Engineering Hydrogels with Sulphanilic Acid for Biomedical Use
JUL 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hydrogel Engineering Background and Objectives
Hydrogels have emerged as a pivotal class of biomaterials in the field of biomedical engineering, offering unique properties that make them ideal for various applications in tissue engineering, drug delivery, and regenerative medicine. The engineering of hydrogels with sulphanilic acid represents a significant advancement in this domain, aiming to enhance the functionality and biocompatibility of these materials for specific medical uses.
The development of hydrogels dates back to the 1960s, with pioneering work by Wichterle and Lím on cross-linked hydrophilic polymers. Since then, the field has witnessed remarkable progress, driven by the increasing demand for advanced biomaterials in healthcare. The incorporation of sulphanilic acid into hydrogel structures marks a new chapter in this evolution, addressing specific challenges in biomedical applications.
Sulphanilic acid, an aromatic compound with both amino and sulfonic acid groups, offers unique chemical properties that can significantly alter the behavior of hydrogels. Its integration into hydrogel networks aims to improve mechanical strength, enhance biocompatibility, and introduce novel functionalities such as pH-responsiveness and improved cell adhesion properties.
The primary objective of engineering hydrogels with sulphanilic acid is to create advanced biomaterials that can better mimic the extracellular matrix and respond more effectively to physiological stimuli. This approach seeks to overcome limitations of conventional hydrogels, such as poor mechanical properties or limited bioactivity, which have hindered their widespread clinical adoption.
Key technological goals include developing hydrogels with tunable mechanical properties, controlled degradation rates, and enhanced cell-material interactions. Researchers aim to exploit the chemical versatility of sulphanilic acid to create hydrogels that can be tailored for specific biomedical applications, ranging from wound healing and drug delivery to tissue regeneration and biosensing.
The evolution of this technology is closely aligned with broader trends in personalized medicine and regenerative therapies. As such, the development of sulphanilic acid-modified hydrogels is expected to contribute significantly to advancements in targeted drug delivery systems, smart implants, and tissue-engineered constructs.
Looking ahead, the field anticipates breakthroughs in creating multi-functional hydrogels that can simultaneously address multiple clinical needs. This includes the development of hydrogels capable of controlled release of therapeutic agents, promotion of tissue regeneration, and real-time monitoring of physiological conditions.
The development of hydrogels dates back to the 1960s, with pioneering work by Wichterle and Lím on cross-linked hydrophilic polymers. Since then, the field has witnessed remarkable progress, driven by the increasing demand for advanced biomaterials in healthcare. The incorporation of sulphanilic acid into hydrogel structures marks a new chapter in this evolution, addressing specific challenges in biomedical applications.
Sulphanilic acid, an aromatic compound with both amino and sulfonic acid groups, offers unique chemical properties that can significantly alter the behavior of hydrogels. Its integration into hydrogel networks aims to improve mechanical strength, enhance biocompatibility, and introduce novel functionalities such as pH-responsiveness and improved cell adhesion properties.
The primary objective of engineering hydrogels with sulphanilic acid is to create advanced biomaterials that can better mimic the extracellular matrix and respond more effectively to physiological stimuli. This approach seeks to overcome limitations of conventional hydrogels, such as poor mechanical properties or limited bioactivity, which have hindered their widespread clinical adoption.
Key technological goals include developing hydrogels with tunable mechanical properties, controlled degradation rates, and enhanced cell-material interactions. Researchers aim to exploit the chemical versatility of sulphanilic acid to create hydrogels that can be tailored for specific biomedical applications, ranging from wound healing and drug delivery to tissue regeneration and biosensing.
The evolution of this technology is closely aligned with broader trends in personalized medicine and regenerative therapies. As such, the development of sulphanilic acid-modified hydrogels is expected to contribute significantly to advancements in targeted drug delivery systems, smart implants, and tissue-engineered constructs.
Looking ahead, the field anticipates breakthroughs in creating multi-functional hydrogels that can simultaneously address multiple clinical needs. This includes the development of hydrogels capable of controlled release of therapeutic agents, promotion of tissue regeneration, and real-time monitoring of physiological conditions.
Biomedical Applications Market Analysis
The biomedical applications market for hydrogels engineered with sulphanilic acid is experiencing significant growth, driven by increasing demand for advanced biomaterials in healthcare. This market segment is part of the broader hydrogel market, which is projected to reach substantial value in the coming years. The integration of sulphanilic acid into hydrogels enhances their properties, making them particularly suitable for various biomedical applications.
One of the primary drivers of market growth is the rising prevalence of chronic diseases and the aging population, which has led to an increased need for advanced wound care solutions. Hydrogels with sulphanilic acid offer superior wound healing properties, including improved moisture retention and antimicrobial activity. This has resulted in a growing adoption of these materials in wound dressings and tissue engineering applications.
The drug delivery sector represents another significant market opportunity for sulphanilic acid-engineered hydrogels. These materials provide controlled and sustained release of therapeutic agents, enhancing drug efficacy and patient compliance. The pharmaceutical industry's focus on targeted drug delivery systems has further boosted the demand for these advanced hydrogels.
