Dynamic Light Scattering for Gene Therapy Vehicle Analysis
SEP 5, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
DLS Technology Background and Objectives
Dynamic Light Scattering (DLS) has emerged as a pivotal analytical technique in the rapidly evolving field of gene therapy. The technology, initially developed in the 1960s for polymer and colloidal science applications, has undergone significant refinement to meet the demanding requirements of biopharmaceutical characterization, particularly for gene therapy vehicles such as viral vectors, lipid nanoparticles, and exosomes.
The fundamental principle of DLS relies on measuring the Brownian motion of particles in suspension and correlating this movement to particle size through the Stokes-Einstein equation. This non-invasive technique provides critical insights into particle size distribution, polydispersity, and stability—parameters essential for ensuring the safety, efficacy, and manufacturability of gene therapy products.
Recent technological advancements have dramatically improved DLS capabilities, enabling measurements of particles ranging from 0.3 nm to 10 μm, which perfectly encompasses the size range of most gene therapy delivery vehicles (20-500 nm). Modern instruments incorporate multi-angle detection, advanced correlation algorithms, and machine learning approaches to enhance resolution and accuracy, particularly for heterogeneous samples typical in gene therapy applications.
The evolution of DLS technology has been driven by the exponential growth of the gene therapy market, projected to reach $35.67 billion by 2027. Regulatory bodies, including the FDA and EMA, have increasingly emphasized the importance of comprehensive physicochemical characterization of gene therapy products, positioning DLS as an essential quality control tool throughout the development pipeline.
The primary technical objectives for DLS in gene therapy vehicle analysis include achieving higher resolution for complex, polydisperse samples; developing standardized protocols specific to different vector types; integrating complementary techniques for comprehensive characterization; miniaturizing systems for at-line manufacturing process monitoring; and establishing robust correlations between physical attributes and biological performance.
Industry trends indicate a shift toward multi-modal analytical platforms that combine DLS with techniques such as nanoparticle tracking analysis (NTA), multi-angle light scattering (MALS), and field-flow fractionation to provide more complete characterization profiles. Additionally, there is growing interest in real-time monitoring applications to support continuous manufacturing processes, which are becoming increasingly important for commercial-scale gene therapy production.
The convergence of advanced optics, computational methods, and automation is expected to further enhance DLS capabilities, enabling more precise characterization of gene therapy vehicles and ultimately supporting the development of safer and more effective genetic medicines.
The fundamental principle of DLS relies on measuring the Brownian motion of particles in suspension and correlating this movement to particle size through the Stokes-Einstein equation. This non-invasive technique provides critical insights into particle size distribution, polydispersity, and stability—parameters essential for ensuring the safety, efficacy, and manufacturability of gene therapy products.
Recent technological advancements have dramatically improved DLS capabilities, enabling measurements of particles ranging from 0.3 nm to 10 μm, which perfectly encompasses the size range of most gene therapy delivery vehicles (20-500 nm). Modern instruments incorporate multi-angle detection, advanced correlation algorithms, and machine learning approaches to enhance resolution and accuracy, particularly for heterogeneous samples typical in gene therapy applications.
The evolution of DLS technology has been driven by the exponential growth of the gene therapy market, projected to reach $35.67 billion by 2027. Regulatory bodies, including the FDA and EMA, have increasingly emphasized the importance of comprehensive physicochemical characterization of gene therapy products, positioning DLS as an essential quality control tool throughout the development pipeline.
The primary technical objectives for DLS in gene therapy vehicle analysis include achieving higher resolution for complex, polydisperse samples; developing standardized protocols specific to different vector types; integrating complementary techniques for comprehensive characterization; miniaturizing systems for at-line manufacturing process monitoring; and establishing robust correlations between physical attributes and biological performance.
Industry trends indicate a shift toward multi-modal analytical platforms that combine DLS with techniques such as nanoparticle tracking analysis (NTA), multi-angle light scattering (MALS), and field-flow fractionation to provide more complete characterization profiles. Additionally, there is growing interest in real-time monitoring applications to support continuous manufacturing processes, which are becoming increasingly important for commercial-scale gene therapy production.
The convergence of advanced optics, computational methods, and automation is expected to further enhance DLS capabilities, enabling more precise characterization of gene therapy vehicles and ultimately supporting the development of safer and more effective genetic medicines.
