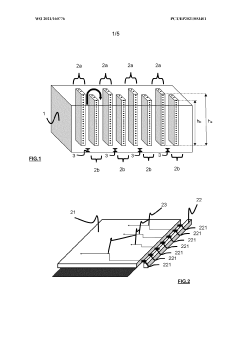
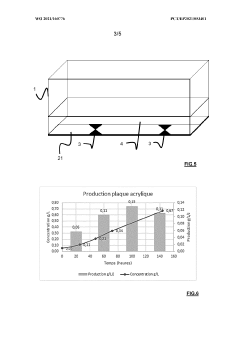
Bioreactor systems that incorporate dynamic light scattering (DLS) technology directly into the reactor design allow for real-time monitoring of particle size, aggregation, and growth during bioprocesses. These integrated systems enable continuous measurement without sample extraction, reducing contamination risks and providing immediate feedback for process control. The integration often involves specialized optical components positioned at strategic locations within the bioreactor to capture scattered light patterns from particles in suspension.- Bioreactor monitoring systems using dynamic light scattering: Dynamic light scattering technology can be integrated into bioreactor systems to monitor various parameters in real-time. These monitoring systems allow for the analysis of particle size distribution, aggregation behavior, and molecular interactions within the bioreactor environment. The technology enables continuous monitoring without disrupting the bioprocess, providing valuable data for process control and optimization.
- Specialized bioreactor designs with integrated DLS capabilities: Specialized bioreactor designs incorporate dynamic light scattering capabilities directly into the vessel structure. These integrated designs feature optical windows, fiber optic probes, or specialized chambers that allow for in-situ measurements. The integration minimizes contamination risks and enables real-time monitoring of cell cultures, fermentation processes, and protein production without sample extraction.
- Flow-through DLS systems for continuous bioprocess monitoring: Flow-through dynamic light scattering systems enable continuous monitoring of bioreactor contents by directing a sample stream through an analysis chamber. These systems can be designed as bypass loops or in-line configurations that allow for automated sampling and analysis without manual intervention. The technology provides real-time data on particle size, molecular weight, and aggregation state during bioprocessing operations.
- Advanced optical configurations for DLS in complex bioreactor media: Advanced optical configurations have been developed to overcome the challenges of performing dynamic light scattering measurements in complex bioreactor media. These designs incorporate multi-angle detection, backscattering analysis, or cross-correlation techniques to mitigate the effects of multiple scattering and high sample turbidity. Such innovations enable accurate measurements even in high-concentration cell cultures or protein solutions.
- Miniaturized and disposable DLS bioreactor systems: Miniaturized and disposable bioreactor systems with integrated dynamic light scattering capabilities have been developed for high-throughput applications. These systems feature reduced volume requirements, parallel processing capabilities, and single-use components to minimize cross-contamination. The technology enables rapid screening of culture conditions, formulation development, and process optimization while maintaining the analytical power of dynamic light scattering.
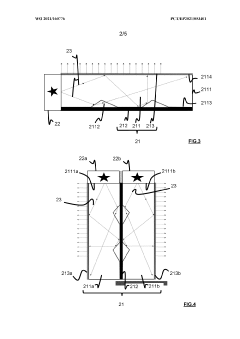
02 Flow-through DLS measurement systems for bioreactors
Flow-through dynamic light scattering systems are designed to continuously sample and analyze culture media from bioreactors. These systems typically include a circulation loop that extracts media from the bioreactor, passes it through a DLS measurement chamber, and returns it to the vessel. This approach allows for automated, periodic measurements without manual sampling while maintaining sterility. Advanced designs incorporate temperature control and pressure regulation to ensure measurement conditions match those inside the bioreactor.Expand Specific Solutions03 Multi-angle DLS detection systems for enhanced bioreactor monitoring
Multi-angle dynamic light scattering systems collect scattered light at various angles simultaneously, providing more comprehensive data about particles in bioreactor media. This approach enables better characterization of complex biological samples containing particles of different sizes and shapes. The technology can distinguish between cell growth, protein aggregation, and other phenomena occurring in bioreactors, offering more detailed insights into bioprocess dynamics and improving control strategies.Expand Specific Solutions04 Miniaturized DLS sensors for small-scale bioreactors
Miniaturized dynamic light scattering sensors are specifically designed for integration into small-scale or microfluidic bioreactors. These compact systems utilize advanced optical components and detection algorithms to achieve high sensitivity despite their reduced size. The miniaturized sensors enable DLS monitoring in space-constrained applications such as parallel small-scale bioreactors used in process development and optimization studies, providing valuable data for scale-up decisions.Expand Specific Solutions05 AI-enhanced DLS data processing for bioreactor control
