How to Optimize Dynamic Light Scattering for Plant Phenotyping
SEP 5, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
DLS Technology Background and Plant Phenotyping Goals
Dynamic Light Scattering (DLS) emerged in the 1960s as a technique for measuring particle size distributions in solutions through the analysis of scattered light intensity fluctuations. Originally developed for applications in physical chemistry and material science, DLS has evolved significantly over the past six decades, transitioning from bulky laboratory equipment to more compact, user-friendly systems with enhanced sensitivity and resolution.
The technology operates on the principle of Brownian motion, where particles in suspension move randomly due to collisions with solvent molecules. By measuring the time-dependent fluctuations in scattered light intensity, DLS can determine the diffusion coefficient of particles, which is inversely related to their hydrodynamic radius. This fundamental relationship enables precise characterization of particle size distributions in the nanometer to micrometer range.
Recent technological advancements have expanded DLS capabilities beyond traditional applications. Modern systems incorporate multi-angle detection, advanced correlation algorithms, and machine learning approaches that significantly improve measurement accuracy and enable the characterization of complex, heterogeneous samples. These developments have opened new possibilities for DLS in biological and agricultural sciences.
In the context of plant phenotyping, DLS presents a promising non-destructive approach for characterizing plant cellular components, monitoring physiological responses to environmental stressors, and detecting early signs of disease. The technology's ability to analyze subcellular structures, protein aggregation states, and membrane vesicles provides valuable insights into plant development and stress responses at the molecular level.
The primary goals for optimizing DLS for plant phenotyping include enhancing measurement sensitivity for complex plant tissues, developing standardized protocols for sample preparation that preserve native cellular structures, and creating data analysis frameworks specifically tailored to plant-specific parameters. Additionally, there is a critical need to establish correlations between DLS measurements and important agronomic traits such as drought tolerance, disease resistance, and yield potential.
Another key objective is to transition DLS from laboratory settings to field-deployable systems that can provide real-time phenotypic data in agricultural environments. This requires addressing challenges related to sample heterogeneity, environmental interferences, and data interpretation in non-controlled conditions. The development of portable, robust DLS instruments with simplified operation protocols would significantly expand the technology's utility in agricultural research and precision farming applications.
The integration of DLS with other phenotyping technologies, such as hyperspectral imaging and chlorophyll fluorescence, represents another important goal. Such multi-modal approaches could provide comprehensive phenotypic profiles that capture both structural and functional plant characteristics across different biological scales, from cellular components to whole-plant responses.
The technology operates on the principle of Brownian motion, where particles in suspension move randomly due to collisions with solvent molecules. By measuring the time-dependent fluctuations in scattered light intensity, DLS can determine the diffusion coefficient of particles, which is inversely related to their hydrodynamic radius. This fundamental relationship enables precise characterization of particle size distributions in the nanometer to micrometer range.
Recent technological advancements have expanded DLS capabilities beyond traditional applications. Modern systems incorporate multi-angle detection, advanced correlation algorithms, and machine learning approaches that significantly improve measurement accuracy and enable the characterization of complex, heterogeneous samples. These developments have opened new possibilities for DLS in biological and agricultural sciences.
In the context of plant phenotyping, DLS presents a promising non-destructive approach for characterizing plant cellular components, monitoring physiological responses to environmental stressors, and detecting early signs of disease. The technology's ability to analyze subcellular structures, protein aggregation states, and membrane vesicles provides valuable insights into plant development and stress responses at the molecular level.
The primary goals for optimizing DLS for plant phenotyping include enhancing measurement sensitivity for complex plant tissues, developing standardized protocols for sample preparation that preserve native cellular structures, and creating data analysis frameworks specifically tailored to plant-specific parameters. Additionally, there is a critical need to establish correlations between DLS measurements and important agronomic traits such as drought tolerance, disease resistance, and yield potential.
Another key objective is to transition DLS from laboratory settings to field-deployable systems that can provide real-time phenotypic data in agricultural environments. This requires addressing challenges related to sample heterogeneity, environmental interferences, and data interpretation in non-controlled conditions. The development of portable, robust DLS instruments with simplified operation protocols would significantly expand the technology's utility in agricultural research and precision farming applications.
The integration of DLS with other phenotyping technologies, such as hyperspectral imaging and chlorophyll fluorescence, represents another important goal. Such multi-modal approaches could provide comprehensive phenotypic profiles that capture both structural and functional plant characteristics across different biological scales, from cellular components to whole-plant responses.