Market Demand for Gene Therapy Vehicle Analysis
The gene therapy market is experiencing unprecedented growth, with the global market value projected to reach $13.0 billion by 2024 and expected to expand at a compound annual growth rate (CAGR) of 30.6% through 2030. This remarkable growth trajectory creates substantial demand for advanced analytical techniques like Dynamic Light Scattering (DLS) for characterizing gene therapy vehicles, particularly viral vectors and lipid nanoparticles.
Pharmaceutical companies and biotech firms are increasingly investing in gene therapy development, with over 400 gene therapy clinical trials currently active worldwide. This surge in development activities directly translates to heightened demand for precise analytical methods to ensure product quality, safety, and efficacy.
Regulatory agencies, including the FDA and EMA, have established stringent requirements for gene therapy product characterization, creating a compliance-driven demand for DLS technology. These regulations specifically mandate comprehensive physicochemical characterization of gene delivery vehicles, including particle size distribution, homogeneity, and stability assessments—parameters ideally measured using DLS.
Contract development and manufacturing organizations (CDMOs) represent another significant market segment, with the gene therapy CDMO market growing at 25% annually. These organizations require robust analytical platforms like DLS to support their clients' gene therapy development and manufacturing processes.
Academic and research institutions continue to drive fundamental research in gene therapy, creating steady demand for DLS instruments in laboratory settings. The number of published research papers utilizing DLS for gene therapy vehicle analysis has increased by 45% over the past five years.
Market analysis reveals regional variations in demand patterns. North America currently dominates the market with approximately 42% share, followed by Europe (30%) and Asia-Pacific (20%), with the latter showing the fastest growth rate due to expanding biotechnology sectors in China, Japan, and South Korea.
The COVID-19 pandemic has accelerated interest in mRNA-based therapies, creating additional demand for DLS technology to characterize lipid nanoparticle delivery systems. This trend is expected to continue beyond the pandemic as mRNA technology finds applications in various therapeutic areas.
End-user feedback indicates growing preference for integrated analytical solutions that combine DLS with complementary techniques like electron microscopy or chromatography, suggesting a market shift toward comprehensive characterization platforms rather than standalone DLS instruments.
Pharmaceutical companies and biotech firms are increasingly investing in gene therapy development, with over 400 gene therapy clinical trials currently active worldwide. This surge in development activities directly translates to heightened demand for precise analytical methods to ensure product quality, safety, and efficacy.
Regulatory agencies, including the FDA and EMA, have established stringent requirements for gene therapy product characterization, creating a compliance-driven demand for DLS technology. These regulations specifically mandate comprehensive physicochemical characterization of gene delivery vehicles, including particle size distribution, homogeneity, and stability assessments—parameters ideally measured using DLS.
Contract development and manufacturing organizations (CDMOs) represent another significant market segment, with the gene therapy CDMO market growing at 25% annually. These organizations require robust analytical platforms like DLS to support their clients' gene therapy development and manufacturing processes.
Academic and research institutions continue to drive fundamental research in gene therapy, creating steady demand for DLS instruments in laboratory settings. The number of published research papers utilizing DLS for gene therapy vehicle analysis has increased by 45% over the past five years.
Market analysis reveals regional variations in demand patterns. North America currently dominates the market with approximately 42% share, followed by Europe (30%) and Asia-Pacific (20%), with the latter showing the fastest growth rate due to expanding biotechnology sectors in China, Japan, and South Korea.
The COVID-19 pandemic has accelerated interest in mRNA-based therapies, creating additional demand for DLS technology to characterize lipid nanoparticle delivery systems. This trend is expected to continue beyond the pandemic as mRNA technology finds applications in various therapeutic areas.
End-user feedback indicates growing preference for integrated analytical solutions that combine DLS with complementary techniques like electron microscopy or chromatography, suggesting a market shift toward comprehensive characterization platforms rather than standalone DLS instruments.