Advanced bioreactor designs incorporate artificial intelligence and machine learning algorithms to process dynamic light scattering data in real-time. These systems can identify patterns in particle behavior that indicate changes in culture conditions, predict potential issues before they become critical, and automatically adjust bioreactor parameters to maintain optimal growth conditions. The integration of AI with DLS technology enables more sophisticated bioprocess control strategies and facilitates the development of fully automated bioreactor systems.Expand Specific Solutions
Leading Companies in DLS Bioreactor Technology
Dynamic Light Scattering (DLS) technology for bioreactor design enhancement is currently in a growth phase, with an expanding market driven by increasing demand for biopharmaceutical production optimization. The global market is experiencing steady growth as biomanufacturing becomes more sophisticated. Technologically, DLS applications in bioreactors show varying maturity levels across different implementations. Leading players include Sartorius Stedim Biotech, which has integrated DLS into advanced monitoring systems, and Ningaloo Biosystems, developing cyber bioproduction systems with real-time control capabilities. Academic institutions like South China Normal University and Colorado State University are contributing fundamental research, while companies like Philips and Hitachi provide complementary technological expertise. The collaboration between research institutions and industrial players indicates a technology approaching commercial viability but still undergoing refinement for widespread adoption.
Sartorius Stedim Biotech GmbH
Technical Solution: Sartorius has developed advanced Dynamic Light Scattering (DLS) integration systems for real-time bioprocess monitoring in their bioreactors. Their technology employs in-situ probes that can continuously measure particle size distributions and molecular interactions without sample extraction. The system incorporates fiber optic technology to transmit laser light directly into bioreactors, with specialized algorithms that can distinguish between cellular components and product molecules even in high-density cultures. Their BioPAT® Spectro platform integrates DLS with spectroscopic methods, allowing simultaneous monitoring of multiple critical quality attributes. This technology enables automated feedback control systems that can adjust process parameters based on real-time DLS measurements, significantly improving process consistency and product quality in continuous bioprocessing applications.
Strengths: Industry-leading integration of DLS with established bioprocess control systems; exceptional measurement precision in high-cell-density environments; validated implementation in GMP manufacturing. Weaknesses: Higher implementation costs compared to offline systems; requires specialized training for optimal operation; potential challenges with fouling during extended continuous processing.
Fraunhofer-Gesellschaft eV
Technical Solution: Fraunhofer has pioneered innovative DLS technologies specifically designed for bioreactor optimization through their BioReactor DLS Monitoring System. Their approach utilizes multi-angle DLS measurements combined with advanced correlation algorithms to provide detailed insights into particle dynamics within complex biological systems. The technology employs specialized optical configurations that can penetrate through high-density cell cultures while minimizing multiple scattering effects. Their system incorporates machine learning algorithms that continuously improve measurement accuracy by adapting to changing media conditions throughout the bioprocess. Fraunhofer has also developed specialized flow-through cells that enable representative sampling from bioreactors while maintaining sterility and process integrity. This technology has been successfully implemented in various scales from bench-top to industrial bioreactors, demonstrating scalability across different bioprocessing platforms.
Strengths: Exceptional adaptability to different bioreactor types and scales; superior data processing algorithms for complex biological media; strong integration capabilities with existing bioprocess equipment. Weaknesses: Requires significant customization for specific applications; higher initial investment compared to conventional monitoring systems; limited commercial deployment compared to established industry players.
Key DLS Patents and Technical Innovations
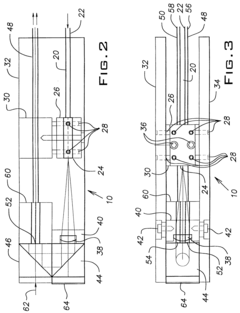
Dynamic light scattering homodyne probe
PatentInactiveUS6469787B1
Innovation
- A novel optical probe design incorporating a beam splitter system with input and output optical fibers, a lens, and adjustable components allows for simultaneous homodyne detection and cross-correlation, stabilizing the phase relationship between local oscillators and enhancing signal-to-noise ratio by using a coherent optical field, thereby overcoming past instabilities and improving measurement accuracy across all challenging regimes.