Market Analysis for DLS in Agricultural Applications
The Dynamic Light Scattering (DLS) technology market in agricultural applications is experiencing significant growth, driven by increasing demand for advanced plant phenotyping solutions. The global agricultural technology market, within which DLS operates, was valued at approximately $17.4 billion in 2020 and is projected to reach $41.2 billion by 2027, growing at a CAGR of 12.1%. Plant phenotyping specifically represents about $850 million of this market, with projected annual growth rates exceeding 15% through 2028.
DLS technology addresses critical market needs in precision agriculture, particularly as climate change and food security concerns intensify. Agricultural stakeholders increasingly require non-destructive, high-throughput methods to assess plant health, growth patterns, and stress responses. Traditional phenotyping methods are labor-intensive and often subjective, creating substantial market opportunity for automated, data-driven solutions like DLS.
Market segmentation reveals distinct customer groups for DLS technology in agriculture. Research institutions and universities currently constitute approximately 45% of the market, utilizing DLS primarily for fundamental plant science research. Commercial seed developers and agricultural biotechnology companies represent 30% of the market, applying DLS for crop improvement programs and genetic screening. The remaining 25% is divided among agricultural service providers, large-scale farming operations, and government agencies.
Geographically, North America leads DLS adoption in agricultural applications with 38% market share, followed by Europe (32%), Asia-Pacific (22%), and other regions (8%). The Asia-Pacific region, particularly China and India, demonstrates the fastest growth trajectory due to increasing agricultural modernization initiatives and government support for precision farming technologies.
Key market drivers include rising global food demand, decreasing arable land, climate change impacts, and increasing investment in agricultural research. The push toward sustainable farming practices further accelerates adoption of technologies that optimize resource utilization and crop yields. Regulatory support for precision agriculture technologies in major agricultural economies also provides favorable market conditions.
Market barriers include high initial investment costs for DLS equipment, technical complexity requiring specialized expertise, and integration challenges with existing agricultural workflows. Additionally, awareness and education gaps regarding DLS benefits among traditional farming communities limit broader market penetration in developing regions.
The DLS agricultural market demonstrates seasonal demand patterns aligned with growing seasons in major agricultural regions, with equipment purchases typically occurring during off-season periods. Subscription-based service models are gaining traction, offering more accessible entry points for smaller agricultural operations.
DLS technology addresses critical market needs in precision agriculture, particularly as climate change and food security concerns intensify. Agricultural stakeholders increasingly require non-destructive, high-throughput methods to assess plant health, growth patterns, and stress responses. Traditional phenotyping methods are labor-intensive and often subjective, creating substantial market opportunity for automated, data-driven solutions like DLS.
Market segmentation reveals distinct customer groups for DLS technology in agriculture. Research institutions and universities currently constitute approximately 45% of the market, utilizing DLS primarily for fundamental plant science research. Commercial seed developers and agricultural biotechnology companies represent 30% of the market, applying DLS for crop improvement programs and genetic screening. The remaining 25% is divided among agricultural service providers, large-scale farming operations, and government agencies.
Geographically, North America leads DLS adoption in agricultural applications with 38% market share, followed by Europe (32%), Asia-Pacific (22%), and other regions (8%). The Asia-Pacific region, particularly China and India, demonstrates the fastest growth trajectory due to increasing agricultural modernization initiatives and government support for precision farming technologies.
Key market drivers include rising global food demand, decreasing arable land, climate change impacts, and increasing investment in agricultural research. The push toward sustainable farming practices further accelerates adoption of technologies that optimize resource utilization and crop yields. Regulatory support for precision agriculture technologies in major agricultural economies also provides favorable market conditions.
Market barriers include high initial investment costs for DLS equipment, technical complexity requiring specialized expertise, and integration challenges with existing agricultural workflows. Additionally, awareness and education gaps regarding DLS benefits among traditional farming communities limit broader market penetration in developing regions.
The DLS agricultural market demonstrates seasonal demand patterns aligned with growing seasons in major agricultural regions, with equipment purchases typically occurring during off-season periods. Subscription-based service models are gaining traction, offering more accessible entry points for smaller agricultural operations.
