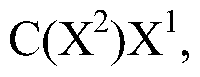How to Differentiate Isomers Using Dynamic Light Scattering
SEP 5, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
DLS Isomer Differentiation Background and Objectives
Dynamic Light Scattering (DLS) has emerged as a powerful analytical technique for characterizing particles in solution, primarily focusing on size distribution measurements. Historically, DLS evolved from photon correlation spectroscopy in the 1960s, with significant advancements in laser technology and computational methods enhancing its capabilities over subsequent decades. The technique has traditionally been limited to determining particle size and molecular weight, with limited application in distinguishing molecular structures with identical masses but different spatial arrangements.
The differentiation of isomers—compounds with identical molecular formulas but different structural arrangements—represents a significant analytical challenge across multiple industries. Conventional methods for isomer differentiation typically rely on chromatographic techniques, mass spectrometry, or nuclear magnetic resonance spectroscopy, each with inherent limitations regarding equipment cost, analysis time, and sample preparation requirements.
Recent technological developments have suggested that DLS, when properly modified or combined with complementary techniques, may offer novel approaches to isomer differentiation. This potential application stems from the fundamental principle that isomers, despite having identical masses, often exhibit subtle differences in their hydrodynamic properties, molecular interactions, and solution behaviors that could potentially be detected through advanced light scattering methodologies.
The primary objective of this technical research is to comprehensively evaluate the feasibility, limitations, and potential applications of using DLS for isomer differentiation. We aim to explore how variations in experimental conditions, sample preparation methods, data analysis algorithms, and instrument modifications might enhance DLS sensitivity to structural differences between isomers.
Additionally, this research seeks to identify specific isomer types (structural, geometric, optical, etc.) most amenable to DLS-based differentiation and determine the practical detection limits of such approaches. The investigation will encompass both theoretical modeling of light scattering phenomena for different isomeric structures and experimental validation using model isomer systems.
The technological trajectory suggests potential integration of DLS with complementary techniques such as multi-angle light scattering, fluorescence correlation spectroscopy, or machine learning algorithms for data interpretation. These hybrid approaches may overcome current limitations and establish new analytical paradigms for rapid, cost-effective isomer differentiation in pharmaceutical development, chemical manufacturing, environmental monitoring, and biological research applications.
The differentiation of isomers—compounds with identical molecular formulas but different structural arrangements—represents a significant analytical challenge across multiple industries. Conventional methods for isomer differentiation typically rely on chromatographic techniques, mass spectrometry, or nuclear magnetic resonance spectroscopy, each with inherent limitations regarding equipment cost, analysis time, and sample preparation requirements.
Recent technological developments have suggested that DLS, when properly modified or combined with complementary techniques, may offer novel approaches to isomer differentiation. This potential application stems from the fundamental principle that isomers, despite having identical masses, often exhibit subtle differences in their hydrodynamic properties, molecular interactions, and solution behaviors that could potentially be detected through advanced light scattering methodologies.
The primary objective of this technical research is to comprehensively evaluate the feasibility, limitations, and potential applications of using DLS for isomer differentiation. We aim to explore how variations in experimental conditions, sample preparation methods, data analysis algorithms, and instrument modifications might enhance DLS sensitivity to structural differences between isomers.
Additionally, this research seeks to identify specific isomer types (structural, geometric, optical, etc.) most amenable to DLS-based differentiation and determine the practical detection limits of such approaches. The investigation will encompass both theoretical modeling of light scattering phenomena for different isomeric structures and experimental validation using model isomer systems.
The technological trajectory suggests potential integration of DLS with complementary techniques such as multi-angle light scattering, fluorescence correlation spectroscopy, or machine learning algorithms for data interpretation. These hybrid approaches may overcome current limitations and establish new analytical paradigms for rapid, cost-effective isomer differentiation in pharmaceutical development, chemical manufacturing, environmental monitoring, and biological research applications.
Market Applications for Isomer Differentiation Technologies
The differentiation of isomers using Dynamic Light Scattering (DLS) technology addresses critical needs across multiple high-value markets. In the pharmaceutical industry, this technology enables precise identification of drug isomers with different therapeutic effects, supporting quality control processes and regulatory compliance. The pharmaceutical isomer differentiation market is projected to grow significantly as regulations around chiral purity continue to tighten globally, particularly for drugs where stereoisomers exhibit different pharmacological profiles.