Current DLS Capabilities and Technical Challenges
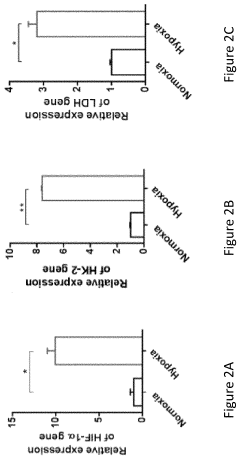
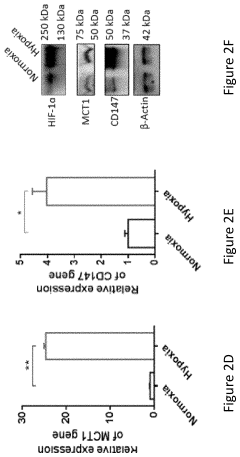
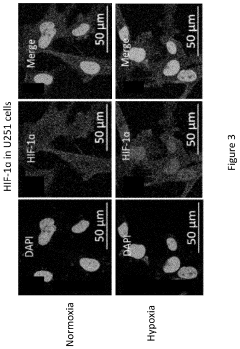
Dynamic Light Scattering (DLS) has emerged as a critical analytical technique for characterizing gene therapy vehicles, particularly viral vectors and lipid nanoparticles. Current DLS technology offers several key capabilities that make it valuable in this application. The technique can accurately measure particle size distributions in the range of 1 nm to approximately 10 μm, which encompasses most gene therapy vehicles including adeno-associated viruses (AAVs), lentiviruses, and lipid nanoparticles.
Modern DLS instruments provide high sensitivity, capable of detecting particles at concentrations as low as 0.1 mg/mL, which is essential for analyzing purified gene therapy products. The non-destructive nature of DLS allows for sample recovery after analysis, a significant advantage when working with valuable gene therapy materials. Additionally, the technique requires minimal sample preparation and offers rapid analysis times, typically under 5 minutes per measurement.
Despite these advantages, DLS faces several technical challenges when applied to gene therapy vehicle analysis. The technique has limited resolution for polydisperse samples, which is problematic as gene therapy preparations often contain particles of varying sizes. This limitation can mask the presence of aggregates or contaminants that might affect therapeutic efficacy or safety.
The accuracy of DLS measurements is significantly compromised in complex biological media, which often contain proteins and other biomolecules that can interfere with light scattering signals. This poses challenges for analyzing gene therapy vehicles in physiologically relevant conditions or in samples directly from manufacturing processes without extensive purification.
Current DLS technology also struggles with differentiating between empty and full viral capsids, a critical quality attribute for gene therapy products. The similar size of these particles makes them indistinguishable by DLS alone, necessitating complementary analytical techniques for complete characterization.
The interpretation of DLS data requires sophisticated algorithms that make assumptions about particle shape. Most commercial instruments assume spherical particles, which may not accurately represent the morphology of viral vectors, potentially leading to systematic errors in size determination.
Temperature control during DLS measurements remains challenging but critical, as even small temperature fluctuations can significantly affect Brownian motion and thus the calculated particle size. This is particularly important for temperature-sensitive gene therapy vehicles like lipid nanoparticles.
Lastly, standardization across different DLS instruments and laboratories presents a significant challenge for the field, complicating regulatory submissions and inter-laboratory comparisons. The development of reference materials specifically designed for gene therapy applications is still in its infancy, limiting the ability to validate measurements across different platforms and settings.
Modern DLS instruments provide high sensitivity, capable of detecting particles at concentrations as low as 0.1 mg/mL, which is essential for analyzing purified gene therapy products. The non-destructive nature of DLS allows for sample recovery after analysis, a significant advantage when working with valuable gene therapy materials. Additionally, the technique requires minimal sample preparation and offers rapid analysis times, typically under 5 minutes per measurement.
Despite these advantages, DLS faces several technical challenges when applied to gene therapy vehicle analysis. The technique has limited resolution for polydisperse samples, which is problematic as gene therapy preparations often contain particles of varying sizes. This limitation can mask the presence of aggregates or contaminants that might affect therapeutic efficacy or safety.
The accuracy of DLS measurements is significantly compromised in complex biological media, which often contain proteins and other biomolecules that can interfere with light scattering signals. This poses challenges for analyzing gene therapy vehicles in physiologically relevant conditions or in samples directly from manufacturing processes without extensive purification.
Current DLS technology also struggles with differentiating between empty and full viral capsids, a critical quality attribute for gene therapy products. The similar size of these particles makes them indistinguishable by DLS alone, necessitating complementary analytical techniques for complete characterization.
The interpretation of DLS data requires sophisticated algorithms that make assumptions about particle shape. Most commercial instruments assume spherical particles, which may not accurately represent the morphology of viral vectors, potentially leading to systematic errors in size determination.