Reactor having an optimized lighting device
PatentWO2021160776A1
Innovation
- A reactor design featuring micro-etched light diffusers with light-emitting diodes arranged along the edges of transparent plates, allowing for homogeneous light distribution and increased illuminated surface area, optimizing photon energy use and reducing operational costs.
Scale-up Considerations for DLS-Enhanced Bioreactors
Scaling up DLS-enhanced bioreactors from laboratory to industrial scale presents significant engineering challenges that must be addressed systematically. The transition requires careful consideration of geometric similarity, maintaining consistent hydrodynamic conditions, and preserving the integrity of DLS measurement capabilities across different scales.
When increasing bioreactor dimensions, the light scattering properties can be dramatically affected due to changes in optical path length and scattering angles. Industrial-scale bioreactors typically feature thicker walls and greater distances between measurement points, necessitating adjustments to laser power, detector sensitivity, and signal processing algorithms to maintain measurement accuracy.
The positioning of DLS probes becomes increasingly critical in larger vessels. While a single probe might suffice for laboratory-scale operations, industrial bioreactors may require multiple strategically placed probes to account for spatial heterogeneity. This multi-point measurement approach enables more comprehensive monitoring but introduces complexity in data integration and interpretation.
Computational fluid dynamics (CFD) modeling plays an essential role in scale-up processes, allowing engineers to predict flow patterns, mixing efficiency, and potential dead zones where particles might accumulate. These models must incorporate the specific optical properties relevant to DLS measurements to ensure that scaling decisions preserve measurement integrity.
Material selection for larger bioreactors must balance structural requirements with optical considerations. Specialized viewing ports or windows may need to be incorporated into vessel designs to facilitate DLS measurements without compromising structural integrity or sterility. These materials must withstand sterilization procedures while maintaining optical clarity.
The increased volume in industrial bioreactors introduces longer mixing times, which can affect the homogeneity of particle distribution being measured by DLS. Engineers must implement enhanced mixing strategies that maintain uniform conditions without introducing excessive shear stress that could damage sensitive biological materials or create microbubbles that interfere with light scattering measurements.
Data handling infrastructure must scale accordingly, as industrial-scale DLS-enhanced bioreactors generate substantially more data than laboratory counterparts. Real-time processing capabilities, robust storage solutions, and advanced analytics platforms become necessary components of the scaled-up system, requiring significant investment in computing infrastructure.
Economic considerations ultimately drive scale-up decisions, with capital expenditure for DLS implementation increasing non-linearly with bioreactor size. A thorough cost-benefit analysis must weigh the enhanced process control and product quality against the substantial investment required for industrial implementation.
When increasing bioreactor dimensions, the light scattering properties can be dramatically affected due to changes in optical path length and scattering angles. Industrial-scale bioreactors typically feature thicker walls and greater distances between measurement points, necessitating adjustments to laser power, detector sensitivity, and signal processing algorithms to maintain measurement accuracy.
The positioning of DLS probes becomes increasingly critical in larger vessels. While a single probe might suffice for laboratory-scale operations, industrial bioreactors may require multiple strategically placed probes to account for spatial heterogeneity. This multi-point measurement approach enables more comprehensive monitoring but introduces complexity in data integration and interpretation.
Computational fluid dynamics (CFD) modeling plays an essential role in scale-up processes, allowing engineers to predict flow patterns, mixing efficiency, and potential dead zones where particles might accumulate. These models must incorporate the specific optical properties relevant to DLS measurements to ensure that scaling decisions preserve measurement integrity.
Material selection for larger bioreactors must balance structural requirements with optical considerations. Specialized viewing ports or windows may need to be incorporated into vessel designs to facilitate DLS measurements without compromising structural integrity or sterility. These materials must withstand sterilization procedures while maintaining optical clarity.
The increased volume in industrial bioreactors introduces longer mixing times, which can affect the homogeneity of particle distribution being measured by DLS. Engineers must implement enhanced mixing strategies that maintain uniform conditions without introducing excessive shear stress that could damage sensitive biological materials or create microbubbles that interfere with light scattering measurements.
Data handling infrastructure must scale accordingly, as industrial-scale DLS-enhanced bioreactors generate substantially more data than laboratory counterparts. Real-time processing capabilities, robust storage solutions, and advanced analytics platforms become necessary components of the scaled-up system, requiring significant investment in computing infrastructure.
Economic considerations ultimately drive scale-up decisions, with capital expenditure for DLS implementation increasing non-linearly with bioreactor size. A thorough cost-benefit analysis must weigh the enhanced process control and product quality against the substantial investment required for industrial implementation.