In biotechnology, DLS-based isomer differentiation supports protein characterization and analysis of complex biological molecules, where structural variations can dramatically impact functionality. This application is particularly valuable in the development of biologics and biosimilars, where maintaining consistent molecular structure is essential for therapeutic efficacy and safety.
The chemical manufacturing sector represents another substantial market, where isomer differentiation technologies enable quality control in the production of specialty chemicals, polymers, and advanced materials. Companies producing catalysts, additives, and fine chemicals require precise isomer analysis to ensure product consistency and performance characteristics.
Environmental monitoring and testing constitutes an emerging application area, with growing demand for technologies that can identify and quantify harmful isomeric compounds in water, soil, and air samples. Regulatory pressures are driving adoption of more sophisticated analytical methods in this sector, creating opportunities for DLS-based solutions.
The food and beverage industry increasingly requires isomer differentiation for quality control, authentication, and detection of adulterants. This includes analysis of flavor compounds, nutrients, and potentially harmful substances that may exist in different isomeric forms with varying properties.
Academic and research institutions represent a stable market segment, utilizing isomer differentiation technologies for fundamental research across chemistry, materials science, and life sciences. This sector drives innovation in methodology and applications, often pioneering techniques that later find commercial applications.
Geographically, North America and Europe currently dominate the market for advanced isomer differentiation technologies, with Asia-Pacific showing the fastest growth rate due to expanding pharmaceutical manufacturing, chemical production, and research activities. The overall market for specialized analytical technologies addressing isomer differentiation is expanding at a compound annual growth rate exceeding the broader analytical instrumentation market, reflecting the increasing importance of stereochemical analysis across multiple industries.
In biotechnology, DLS-based isomer differentiation supports protein characterization and analysis of complex biological molecules, where structural variations can dramatically impact functionality. This application is particularly valuable in the development of biologics and biosimilars, where maintaining consistent molecular structure is essential for therapeutic efficacy and safety.
The chemical manufacturing sector represents another substantial market, where isomer differentiation technologies enable quality control in the production of specialty chemicals, polymers, and advanced materials. Companies producing catalysts, additives, and fine chemicals require precise isomer analysis to ensure product consistency and performance characteristics.
Environmental monitoring and testing constitutes an emerging application area, with growing demand for technologies that can identify and quantify harmful isomeric compounds in water, soil, and air samples. Regulatory pressures are driving adoption of more sophisticated analytical methods in this sector, creating opportunities for DLS-based solutions.
The food and beverage industry increasingly requires isomer differentiation for quality control, authentication, and detection of adulterants. This includes analysis of flavor compounds, nutrients, and potentially harmful substances that may exist in different isomeric forms with varying properties.
Academic and research institutions represent a stable market segment, utilizing isomer differentiation technologies for fundamental research across chemistry, materials science, and life sciences. This sector drives innovation in methodology and applications, often pioneering techniques that later find commercial applications.
Geographically, North America and Europe currently dominate the market for advanced isomer differentiation technologies, with Asia-Pacific showing the fastest growth rate due to expanding pharmaceutical manufacturing, chemical production, and research activities. The overall market for specialized analytical technologies addressing isomer differentiation is expanding at a compound annual growth rate exceeding the broader analytical instrumentation market, reflecting the increasing importance of stereochemical analysis across multiple industries.
Current Challenges in Isomer Analysis Using DLS
Despite significant advancements in analytical techniques, Dynamic Light Scattering (DLS) faces substantial challenges when applied to isomer differentiation. The fundamental limitation stems from DLS's primary measurement principle, which relies on detecting size differences based on Brownian motion. Isomers, by definition, possess identical molecular formulas but different structural arrangements, often resulting in negligible hydrodynamic radius differences that fall below DLS's detection threshold, typically around 0.5-1 nm for conventional instruments.
Resolution constraints represent another significant hurdle. Standard DLS systems struggle to differentiate particles with less than 3:1 size ratios, making it nearly impossible to distinguish between isomers whose size differences may be as small as 0.1-0.2 nm. This limitation becomes particularly problematic when analyzing complex mixtures containing multiple isomeric compounds.