Current Challenges in DLS for Plant Phenotyping
Despite the promising potential of Dynamic Light Scattering (DLS) for plant phenotyping, several significant challenges currently limit its widespread adoption and effectiveness in this field. The primary technical obstacle remains the complex optical properties of plant tissues, which cause multiple scattering events that can confound traditional DLS algorithms. Plant cells contain numerous organelles, cell walls, and intercellular spaces that create a heterogeneous medium, resulting in light path complexity that standard DLS systems struggle to interpret accurately.
Sample preparation presents another substantial hurdle. Unlike solutions or simple biological samples, plant tissues require specialized preparation techniques that preserve cellular structures while enabling light penetration. Current protocols often damage delicate plant structures or alter their natural state, compromising measurement accuracy. The trade-off between maintaining tissue integrity and achieving optical clarity remains unresolved in many applications.
Instrument sensitivity and calibration issues further complicate DLS implementation in plant phenotyping. Most commercial DLS systems were designed for homogeneous solutions or simple biological samples, not the complex hierarchical structures found in plant tissues. The dynamic range required to capture both nanoscale cellular components and larger tissue structures exceeds the capabilities of many existing instruments, necessitating compromises in measurement parameters.
Data interpretation represents perhaps the most significant challenge. The algorithms used to convert DLS measurements into meaningful biological parameters were largely developed for simpler systems. Plant tissues generate complex correlation functions that current mathematical models struggle to deconvolute. This leads to ambiguity in distinguishing between different cellular components and processes, limiting the biological insights that can be extracted from the data.
Environmental factors introduce additional variability in DLS measurements of plant samples. Temperature fluctuations, ambient light, and sample hydration status can dramatically affect scattering patterns. Unlike controlled laboratory samples, living plant tissues continue metabolic processes during measurement, creating temporal dynamics that further complicate data acquisition and interpretation.
Standardization across the field remains inadequate, with different research groups employing varied protocols, instruments, and analysis methods. This lack of standardization hampers comparative studies and slows the development of robust phenotyping methodologies. The absence of reference materials specifically designed for plant tissue DLS calibration further exacerbates this problem, making cross-laboratory validation challenging.
Integration with other phenotyping technologies represents a final challenge. To realize its full potential, DLS must be effectively combined with complementary techniques such as hyperspectral imaging or fluorescence-based methods. Current integration approaches often suffer from alignment issues, data format incompatibilities, and analytical frameworks that fail to leverage the strengths of each technology.
Sample preparation presents another substantial hurdle. Unlike solutions or simple biological samples, plant tissues require specialized preparation techniques that preserve cellular structures while enabling light penetration. Current protocols often damage delicate plant structures or alter their natural state, compromising measurement accuracy. The trade-off between maintaining tissue integrity and achieving optical clarity remains unresolved in many applications.
Instrument sensitivity and calibration issues further complicate DLS implementation in plant phenotyping. Most commercial DLS systems were designed for homogeneous solutions or simple biological samples, not the complex hierarchical structures found in plant tissues. The dynamic range required to capture both nanoscale cellular components and larger tissue structures exceeds the capabilities of many existing instruments, necessitating compromises in measurement parameters.
Data interpretation represents perhaps the most significant challenge. The algorithms used to convert DLS measurements into meaningful biological parameters were largely developed for simpler systems. Plant tissues generate complex correlation functions that current mathematical models struggle to deconvolute. This leads to ambiguity in distinguishing between different cellular components and processes, limiting the biological insights that can be extracted from the data.
Environmental factors introduce additional variability in DLS measurements of plant samples. Temperature fluctuations, ambient light, and sample hydration status can dramatically affect scattering patterns. Unlike controlled laboratory samples, living plant tissues continue metabolic processes during measurement, creating temporal dynamics that further complicate data acquisition and interpretation.
Standardization across the field remains inadequate, with different research groups employing varied protocols, instruments, and analysis methods. This lack of standardization hampers comparative studies and slows the development of robust phenotyping methodologies. The absence of reference materials specifically designed for plant tissue DLS calibration further exacerbates this problem, making cross-laboratory validation challenging.
Integration with other phenotyping technologies represents a final challenge. To realize its full potential, DLS must be effectively combined with complementary techniques such as hyperspectral imaging or fluorescence-based methods. Current integration approaches often suffer from alignment issues, data format incompatibilities, and analytical frameworks that fail to leverage the strengths of each technology.