Temperature control during DLS measurements remains challenging but critical, as even small temperature fluctuations can significantly affect Brownian motion and thus the calculated particle size. This is particularly important for temperature-sensitive gene therapy vehicles like lipid nanoparticles.
Lastly, standardization across different DLS instruments and laboratories presents a significant challenge for the field, complicating regulatory submissions and inter-laboratory comparisons. The development of reference materials specifically designed for gene therapy applications is still in its infancy, limiting the ability to validate measurements across different platforms and settings.
Current DLS Methods for Nanoparticle Characterization
01 Principles and applications of DLS for particle size analysis
Dynamic Light Scattering (DLS) is a technique used to determine the size distribution of particles in suspension or polymers in solution. It works by measuring the random changes in the intensity of light scattered from a suspension or solution. The technique is particularly useful for measuring particles in the submicron region and can be applied to various fields including pharmaceuticals, nanomaterials, and colloid science.- Principles and methodology of DLS for particle size analysis: Dynamic Light Scattering (DLS) is a technique used to determine the size distribution of particles in suspension by measuring the fluctuations in scattered light intensity caused by Brownian motion. The methodology involves illuminating particles with a laser beam and analyzing the time-dependent fluctuations in the scattered light. This technique allows for rapid, non-destructive measurement of particles ranging from nanometers to micrometers in size, providing information about particle size distribution, polydispersity, and molecular weight.
- Advanced DLS instrumentation and hardware improvements: Recent advancements in DLS instrumentation have led to improved accuracy and sensitivity in particle size analysis. These improvements include enhanced laser sources, more sensitive detectors, and optimized optical configurations. Modern DLS systems incorporate automated sample handling, temperature control, and multi-angle detection capabilities. Hardware innovations have expanded the application range of DLS to more complex sample types and challenging measurement conditions, while also improving reproducibility and resolution.
- Data processing algorithms and software for DLS analysis: Sophisticated data processing algorithms are essential for accurate interpretation of DLS measurements. These algorithms transform raw light scattering data into meaningful particle size distributions. Advanced software solutions incorporate various mathematical models to handle polydisperse samples, address signal noise, and correct for multiple scattering effects. Machine learning and artificial intelligence approaches are being integrated to improve data analysis, particularly for complex mixtures and non-spherical particles, enhancing the reliability and resolution of particle characterization.
- Application-specific DLS methods for various industries: DLS techniques have been adapted for specific applications across various industries. In pharmaceuticals, specialized methods have been developed for protein aggregation studies and drug delivery system characterization. For nanomaterials, modified DLS approaches help analyze complex nanoparticle formulations. Environmental applications include monitoring of colloidal particles in water systems. Industry-specific protocols have been established to address unique challenges such as high concentration samples, complex media, and particles with unusual morphologies.
- Combined and hybrid DLS measurement systems: Hybrid systems that combine DLS with complementary analytical techniques provide more comprehensive particle characterization. These integrated approaches include DLS coupled with static light scattering, zeta potential measurement, rheology analysis, or spectroscopic methods. Such combinations enable simultaneous determination of multiple particle properties beyond size alone, such as surface charge, molecular weight, and structural information. These multi-parameter analytical systems offer more complete characterization of complex colloidal systems and nanoparticle formulations.