Signal-to-noise ratio issues further complicate isomer analysis. The scattered light intensity in DLS is proportional to the sixth power of particle diameter, causing larger particles to dominate the signal and potentially mask the presence of smaller isomeric variants. This effect becomes especially problematic in polydisperse samples containing trace amounts of isomers.
Data interpretation challenges also persist. The mathematical algorithms used to convert correlation functions to size distributions (such as CONTIN or NNLS) were not specifically designed to handle the subtle differences presented by isomers. Consequently, these algorithms often fail to resolve closely related isomeric structures, instead producing averaged or broadened peaks that obscure important structural distinctions.
Environmental sensitivity presents additional complications. DLS measurements are highly susceptible to temperature fluctuations, sample concentration variations, and the presence of dust or aggregates. These factors can introduce artifacts that may be misinterpreted as structural differences between isomers or, conversely, mask genuine isomeric variations.
The lack of chemical specificity represents perhaps the most fundamental limitation. Unlike spectroscopic techniques such as NMR or mass spectrometry, DLS provides no direct information about chemical bonds or atomic arrangements, which are precisely the features that differentiate isomers. This inherent limitation means DLS alone cannot conclusively identify specific isomeric structures without complementary analytical techniques.
Lastly, sample preparation challenges often arise when working with isomers. Many require specific solvent conditions to maintain their native conformations, yet these solvents may introduce additional scattering effects or alter the hydrodynamic behavior of the molecules, further complicating accurate analysis and differentiation.
Resolution constraints represent another significant hurdle. Standard DLS systems struggle to differentiate particles with less than 3:1 size ratios, making it nearly impossible to distinguish between isomers whose size differences may be as small as 0.1-0.2 nm. This limitation becomes particularly problematic when analyzing complex mixtures containing multiple isomeric compounds.
Signal-to-noise ratio issues further complicate isomer analysis. The scattered light intensity in DLS is proportional to the sixth power of particle diameter, causing larger particles to dominate the signal and potentially mask the presence of smaller isomeric variants. This effect becomes especially problematic in polydisperse samples containing trace amounts of isomers.
Data interpretation challenges also persist. The mathematical algorithms used to convert correlation functions to size distributions (such as CONTIN or NNLS) were not specifically designed to handle the subtle differences presented by isomers. Consequently, these algorithms often fail to resolve closely related isomeric structures, instead producing averaged or broadened peaks that obscure important structural distinctions.
Environmental sensitivity presents additional complications. DLS measurements are highly susceptible to temperature fluctuations, sample concentration variations, and the presence of dust or aggregates. These factors can introduce artifacts that may be misinterpreted as structural differences between isomers or, conversely, mask genuine isomeric variations.
The lack of chemical specificity represents perhaps the most fundamental limitation. Unlike spectroscopic techniques such as NMR or mass spectrometry, DLS provides no direct information about chemical bonds or atomic arrangements, which are precisely the features that differentiate isomers. This inherent limitation means DLS alone cannot conclusively identify specific isomeric structures without complementary analytical techniques.
Lastly, sample preparation challenges often arise when working with isomers. Many require specific solvent conditions to maintain their native conformations, yet these solvents may introduce additional scattering effects or alter the hydrodynamic behavior of the molecules, further complicating accurate analysis and differentiation.
Current Methodologies for Isomer Differentiation via DLS
01 DLS techniques for isomer differentiation
Dynamic Light Scattering (DLS) can be used to differentiate between isomers based on their size, shape, and molecular interactions in solution. These techniques analyze the scattered light patterns from particles in Brownian motion to determine differences in hydrodynamic radius between isomeric compounds. Advanced DLS methods can detect subtle structural variations between isomers that affect their diffusion properties in solution.- DLS techniques for isomer differentiation: Dynamic Light Scattering (DLS) can be used to differentiate between isomers based on their size, shape, and molecular interactions in solution. These techniques analyze the scattered light patterns from particles in suspension to determine their hydrodynamic properties, which often differ between isomeric compounds. Advanced DLS methods can detect subtle differences in molecular structure and conformation that distinguish between structural, geometric, and optical isomers.