Current DLS Implementation Methods for Plant Analysis
01 Optimization of DLS measurement parameters
Dynamic Light Scattering (DLS) measurement accuracy can be improved by optimizing various parameters such as laser intensity, detector angle, sample concentration, and temperature control. These optimizations help reduce noise, improve signal quality, and enhance the resolution of particle size distributions. Advanced algorithms can automatically adjust these parameters based on sample characteristics to achieve optimal measurement conditions.- Optimization of DLS measurement parameters: Dynamic Light Scattering (DLS) measurement accuracy can be improved by optimizing various parameters such as laser intensity, detector angle, sample concentration, and temperature control. These optimizations help reduce noise, improve signal quality, and enhance the reliability of particle size distribution measurements. Advanced algorithms can automatically adjust these parameters based on sample characteristics to achieve optimal measurement conditions.
- Data processing algorithms for DLS analysis: Sophisticated data processing algorithms are essential for accurate DLS analysis. These include correlation function analysis, cumulant analysis, and regularization methods that transform raw scattered light data into meaningful particle size distributions. Machine learning and AI-based approaches can further enhance data interpretation by filtering noise, identifying outliers, and improving resolution between particle populations of different sizes.
- Sample preparation techniques for DLS: Proper sample preparation is critical for reliable DLS measurements. This includes methods for controlling dust contamination, preventing aggregation, achieving optimal sample concentration, and ensuring sample homogeneity. Specialized filtration techniques, buffer optimization, and stabilizing additives can significantly improve measurement quality by reducing unwanted scattering events and maintaining sample integrity during analysis.
- Multi-angle and multi-wavelength DLS systems: Advanced DLS systems utilize measurements at multiple angles and/or multiple wavelengths to enhance resolution and accuracy. These approaches provide complementary information about particle characteristics, allowing for better discrimination between different particle populations and more accurate size determination. The combination of data from different angles and wavelengths enables more robust analysis of complex, polydisperse samples.
- Integration of DLS with other analytical techniques: Combining DLS with complementary analytical techniques creates powerful hybrid systems that provide more comprehensive characterization of particles. Integration with techniques such as Raman spectroscopy, refractive index detection, or chromatography methods enables simultaneous measurement of size, composition, and other physical properties. These integrated approaches overcome the limitations of standalone DLS and provide more detailed insights into complex samples.
02 Advanced data processing algorithms for DLS
Sophisticated algorithms are developed to process raw DLS data, including correlation function analysis, noise filtering, and mathematical transformations. These algorithms can handle polydisperse samples, extract multiple particle populations, and provide more accurate size distributions. Machine learning and AI approaches are increasingly being applied to improve data interpretation and reduce measurement artifacts.Expand Specific Solutions03 Sample preparation techniques for DLS optimization
Proper sample preparation is crucial for reliable DLS measurements. This includes methods for controlling dust contamination, optimizing sample concentration, adjusting ionic strength, and stabilizing dispersions. Specialized filtration techniques, buffer selection, and surface modification approaches can significantly improve measurement quality by reducing aggregation and ensuring sample stability during analysis.Expand Specific Solutions04 Integration of DLS with complementary techniques
Combining DLS with other analytical methods such as static light scattering, microscopy, or spectroscopic techniques provides more comprehensive characterization of complex samples. These integrated approaches allow for validation of results, extension of the measurable size range, and acquisition of additional physicochemical properties beyond particle size, such as molecular weight, shape, and surface characteristics.Expand Specific Solutions05 Specialized DLS instrumentation design
Innovations in DLS hardware design focus on improving optical components, detector sensitivity, and mechanical stability. Advanced instruments incorporate features such as multi-angle detection, fiber optics for remote sampling, temperature control systems, and automated sample handling. These design optimizations enhance measurement precision, extend the application range, and improve the reliability of DLS analysis for challenging samples.Expand Specific Solutions
Key Industry Players and Research Institutions
Dynamic Light Scattering (DLS) optimization for plant phenotyping is emerging as a promising technology in the early growth stage of market development. The global market is expanding rapidly, driven by increasing demands for precision agriculture and sustainable crop production. Currently, the technology demonstrates moderate maturity, with key players developing specialized applications. Companies like Heliospectra AB and Valoya Oy are pioneering LED lighting solutions optimized for plant growth, while research institutions such as Nanjing Agricultural University and Purdue Research Foundation are advancing fundamental research. Commercial entities including KWS SAAT and Gardin Ltd are integrating DLS into practical phenotyping systems. The competitive landscape shows a balanced mix of specialized lighting manufacturers, agricultural biotechnology companies, and academic institutions collaborating to overcome technical challenges in light scattering measurement accuracy and data interpretation for diverse plant species.