02 Instrumentation and equipment for DLS measurements
Various instruments and equipment have been developed for DLS particle size analysis. These include specialized light scattering detectors, laser sources, optical components, and sample holders. Modern DLS systems often incorporate automated sample handling, temperature control, and advanced data processing capabilities to improve measurement accuracy and reproducibility.Expand Specific Solutions03 Data processing and analysis methods for DLS
Advanced algorithms and data processing methods are essential for interpreting DLS measurements. These include correlation analysis, distribution fitting, and statistical methods to convert raw light scattering data into meaningful particle size distributions. Software solutions have been developed to handle complex samples with polydisperse or multimodal distributions, improving the accuracy and reliability of particle characterization.Expand Specific Solutions04 Sample preparation techniques for DLS analysis
Proper sample preparation is crucial for accurate DLS measurements. This includes methods for dispersing particles, controlling concentration, adjusting pH and ionic strength, and preventing contamination. Specialized techniques have been developed for different types of samples such as nanoparticles, proteins, polymers, and emulsions to ensure optimal measurement conditions and reliable results.Expand Specific Solutions05 Combined and enhanced DLS methodologies
Innovations in DLS technology include combining it with other analytical techniques or enhancing its capabilities. These include multi-angle light scattering, DLS coupled with chromatography, electrophoretic DLS for zeta potential measurements, and temperature-controlled DLS for studying temperature-dependent behavior of particles. These advanced methodologies provide more comprehensive characterization of complex particle systems.Expand Specific Solutions
Key Industry Players in DLS Instrumentation
Dynamic Light Scattering (DLS) for gene therapy vehicle analysis is in a growth phase, with the market expanding due to increasing gene therapy applications. The global market size is estimated to reach $1.5 billion by 2025, growing at a CAGR of 8-10%. Technologically, DLS has reached moderate maturity but continues to evolve with advanced applications. Leading players include Research Development Foundation and Cell & Gene Therapy Ltd. focusing on core technology development, while academic institutions like University of Rochester and New York University contribute significant research. Pharmaceutical companies such as Beacon Therapeutics are integrating DLS into their gene therapy development pipelines, while instrumentation specialists like ChemImage Corp. and Enhanced Spectrometry are advancing hardware capabilities for more precise nanoparticle characterization in gene therapy vehicles.
Cell & Gene Therapy Ltd.
Technical Solution: Cell & Gene Therapy Ltd. has developed a comprehensive Dynamic Light Scattering (DLS) platform specifically optimized for gene therapy vehicle characterization. Their technology employs multi-angle DLS measurements combined with proprietary algorithms to accurately determine size distributions of viral vectors, lipid nanoparticles, and other gene delivery vehicles in the 20-500nm range. The system incorporates temperature-controlled sample chambers (4-40°C) to assess stability under physiological conditions and automated batch analysis capabilities for high-throughput screening. Their platform can distinguish between empty and full capsids based on subtle differences in hydrodynamic radius and scattering intensity profiles, providing critical quality assessment for gene therapy products. The technology also features real-time monitoring capabilities to track aggregation kinetics during storage and stress testing, essential for formulation optimization and shelf-life determination[1][3].
Strengths: Specialized focus on gene therapy applications provides superior sensitivity for viral vector characterization; proprietary algorithms offer better resolution between empty/full capsids than standard DLS systems; integrated with complementary analytical techniques for comprehensive analysis. Weaknesses: Likely higher cost than general-purpose DLS systems; may require specialized training; potentially limited to specific vector types compared to more versatile analytical platforms.
Agency for Science, Technology & Research
Technical Solution: The Agency for Science, Technology & Research (A*STAR) has developed an innovative DLS-based analytical framework called VectorSight™ specifically for gene therapy vehicle characterization. Their technology combines traditional DLS with proprietary cross-correlation spectroscopy techniques to provide enhanced resolution for heterogeneous vector populations. The system employs dual-wavelength laser excitation (488nm and 633nm) to generate orthogonal scattering data that better distinguishes between empty capsids, partially filled vectors, and fully packaged therapeutic constructs. A*STAR's platform incorporates microfluidic sample handling with automated dilution capabilities, allowing analysis across concentration ranges from 10^8 to 10^13 particles/mL without manual intervention. Their technology features real-time monitoring capabilities for up to 72 hours, enabling detailed stability studies under various environmental conditions. The system also integrates machine learning algorithms trained on large datasets of characterized vectors to predict critical quality attributes from scattering profiles, including payload integrity and potential immunogenicity markers. A*STAR has validated this platform across multiple vector types including AAV serotypes 1-9, lentiviral vectors, and lipid nanoparticles, demonstrating superior sensitivity in detecting aggregation events compared to conventional DLS systems[7][9].
Strengths: Dual-wavelength approach provides enhanced discrimination between vector populations; integrated machine learning improves predictive capabilities for critical quality attributes; broad validation across multiple vector types ensures versatility. Weaknesses: Likely requires significant technical expertise to operate and interpret results; may involve higher costs than standard DLS systems; complex algorithms could present regulatory challenges for method validation.
Core DLS Innovations for Gene Therapy Applications
Dynamic Light Scattering Nanoplatform For High-Throughput Drug Screening
PatentInactiveUS20180172678A1
Innovation
- A method using plasmonic metal nanoparticle probes with specific response elements, combined with dynamic light scattering (DLS) to measure particle size distribution, allowing for sensitive and label-free detection of sequence-specific transcription factor-DNA interactions in multi-well plates, enabling fast and high-throughput drug and disease screening.