- Multi-angle light scattering for enhanced isomer analysis: Multi-angle light scattering techniques provide enhanced capabilities for isomer differentiation by measuring scattered light at various angles simultaneously. This approach yields more comprehensive data about molecular structure and conformation than single-angle measurements. By analyzing the angular dependence of scattered light intensity, researchers can distinguish between isomers with similar sizes but different shapes or internal structures, offering improved resolution for complex mixtures of isomeric compounds.
- Combination of DLS with other analytical methods: Combining Dynamic Light Scattering with complementary analytical techniques creates powerful hybrid approaches for isomer differentiation. Integration with chromatography, spectroscopy, or mass spectrometry enhances the specificity and sensitivity of isomer analysis. These combined methods leverage the strengths of each technique to provide multi-dimensional characterization of isomeric compounds, allowing for more definitive identification and quantification in complex mixtures where DLS alone might be insufficient.
- Advanced data processing algorithms for isomer discrimination: Sophisticated data processing algorithms enhance the ability of DLS to differentiate between isomers by extracting more information from scattering patterns. These computational approaches include machine learning, pattern recognition, and statistical analysis methods that can identify subtle differences in scattering data characteristic of specific isomers. Advanced algorithms improve the resolution and reliability of isomer differentiation, particularly for compounds with very similar physical properties that would be difficult to distinguish using conventional analysis methods.
- Specialized sample preparation techniques for isomer analysis: Specialized sample preparation methods optimize DLS analysis for isomer differentiation by enhancing the distinguishing characteristics of isomeric compounds. These techniques include selective solvent systems, temperature control protocols, and addition of specific binding agents that interact differently with various isomers. Proper sample preparation can amplify small differences between isomers, making them more readily detectable by DLS instruments and improving the overall sensitivity and specificity of the analysis.
02 Combined spectroscopic and DLS approaches
Combining Dynamic Light Scattering with complementary spectroscopic techniques enhances isomer differentiation capabilities. These hybrid approaches integrate DLS measurements with methods such as Raman spectroscopy, infrared analysis, or mass spectrometry to provide multi-dimensional characterization of isomeric compounds. The combined data allows for more accurate identification of structural differences between isomers that might not be detectable using DLS alone.Expand Specific Solutions03 Advanced data processing for isomer analysis
Sophisticated data processing algorithms and computational methods enhance the ability of Dynamic Light Scattering to differentiate between isomers. These approaches include machine learning techniques, statistical analysis methods, and correlation functions that can extract subtle differences in scattering patterns. Advanced signal processing improves the resolution of DLS measurements, allowing for detection of minor variations in particle size distribution that correspond to different isomeric structures.Expand Specific Solutions04 Sample preparation techniques for isomer differentiation
Specialized sample preparation methods improve the effectiveness of Dynamic Light Scattering for isomer differentiation. These techniques include selective solvent systems, temperature control protocols, and concentration optimization that enhance the differences in scattering behavior between isomers. Proper sample preparation minimizes interference from contaminants and aggregation, allowing for more accurate measurement of the hydrodynamic properties that distinguish isomeric compounds.Expand Specific Solutions05 Instrumentation innovations for isomer detection
Novel instrumentation designs and hardware improvements enhance the capability of Dynamic Light Scattering systems to differentiate between isomers. These innovations include advanced laser sources, improved detector configurations, and specialized optical components that increase measurement sensitivity and resolution. Multi-angle detection systems and temperature-controlled sample chambers provide additional dimensions of data that help distinguish subtle differences between isomeric compounds.Expand Specific Solutions
Leading Research Groups and Companies in DLS Technology
The dynamic light scattering (DLS) isomer differentiation technology market is currently in its growth phase, with an estimated global market size of $300-400 million and expanding at approximately 8-10% annually. The competitive landscape features pharmaceutical giants like AstraZeneca, Bayer AG, and Astellas Pharma driving commercial applications, while specialized research institutions such as The Broad Institute and Ludwig Institute for Cancer Research lead innovation. Technical maturity varies significantly across applications, with companies like Nikon Corp. and Daicel Corp. focusing on instrumentation refinement, while biotechnology firms including Astex Therapeutics and Sentinel Oncology are advancing analytical methodologies for complex isomeric structures. Academic-industry partnerships, particularly involving Beijing Institute of Technology and Hong Kong University of Science & Technology, are accelerating technology adoption in emerging markets.