Purdue Research Foundation
Technical Solution: Purdue has developed an innovative dynamic light scattering platform specifically designed for agricultural applications in plant phenotyping. Their system utilizes multi-angle light scattering techniques combined with specialized sample preparation protocols that preserve the native state of plant tissues during analysis. The technology incorporates temperature-controlled measurement chambers that allow researchers to simulate various environmental conditions while monitoring plant responses through changes in light scattering patterns. Purdue's approach includes correlation of scattering data with traditional phenotypic measurements to establish robust relationships between optical properties and plant traits of interest. Their platform features open-source software components that facilitate data integration with other phenotyping technologies and enable customized analysis workflows for specific research applications.
Strengths: Strong integration with traditional phenotyping methods enhances data interpretation and validation; open-source components promote collaborative development and customization. Weaknesses: Requires careful sample preparation that may limit throughput; system optimization needs significant expertise in both plant biology and optical physics.
Fraunhofer-Gesellschaft eV
Technical Solution: Fraunhofer has developed comprehensive dynamic light scattering solutions for plant phenotyping that integrate multiple optical technologies. Their system combines traditional DLS with spatial light interference microscopy to achieve unprecedented resolution in monitoring plant cellular dynamics. The technology employs coherent light sources with precise control over scattering angles and detection parameters, optimized specifically for plant tissue analysis. Fraunhofer's approach includes modular components that can be customized for different research applications, from root architecture analysis to leaf physiological monitoring. Their platform incorporates automated calibration procedures that account for the complex optical properties of plant tissues, ensuring accurate and reproducible measurements across diverse plant species and growth conditions.
Strengths: Highly customizable system architecture allows adaptation to diverse research questions; exceptional measurement precision enables detection of subtle phenotypic differences. Weaknesses: Significant technical expertise required for system optimization; higher cost compared to simpler phenotyping approaches limits accessibility for smaller research groups.
Technical Innovations in DLS Sensor Technology
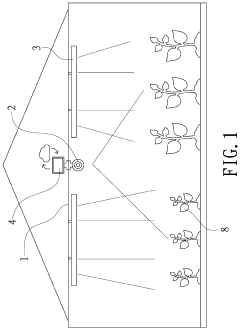
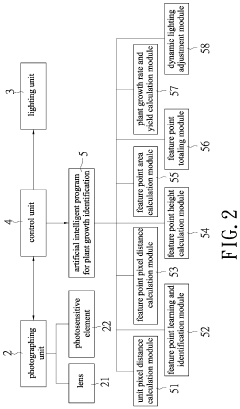
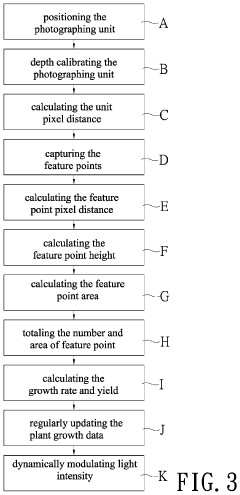
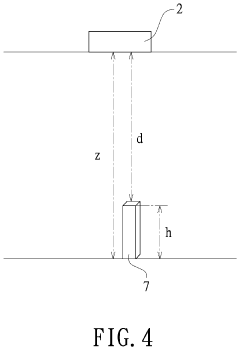
Plant growth identification method and system therefor
PatentInactiveUS20230177830A1
Innovation
- A plant growth identification system using deep photography and artificial intelligence to calculate pixel distances, feature points, and dynamically adjust lighting, enabling precise control of light spectrum and intensity based on plant growth data.