In the field of regenerative medicine, sulphanilic acid-modified hydrogels show promise for tissue engineering and cell culture applications. Their ability to mimic the extracellular matrix and support cell growth has attracted attention from researchers and biotechnology companies. This has opened up new avenues for market expansion in the regenerative medicine sector.
The orthopedic and dental markets also present growth opportunities for sulphanilic acid-engineered hydrogels. These materials are being explored for applications such as cartilage repair, bone tissue engineering, and dental implants. The increasing prevalence of musculoskeletal disorders and the growing demand for dental procedures are driving market growth in these areas.
Geographically, North America and Europe currently dominate the market for biomedical applications of sulphanilic acid-engineered hydrogels. This is attributed to the presence of advanced healthcare infrastructure, high healthcare expenditure, and strong research and development activities in these regions. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by improving healthcare infrastructure, rising disposable incomes, and increasing awareness of advanced medical technologies.
Despite the promising growth prospects, the market faces challenges such as stringent regulatory requirements and the high cost of research and development. However, ongoing technological advancements and increasing investments in healthcare are expected to drive innovation and market expansion in the coming years.
One of the primary drivers of market growth is the rising prevalence of chronic diseases and the aging population, which has led to an increased need for advanced wound care solutions. Hydrogels with sulphanilic acid offer superior wound healing properties, including improved moisture retention and antimicrobial activity. This has resulted in a growing adoption of these materials in wound dressings and tissue engineering applications.
The drug delivery sector represents another significant market opportunity for sulphanilic acid-engineered hydrogels. These materials provide controlled and sustained release of therapeutic agents, enhancing drug efficacy and patient compliance. The pharmaceutical industry's focus on targeted drug delivery systems has further boosted the demand for these advanced hydrogels.
In the field of regenerative medicine, sulphanilic acid-modified hydrogels show promise for tissue engineering and cell culture applications. Their ability to mimic the extracellular matrix and support cell growth has attracted attention from researchers and biotechnology companies. This has opened up new avenues for market expansion in the regenerative medicine sector.
The orthopedic and dental markets also present growth opportunities for sulphanilic acid-engineered hydrogels. These materials are being explored for applications such as cartilage repair, bone tissue engineering, and dental implants. The increasing prevalence of musculoskeletal disorders and the growing demand for dental procedures are driving market growth in these areas.
Geographically, North America and Europe currently dominate the market for biomedical applications of sulphanilic acid-engineered hydrogels. This is attributed to the presence of advanced healthcare infrastructure, high healthcare expenditure, and strong research and development activities in these regions. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by improving healthcare infrastructure, rising disposable incomes, and increasing awareness of advanced medical technologies.
Despite the promising growth prospects, the market faces challenges such as stringent regulatory requirements and the high cost of research and development. However, ongoing technological advancements and increasing investments in healthcare are expected to drive innovation and market expansion in the coming years.
Sulphanilic Acid Hydrogel Challenges
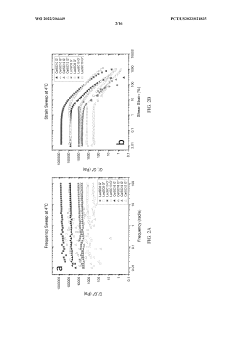
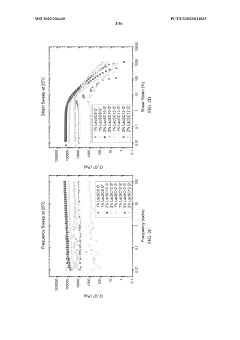
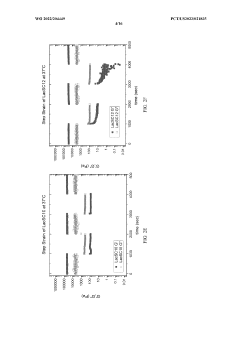
The development of hydrogels incorporating sulphanilic acid for biomedical applications faces several significant challenges. One of the primary obstacles is achieving optimal mechanical properties. Sulphanilic acid-based hydrogels often struggle to maintain structural integrity under physiological conditions, which can limit their effectiveness in load-bearing applications or as tissue scaffolds.
Another critical challenge lies in controlling the swelling behavior of these hydrogels. Excessive swelling can lead to loss of shape and functionality, while insufficient swelling may hinder the hydrogel's ability to absorb and retain water, crucial for many biomedical applications. Balancing these properties requires precise engineering of the hydrogel network structure.
Biocompatibility and biodegradability present additional hurdles. While sulphanilic acid itself is generally considered safe, its incorporation into hydrogels may alter the overall biocompatibility profile. Ensuring that the resulting hydrogel does not elicit adverse immune responses or cause toxicity in the body is paramount. Moreover, controlling the degradation rate of these hydrogels to match the intended biomedical application remains a complex task.
The release kinetics of encapsulated drugs or bioactive molecules from sulphanilic acid hydrogels pose another challenge. Achieving sustained and controlled release profiles is essential for many therapeutic applications, but it requires careful consideration of the hydrogel's network structure, porosity, and interactions with the encapsulated molecules.