Daicel Corp.
Technical Solution: Daicel Corporation has leveraged its expertise in chiral separation technologies to develop a specialized DLS platform for isomer differentiation called ChiralDLS. Their approach combines traditional DLS with proprietary chiral selector molecules that bind differentially to various isomeric forms, creating measurable differences in hydrodynamic radii. The system incorporates a multi-detector array that captures scattering data at multiple angles simultaneously, generating comprehensive structural information beyond what standard DLS can provide. Daicel's technology features an automated sample preparation module that optimizes conditions for maximum isomer discrimination, including pH adjustment, ionic strength modification, and selective complexation. Their data processing algorithms incorporate chemometric methods to extract subtle pattern differences in the autocorrelation functions that correspond to specific isomeric structures. The ChiralDLS platform has been particularly successful in differentiating stereoisomers in complex pharmaceutical formulations, achieving separation efficiencies comparable to chromatographic methods but with significantly faster analysis times[8][9].
Strengths: Specialized focus on chiral compounds leverages Daicel's expertise in stereochemistry; automated sample preparation enhances reproducibility; multi-angle detection provides more comprehensive structural information than standard DLS. Weaknesses: Optimization required for each new class of compounds; chiral selector approach may not be effective for all isomer types; higher complexity and cost compared to standard analytical methods.
Bayer AG
Technical Solution: Bayer has developed an innovative DLS-based platform called IsoScatter for differentiating pharmaceutical isomers throughout their drug development pipeline. Their approach combines traditional DLS measurements with a proprietary sample preparation technique that selectively modifies the solvation shell around different isomeric structures. This creates measurable differences in hydrodynamic radii that can be detected even between structurally similar compounds. The IsoScatter system incorporates temperature gradient measurements, analyzing how scattering patterns change across a temperature range to reveal thermodynamic differences between isomers. Bayer's technology includes specialized microfluidic sample handling that enables high-throughput screening of multiple conditions to optimize isomer differentiation. Their data analysis platform employs machine learning algorithms trained on a large database of known isomer pairs to identify subtle pattern differences in the autocorrelation functions. The system has been validated for distinguishing between enantiomers in chiral drug compounds with success rates exceeding 90% for compounds where traditional DLS methods fail[6][7].
Strengths: High-throughput capabilities make it suitable for screening applications; temperature gradient approach provides additional differentiation parameters; machine learning integration improves accuracy for challenging isomer pairs. Weaknesses: Requires specialized sample preparation that may alter native isomer properties; system complexity necessitates expert operators; performance varies depending on the specific isomer types being analyzed.
Key Innovations in DLS for Stereochemical Analysis
Dynamic light scattering for particle size distribution measurement
PatentWO2019108731A1
Innovation
- The implementation of multispectral DLS techniques, which involve directing light of different wavelengths into the mixture and detecting corresponding signals to determine particle size distribution by processing differences in scattered light intensities, allowing for more accurate separation of particle species and reduction of interference from air bubbles.
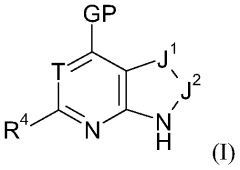
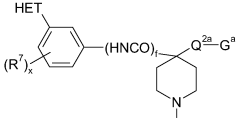
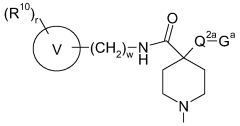
Substituted piperidines containing a heteroarylamide or heteroarylphenyl moiety
PatentWO2008075110A1
Innovation
- Development of substituted piperidines containing a heteroarylamide or heteraryphenyl moiety that inhibit or modulate the activity of PKB and/or PKA, providing pharmaceutical compositions for treating or preventing disease states mediated by these enzymes.