Method and means for enhancing greenhouse lights
PatentActiveUS20130047503A1
Innovation
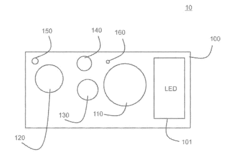
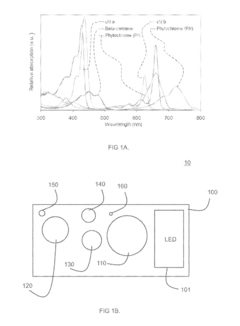
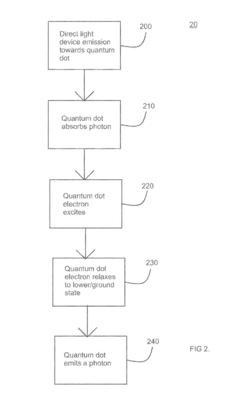
- A system and method using quantum dots to up-convert LED emission, achieving an optimized spectrum for plant cultivation by arranging quantum dots of different sizes to absorb and re-emit photons at longer wavelengths, thereby producing favorable photomorphogenetic effects and enhancing photosynthesis processes.
Standardization and Calibration Protocols
The standardization and calibration of Dynamic Light Scattering (DLS) systems for plant phenotyping applications requires rigorous protocols to ensure measurement reliability and reproducibility across different laboratory settings. Establishing these protocols begins with the selection of appropriate reference materials that closely mimic the optical properties of plant tissues or cellular components being studied. Polystyrene latex beads of known sizes (typically ranging from 50nm to 500nm) serve as primary calibration standards due to their uniform size distribution and stable light scattering properties.
Calibration procedures must address both instrument-specific parameters and environmental variables that influence DLS measurements. Temperature control represents a critical factor, as fluctuations as small as 0.5°C can significantly alter the Brownian motion of particles and consequently affect size distribution calculations. Standardized protocols should mandate temperature stabilization periods of at least 15 minutes before measurements and continuous monitoring throughout data collection sessions.
Sample preparation standardization constitutes another essential component of robust DLS protocols for plant phenotyping. This includes defining consistent procedures for tissue homogenization, filtration thresholds (typically 0.45μm filters for cellular components), and buffer composition. The ionic strength and pH of suspension media must be carefully controlled, as these factors directly influence the electrical double layer around particles and subsequently their hydrodynamic behavior during measurement.
Measurement parameters require explicit standardization, including laser power settings, detector angles, and acquisition times. For plant samples with inherent heterogeneity, longer acquisition times (typically 60-120 seconds per measurement) with multiple measurement cycles (minimum 10-15 repetitions) are necessary to achieve statistically significant results. Signal processing algorithms must also be standardized, with clear documentation of the mathematical models applied for converting correlation functions to size distributions.
Inter-laboratory validation represents the culmination of standardization efforts, requiring round-robin testing across multiple facilities using identical reference materials and protocols. Statistical analysis of these comparative measurements establishes acceptable variance thresholds for different plant tissue types and cellular components. Regular proficiency testing using these established benchmarks ensures ongoing measurement quality and facilitates meaningful comparison of plant phenotyping data across research groups and experimental conditions.
Documentation standards form the final critical element of DLS calibration protocols, requiring comprehensive recording of all calibration parameters, reference material certificates, environmental conditions, and raw correlation data alongside processed results. This documentation chain ensures traceability and enables retrospective analysis when comparing phenotypic measurements across different growth conditions or genetic variants in plant research applications.
Calibration procedures must address both instrument-specific parameters and environmental variables that influence DLS measurements. Temperature control represents a critical factor, as fluctuations as small as 0.5°C can significantly alter the Brownian motion of particles and consequently affect size distribution calculations. Standardized protocols should mandate temperature stabilization periods of at least 15 minutes before measurements and continuous monitoring throughout data collection sessions.
Sample preparation standardization constitutes another essential component of robust DLS protocols for plant phenotyping. This includes defining consistent procedures for tissue homogenization, filtration thresholds (typically 0.45μm filters for cellular components), and buffer composition. The ionic strength and pH of suspension media must be carefully controlled, as these factors directly influence the electrical double layer around particles and subsequently their hydrodynamic behavior during measurement.
Measurement parameters require explicit standardization, including laser power settings, detector angles, and acquisition times. For plant samples with inherent heterogeneity, longer acquisition times (typically 60-120 seconds per measurement) with multiple measurement cycles (minimum 10-15 repetitions) are necessary to achieve statistically significant results. Signal processing algorithms must also be standardized, with clear documentation of the mathematical models applied for converting correlation functions to size distributions.