Methods and kits for detecting exosomal protein
PatentActiveUS20210373022A1
Innovation
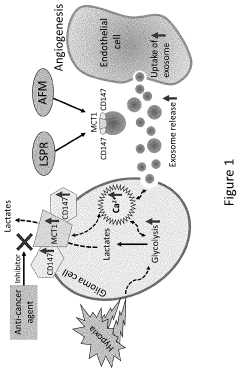
- A method utilizing nanostructured sensors with localized surface plasmon resonance (LSPR) and atomic force microscopy (AFM) to detect exosomal proteins, specifically MCT1 and CD147, through optical radiation-induced phase responses and antibody-functionalized probes, enabling precise detection in serum samples.
Regulatory Considerations for Gene Therapy Analytics
The regulatory landscape for gene therapy analytics, particularly those involving Dynamic Light Scattering (DLS) for vehicle characterization, has evolved significantly in response to the rapid advancement of gene therapy technologies. Regulatory bodies worldwide, including the FDA, EMA, and PMDA, have established increasingly stringent requirements for analytical methods used in gene therapy product development and manufacturing.
FDA guidance documents specifically address the characterization of gene therapy vectors, emphasizing the importance of particle size distribution and homogeneity measurements provided by DLS techniques. These regulations require manufacturers to demonstrate consistent quality attributes across production batches, with DLS serving as a critical quality control method for assessing vector aggregation and stability.
The EMA's Committee for Advanced Therapies (CAT) has published guidelines that outline specific validation parameters for analytical methods like DLS when applied to gene therapy products. These include accuracy, precision, specificity, detection limit, and robustness considerations tailored to the unique challenges of viral and non-viral delivery systems.
Regulatory submissions for gene therapy products must include comprehensive method validation packages for DLS analytics, demonstrating that the technique can reliably detect changes in critical quality attributes of gene therapy vehicles. This includes validation across the product's shelf life and under various stress conditions to ensure stability.
International Conference on Harmonisation (ICH) guidelines, particularly ICH Q2(R1) for analytical procedure validation, provide the framework for validating DLS methods in gene therapy applications. However, regulatory agencies recognize the novel nature of gene therapies and often require case-by-case considerations beyond standard pharmaceutical analytics.
Recent regulatory trends indicate increasing focus on orthogonal analytical approaches, where DLS data must be complemented by other techniques such as nanoparticle tracking analysis (NTA) or transmission electron microscopy (TEM) to provide comprehensive characterization of gene therapy vehicles. This multi-method approach addresses the limitations of any single analytical technique.
Regulatory agencies are also emphasizing the need for reference standards and control materials specific to gene therapy analytics. The development of these standards remains challenging due to the complex and diverse nature of gene therapy vehicles, creating opportunities for industry collaboration with organizations like NIST and USP to establish standardized approaches for DLS implementation.
FDA guidance documents specifically address the characterization of gene therapy vectors, emphasizing the importance of particle size distribution and homogeneity measurements provided by DLS techniques. These regulations require manufacturers to demonstrate consistent quality attributes across production batches, with DLS serving as a critical quality control method for assessing vector aggregation and stability.
The EMA's Committee for Advanced Therapies (CAT) has published guidelines that outline specific validation parameters for analytical methods like DLS when applied to gene therapy products. These include accuracy, precision, specificity, detection limit, and robustness considerations tailored to the unique challenges of viral and non-viral delivery systems.
Regulatory submissions for gene therapy products must include comprehensive method validation packages for DLS analytics, demonstrating that the technique can reliably detect changes in critical quality attributes of gene therapy vehicles. This includes validation across the product's shelf life and under various stress conditions to ensure stability.
International Conference on Harmonisation (ICH) guidelines, particularly ICH Q2(R1) for analytical procedure validation, provide the framework for validating DLS methods in gene therapy applications. However, regulatory agencies recognize the novel nature of gene therapies and often require case-by-case considerations beyond standard pharmaceutical analytics.
Recent regulatory trends indicate increasing focus on orthogonal analytical approaches, where DLS data must be complemented by other techniques such as nanoparticle tracking analysis (NTA) or transmission electron microscopy (TEM) to provide comprehensive characterization of gene therapy vehicles. This multi-method approach addresses the limitations of any single analytical technique.