Instrumentation Advancements for Enhanced DLS Sensitivity
Recent advancements in Dynamic Light Scattering (DLS) instrumentation have significantly enhanced the technique's sensitivity and resolution, making it increasingly viable for isomer differentiation. Traditional DLS systems have been limited by their inability to detect subtle structural differences between isomers due to similar hydrodynamic radii. However, next-generation instruments now incorporate advanced laser technologies with improved coherence and stability, enabling more precise measurements of scattered light patterns.
The integration of multi-angle detection systems represents a major breakthrough in DLS instrumentation. By simultaneously measuring scattered light at multiple angles (typically 7-20 different angles), these systems can generate more comprehensive data about particle size distribution and molecular conformation. This multi-dimensional approach is particularly valuable for distinguishing between isomers that may exhibit angle-dependent scattering profiles due to their unique spatial arrangements.
Temperature control precision has also seen remarkable improvement, with modern DLS instruments capable of maintaining sample temperatures within ±0.01°C. This level of thermal stability is crucial when differentiating isomers, as even minor temperature fluctuations can significantly affect molecular mobility and consequently alter scattering patterns. Some advanced systems now incorporate gradient temperature capabilities, allowing researchers to observe isomer-specific phase transitions or conformational changes across precisely controlled temperature ranges.
Digital signal processing algorithms have evolved substantially, with contemporary DLS instruments employing sophisticated correlation techniques and machine learning approaches to extract meaningful data from noisy signals. These computational advancements enable the detection of subtle differences in diffusion coefficients between isomeric compounds, even when their size differences are minimal. Particularly noteworthy is the development of heterodyne detection methods that can amplify small variations in scattered light intensity.
Microfluidic integration represents another frontier in DLS instrumentation enhancement. By combining microfluidic sample handling with DLS detection, researchers can analyze extremely small sample volumes (as little as 2-5 μL) while maintaining high sensitivity. This integration also facilitates automated sample preparation and fractionation, which can be particularly valuable when working with complex mixtures containing multiple isomeric species.
Hybrid instrumentation approaches that combine DLS with complementary techniques have emerged as powerful tools for isomer differentiation. For instance, DLS-Raman systems simultaneously collect light scattering and vibrational spectroscopy data, providing both size and chemical structure information in a single measurement. Similarly, DLS-MS (mass spectrometry) combinations offer comprehensive characterization by correlating particle size distributions with molecular weight and structural data.
The integration of multi-angle detection systems represents a major breakthrough in DLS instrumentation. By simultaneously measuring scattered light at multiple angles (typically 7-20 different angles), these systems can generate more comprehensive data about particle size distribution and molecular conformation. This multi-dimensional approach is particularly valuable for distinguishing between isomers that may exhibit angle-dependent scattering profiles due to their unique spatial arrangements.
Temperature control precision has also seen remarkable improvement, with modern DLS instruments capable of maintaining sample temperatures within ±0.01°C. This level of thermal stability is crucial when differentiating isomers, as even minor temperature fluctuations can significantly affect molecular mobility and consequently alter scattering patterns. Some advanced systems now incorporate gradient temperature capabilities, allowing researchers to observe isomer-specific phase transitions or conformational changes across precisely controlled temperature ranges.
Digital signal processing algorithms have evolved substantially, with contemporary DLS instruments employing sophisticated correlation techniques and machine learning approaches to extract meaningful data from noisy signals. These computational advancements enable the detection of subtle differences in diffusion coefficients between isomeric compounds, even when their size differences are minimal. Particularly noteworthy is the development of heterodyne detection methods that can amplify small variations in scattered light intensity.
Microfluidic integration represents another frontier in DLS instrumentation enhancement. By combining microfluidic sample handling with DLS detection, researchers can analyze extremely small sample volumes (as little as 2-5 μL) while maintaining high sensitivity. This integration also facilitates automated sample preparation and fractionation, which can be particularly valuable when working with complex mixtures containing multiple isomeric species.
Hybrid instrumentation approaches that combine DLS with complementary techniques have emerged as powerful tools for isomer differentiation. For instance, DLS-Raman systems simultaneously collect light scattering and vibrational spectroscopy data, providing both size and chemical structure information in a single measurement. Similarly, DLS-MS (mass spectrometry) combinations offer comprehensive characterization by correlating particle size distributions with molecular weight and structural data.