Regulatory Compliance for DLS Bioprocessing Systems
The regulatory landscape for Dynamic Light Scattering (DLS) in bioreactor applications presents a complex framework that manufacturers and operators must navigate. FDA regulations in the United States require that all analytical methods used in bioprocessing, including DLS technology, must be validated according to ICH Q2(R1) guidelines. This validation process must demonstrate accuracy, precision, specificity, linearity, range, and robustness of the DLS measurements when applied to bioreactor monitoring systems.
In the European Union, the European Medicines Agency (EMA) mandates compliance with GMP Annex 15, which specifically addresses qualification and validation of analytical equipment used in bioprocessing. DLS systems integrated into bioreactors must meet these requirements, with particular emphasis on data integrity and traceability as outlined in EU GMP Annex 11 for computerized systems.
ISO standards also play a crucial role in regulatory compliance for DLS bioprocessing systems. ISO 13485 for medical devices and ISO 14644 for cleanroom environments establish parameters that affect the design and operation of DLS-enhanced bioreactors. Additionally, ISO 21501-2, which addresses light scattering aerosol spectrometers, provides relevant guidance for calibration and performance verification of DLS instruments.
Regulatory bodies increasingly focus on Process Analytical Technology (PAT) frameworks, as outlined in FDA's guidance documents. DLS systems must be designed to support Quality by Design (QbD) principles, allowing for real-time monitoring and control of critical quality attributes during bioprocessing. This requires robust data management systems that comply with 21 CFR Part 11 for electronic records and signatures.
Risk management is another critical aspect of regulatory compliance. Manufacturers must conduct comprehensive risk assessments following ICH Q9 guidelines to identify potential failure modes in DLS measurement systems and implement appropriate control strategies. This includes validation of software algorithms used for particle size analysis and distribution calculations.
Global harmonization efforts, such as those through the International Council for Harmonisation (ICH), are working to standardize requirements across different regions. However, significant regional variations remain, particularly regarding the implementation of continuous manufacturing technologies that incorporate real-time DLS monitoring. Companies developing DLS-enhanced bioreactors must therefore maintain awareness of evolving regulatory expectations across all target markets.
Compliance documentation for DLS systems must include detailed validation protocols, calibration procedures, maintenance schedules, and training requirements for operators. These documents form part of the broader quality management system and may be subject to inspection during regulatory audits. Manufacturers should establish clear change control procedures to manage updates to DLS hardware or software while maintaining regulatory compliance throughout the system lifecycle.
In the European Union, the European Medicines Agency (EMA) mandates compliance with GMP Annex 15, which specifically addresses qualification and validation of analytical equipment used in bioprocessing. DLS systems integrated into bioreactors must meet these requirements, with particular emphasis on data integrity and traceability as outlined in EU GMP Annex 11 for computerized systems.
ISO standards also play a crucial role in regulatory compliance for DLS bioprocessing systems. ISO 13485 for medical devices and ISO 14644 for cleanroom environments establish parameters that affect the design and operation of DLS-enhanced bioreactors. Additionally, ISO 21501-2, which addresses light scattering aerosol spectrometers, provides relevant guidance for calibration and performance verification of DLS instruments.
Regulatory bodies increasingly focus on Process Analytical Technology (PAT) frameworks, as outlined in FDA's guidance documents. DLS systems must be designed to support Quality by Design (QbD) principles, allowing for real-time monitoring and control of critical quality attributes during bioprocessing. This requires robust data management systems that comply with 21 CFR Part 11 for electronic records and signatures.
Risk management is another critical aspect of regulatory compliance. Manufacturers must conduct comprehensive risk assessments following ICH Q9 guidelines to identify potential failure modes in DLS measurement systems and implement appropriate control strategies. This includes validation of software algorithms used for particle size analysis and distribution calculations.
Global harmonization efforts, such as those through the International Council for Harmonisation (ICH), are working to standardize requirements across different regions. However, significant regional variations remain, particularly regarding the implementation of continuous manufacturing technologies that incorporate real-time DLS monitoring. Companies developing DLS-enhanced bioreactors must therefore maintain awareness of evolving regulatory expectations across all target markets.
Compliance documentation for DLS systems must include detailed validation protocols, calibration procedures, maintenance schedules, and training requirements for operators. These documents form part of the broader quality management system and may be subject to inspection during regulatory audits. Manufacturers should establish clear change control procedures to manage updates to DLS hardware or software while maintaining regulatory compliance throughout the system lifecycle.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!