Regulatory agencies are also emphasizing the need for reference standards and control materials specific to gene therapy analytics. The development of these standards remains challenging due to the complex and diverse nature of gene therapy vehicles, creating opportunities for industry collaboration with organizations like NIST and USP to establish standardized approaches for DLS implementation.
Data Processing Algorithms for DLS Measurements
Data processing algorithms are the cornerstone of accurate and reliable Dynamic Light Scattering (DLS) measurements for gene therapy vehicle analysis. The fundamental algorithm in DLS data processing is the autocorrelation function, which measures the similarity between scattered light intensity at different time intervals. For gene therapy vehicles such as viral vectors, lipid nanoparticles, and exosomes, this function must be optimized to account for their unique physical properties and heterogeneity.
Modern DLS systems employ multiple algorithms to transform raw scattered light data into meaningful size distributions. The cumulants method represents the simplest approach, providing the z-average diameter and polydispersity index (PDI) by analyzing the initial decay rate of the correlation function. While efficient for monodisperse samples, this method has limitations when analyzing the complex, multimodal distributions typical of gene therapy vehicles.
CONTIN and non-negative least squares (NNLS) algorithms offer more sophisticated approaches for heterogeneous samples. These iterative methods decompose the correlation function into multiple exponential decays, enabling the resolution of multiple particle populations within gene therapy formulations. NNLS particularly excels at resolving bimodal distributions, which is crucial when monitoring aggregation or the presence of empty versus loaded vectors.
Machine learning algorithms represent the cutting edge of DLS data processing. Neural networks and support vector machines can be trained to recognize patterns in correlation functions that correspond to specific vector characteristics. These approaches show promise in distinguishing subtle differences between similar-sized particles based on their scattering properties, potentially enabling differentiation between empty and nucleic acid-loaded vectors.
Regularization techniques play a vital role in improving algorithm stability and reducing noise-induced artifacts. Tikhonov regularization and maximum entropy methods help balance the trade-off between resolution and stability in size distribution calculations. For gene therapy applications, where precise size determination is critical for efficacy and safety assessment, these techniques help ensure reliable results even with challenging samples.
Real-time processing algorithms enable continuous monitoring during vector production and formulation. Adaptive algorithms that can adjust parameters based on sample characteristics are particularly valuable for process analytical technology (PAT) applications in gene therapy manufacturing. These algorithms incorporate feedback mechanisms to optimize measurement conditions based on initial scattering intensity readings, ensuring optimal data quality across diverse sample types.
Modern DLS systems employ multiple algorithms to transform raw scattered light data into meaningful size distributions. The cumulants method represents the simplest approach, providing the z-average diameter and polydispersity index (PDI) by analyzing the initial decay rate of the correlation function. While efficient for monodisperse samples, this method has limitations when analyzing the complex, multimodal distributions typical of gene therapy vehicles.
CONTIN and non-negative least squares (NNLS) algorithms offer more sophisticated approaches for heterogeneous samples. These iterative methods decompose the correlation function into multiple exponential decays, enabling the resolution of multiple particle populations within gene therapy formulations. NNLS particularly excels at resolving bimodal distributions, which is crucial when monitoring aggregation or the presence of empty versus loaded vectors.
Machine learning algorithms represent the cutting edge of DLS data processing. Neural networks and support vector machines can be trained to recognize patterns in correlation functions that correspond to specific vector characteristics. These approaches show promise in distinguishing subtle differences between similar-sized particles based on their scattering properties, potentially enabling differentiation between empty and nucleic acid-loaded vectors.
Regularization techniques play a vital role in improving algorithm stability and reducing noise-induced artifacts. Tikhonov regularization and maximum entropy methods help balance the trade-off between resolution and stability in size distribution calculations. For gene therapy applications, where precise size determination is critical for efficacy and safety assessment, these techniques help ensure reliable results even with challenging samples.
Real-time processing algorithms enable continuous monitoring during vector production and formulation. Adaptive algorithms that can adjust parameters based on sample characteristics are particularly valuable for process analytical technology (PAT) applications in gene therapy manufacturing. These algorithms incorporate feedback mechanisms to optimize measurement conditions based on initial scattering intensity readings, ensuring optimal data quality across diverse sample types.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!