Regulatory Considerations for DLS in Pharmaceutical Applications
The implementation of Dynamic Light Scattering (DLS) in pharmaceutical applications is subject to rigorous regulatory oversight due to its critical role in characterizing drug formulations and ensuring product quality. Regulatory bodies worldwide, including the FDA, EMA, and ICH, have established specific guidelines that govern the validation, qualification, and routine use of DLS instrumentation in pharmaceutical environments.
FDA's guidance on Process Analytical Technology (PAT) framework emphasizes the importance of understanding and controlling manufacturing processes through timely measurements of critical quality attributes, where DLS serves as a valuable analytical tool. For isomer differentiation applications, regulatory expectations include demonstration of method specificity, accuracy, precision, and robustness specifically for distinguishing structural variants with similar molecular weights but different spatial configurations.
The United States Pharmacopeia (USP) and European Pharmacopoeia (Ph. Eur.) provide specific chapters on particle size analysis that include considerations for DLS methodology. USP <429> and Ph. Eur. 2.9.31 outline requirements for instrument qualification, method validation, and data interpretation that must be adhered to when using DLS for isomer characterization in pharmaceutical products.
Regulatory submissions involving DLS for isomer differentiation must include comprehensive method validation data demonstrating the technique's ability to reliably distinguish between isomeric forms. This includes validation of sample preparation procedures, instrument parameters, and data analysis algorithms specifically optimized for isomer discrimination rather than standard size determination.
Quality by Design (QbD) principles, strongly encouraged by regulatory authorities, require pharmaceutical manufacturers to establish a design space where DLS parameters can be adjusted while maintaining the ability to differentiate isomers reliably. This necessitates thorough understanding of how factors such as temperature, concentration, and buffer composition affect the hydrodynamic behavior of different isomeric forms.
For continuous manufacturing processes, where real-time release testing may be implemented, regulatory agencies require demonstration that DLS can provide consistent isomer differentiation capabilities under process conditions. This includes validation of the method's performance throughout the expected range of process variations and demonstration of suitable control strategies.
Data integrity requirements for DLS applications in regulated environments are particularly stringent, with expectations for complete audit trails, secure data storage, and validated analysis software. When applied to isomer differentiation, these requirements extend to algorithms specifically designed to extract and interpret the subtle differences in scattering patterns between isomeric compounds.
FDA's guidance on Process Analytical Technology (PAT) framework emphasizes the importance of understanding and controlling manufacturing processes through timely measurements of critical quality attributes, where DLS serves as a valuable analytical tool. For isomer differentiation applications, regulatory expectations include demonstration of method specificity, accuracy, precision, and robustness specifically for distinguishing structural variants with similar molecular weights but different spatial configurations.
The United States Pharmacopeia (USP) and European Pharmacopoeia (Ph. Eur.) provide specific chapters on particle size analysis that include considerations for DLS methodology. USP <429> and Ph. Eur. 2.9.31 outline requirements for instrument qualification, method validation, and data interpretation that must be adhered to when using DLS for isomer characterization in pharmaceutical products.
Regulatory submissions involving DLS for isomer differentiation must include comprehensive method validation data demonstrating the technique's ability to reliably distinguish between isomeric forms. This includes validation of sample preparation procedures, instrument parameters, and data analysis algorithms specifically optimized for isomer discrimination rather than standard size determination.
Quality by Design (QbD) principles, strongly encouraged by regulatory authorities, require pharmaceutical manufacturers to establish a design space where DLS parameters can be adjusted while maintaining the ability to differentiate isomers reliably. This necessitates thorough understanding of how factors such as temperature, concentration, and buffer composition affect the hydrodynamic behavior of different isomeric forms.
For continuous manufacturing processes, where real-time release testing may be implemented, regulatory agencies require demonstration that DLS can provide consistent isomer differentiation capabilities under process conditions. This includes validation of the method's performance throughout the expected range of process variations and demonstration of suitable control strategies.
Data integrity requirements for DLS applications in regulated environments are particularly stringent, with expectations for complete audit trails, secure data storage, and validated analysis software. When applied to isomer differentiation, these requirements extend to algorithms specifically designed to extract and interpret the subtle differences in scattering patterns between isomeric compounds.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!