Inter-laboratory validation represents the culmination of standardization efforts, requiring round-robin testing across multiple facilities using identical reference materials and protocols. Statistical analysis of these comparative measurements establishes acceptable variance thresholds for different plant tissue types and cellular components. Regular proficiency testing using these established benchmarks ensures ongoing measurement quality and facilitates meaningful comparison of plant phenotyping data across research groups and experimental conditions.
Documentation standards form the final critical element of DLS calibration protocols, requiring comprehensive recording of all calibration parameters, reference material certificates, environmental conditions, and raw correlation data alongside processed results. This documentation chain ensures traceability and enables retrospective analysis when comparing phenotypic measurements across different growth conditions or genetic variants in plant research applications.
Environmental Factors Affecting DLS Measurements
Dynamic Light Scattering (DLS) measurements for plant phenotyping are significantly influenced by various environmental factors that can alter data quality and interpretation. Temperature fluctuations represent one of the most critical environmental variables affecting DLS performance. Even minor temperature changes can modify the viscosity of the suspension medium, directly impacting the Brownian motion of particles and consequently altering the measured particle size distribution. Research indicates that a temperature variation of just 1°C can result in measurement deviations of up to 2-3% in particle size determination.
Humidity levels in the laboratory environment also play a substantial role in DLS measurement stability. High humidity can lead to condensation on optical components, causing light scattering artifacts and signal noise. Conversely, extremely low humidity may generate static electricity that interferes with sample preparation and handling, particularly when dealing with plant tissue samples that contain charged biomolecules.
Ambient light contamination presents another significant challenge for DLS applications in plant phenotyping. Stray light entering the detection system can overwhelm the scattered signal from particles, especially when measuring samples with low concentration or small particle sizes typical in plant cellular components. Modern DLS instruments incorporate light-tight chambers, but measurements conducted in environments with intense lighting may still require additional shielding protocols.
Vibration interference constitutes a persistent environmental concern that can compromise DLS data quality. Even minor mechanical vibrations from nearby equipment, HVAC systems, or foot traffic can introduce artifacts in the correlation function, leading to erroneous size distributions. Studies have demonstrated that vibration isolation platforms can improve measurement reproducibility by up to 40% in challenging laboratory environments.
Air quality factors, particularly airborne particulates, can contaminate samples during preparation and measurement phases. Plant phenotyping applications often involve delicate sample preparation steps where contamination risks are elevated. Dust particles as small as 1μm can significantly skew size distribution results, especially when analyzing nanoscale plant components such as membrane fragments or protein complexes.
Electromagnetic interference from nearby electronic equipment can also disrupt the sensitive photon detection systems employed in DLS instruments. This is particularly relevant in modern laboratory settings where multiple electronic devices operate simultaneously. Proper grounding and electromagnetic shielding have been shown to reduce measurement noise by up to 30% in controlled experiments.
Humidity levels in the laboratory environment also play a substantial role in DLS measurement stability. High humidity can lead to condensation on optical components, causing light scattering artifacts and signal noise. Conversely, extremely low humidity may generate static electricity that interferes with sample preparation and handling, particularly when dealing with plant tissue samples that contain charged biomolecules.
Ambient light contamination presents another significant challenge for DLS applications in plant phenotyping. Stray light entering the detection system can overwhelm the scattered signal from particles, especially when measuring samples with low concentration or small particle sizes typical in plant cellular components. Modern DLS instruments incorporate light-tight chambers, but measurements conducted in environments with intense lighting may still require additional shielding protocols.
Vibration interference constitutes a persistent environmental concern that can compromise DLS data quality. Even minor mechanical vibrations from nearby equipment, HVAC systems, or foot traffic can introduce artifacts in the correlation function, leading to erroneous size distributions. Studies have demonstrated that vibration isolation platforms can improve measurement reproducibility by up to 40% in challenging laboratory environments.
Air quality factors, particularly airborne particulates, can contaminate samples during preparation and measurement phases. Plant phenotyping applications often involve delicate sample preparation steps where contamination risks are elevated. Dust particles as small as 1μm can significantly skew size distribution results, especially when analyzing nanoscale plant components such as membrane fragments or protein complexes.
Electromagnetic interference from nearby electronic equipment can also disrupt the sensitive photon detection systems employed in DLS instruments. This is particularly relevant in modern laboratory settings where multiple electronic devices operate simultaneously. Proper grounding and electromagnetic shielding have been shown to reduce measurement noise by up to 30% in controlled experiments.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!