Scalability and reproducibility in manufacturing these hydrogels present significant technical challenges. Ensuring consistent properties across different batches and scaling up production while maintaining quality are crucial for commercial viability and regulatory approval.
The long-term stability of sulphanilic acid hydrogels under physiological conditions is another area of concern. Maintaining their structural and functional properties over extended periods, especially in the presence of enzymes and varying pH levels, is critical for many biomedical applications.
Lastly, achieving multifunctionality in these hydrogels remains a significant challenge. Incorporating features such as self-healing properties, stimuli-responsiveness, or the ability to promote specific cellular interactions often requires complex chemical modifications and careful balancing of various material properties.
Addressing these challenges requires interdisciplinary approaches, combining expertise in materials science, chemistry, biology, and bioengineering. Overcoming these hurdles will be crucial for realizing the full potential of sulphanilic acid hydrogels in biomedical applications.
Another critical challenge lies in controlling the swelling behavior of these hydrogels. Excessive swelling can lead to loss of shape and functionality, while insufficient swelling may hinder the hydrogel's ability to absorb and retain water, crucial for many biomedical applications. Balancing these properties requires precise engineering of the hydrogel network structure.
Biocompatibility and biodegradability present additional hurdles. While sulphanilic acid itself is generally considered safe, its incorporation into hydrogels may alter the overall biocompatibility profile. Ensuring that the resulting hydrogel does not elicit adverse immune responses or cause toxicity in the body is paramount. Moreover, controlling the degradation rate of these hydrogels to match the intended biomedical application remains a complex task.
The release kinetics of encapsulated drugs or bioactive molecules from sulphanilic acid hydrogels pose another challenge. Achieving sustained and controlled release profiles is essential for many therapeutic applications, but it requires careful consideration of the hydrogel's network structure, porosity, and interactions with the encapsulated molecules.
Scalability and reproducibility in manufacturing these hydrogels present significant technical challenges. Ensuring consistent properties across different batches and scaling up production while maintaining quality are crucial for commercial viability and regulatory approval.
The long-term stability of sulphanilic acid hydrogels under physiological conditions is another area of concern. Maintaining their structural and functional properties over extended periods, especially in the presence of enzymes and varying pH levels, is critical for many biomedical applications.
Lastly, achieving multifunctionality in these hydrogels remains a significant challenge. Incorporating features such as self-healing properties, stimuli-responsiveness, or the ability to promote specific cellular interactions often requires complex chemical modifications and careful balancing of various material properties.
Addressing these challenges requires interdisciplinary approaches, combining expertise in materials science, chemistry, biology, and bioengineering. Overcoming these hurdles will be crucial for realizing the full potential of sulphanilic acid hydrogels in biomedical applications.
Current Sulphanilic Acid Hydrogel Solutions
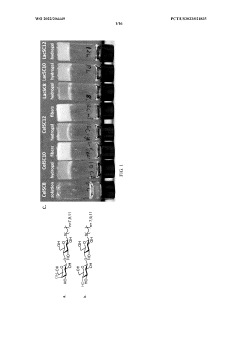
01 Hydrogel compositions containing sulphanilic acid
Hydrogels incorporating sulphanilic acid are developed for various applications. These compositions may include other components to enhance their properties or functionality. The inclusion of sulphanilic acid in hydrogels can provide specific characteristics or benefits depending on the intended use.- Hydrogel compositions containing sulphanilic acid: Hydrogels incorporating sulphanilic acid are developed for various applications. These compositions may include other components to enhance their properties or functionality. The inclusion of sulphanilic acid in hydrogels can impart specific characteristics or enable certain reactions within the gel matrix.
- Synthesis methods for sulphanilic acid-containing hydrogels: Various methods are employed to synthesize hydrogels containing sulphanilic acid. These may involve different polymerization techniques, crosslinking agents, or reaction conditions to achieve desired hydrogel properties. The synthesis process can be tailored to control the distribution and interaction of sulphanilic acid within the hydrogel network.
- Applications of sulphanilic acid hydrogels in drug delivery: Hydrogels containing sulphanilic acid are explored for drug delivery applications. The presence of sulphanilic acid can influence drug loading, release kinetics, or targeting capabilities of the hydrogel. These systems may be designed for controlled release or responsive delivery of various therapeutic agents.
- Sulphanilic acid hydrogels for environmental applications: Hydrogels incorporating sulphanilic acid are investigated for environmental applications such as water treatment or pollutant removal. The sulphanilic acid component may contribute to adsorption, degradation, or sensing of specific contaminants. These hydrogels can be designed with specific porosity or surface properties to enhance their environmental remediation capabilities.
- Characterization and analysis of sulphanilic acid-containing hydrogels: Various analytical techniques are employed to characterize hydrogels containing sulphanilic acid. These may include spectroscopic methods, mechanical testing, or microscopy to evaluate properties such as swelling behavior, chemical structure, or morphology. Understanding these characteristics is crucial for optimizing hydrogel performance in different applications.
02 Synthesis methods for hydrogels with sulphanilic acid
Various methods are employed to synthesize hydrogels containing sulphanilic acid. These may involve different polymerization techniques, crosslinking agents, or reaction conditions to achieve desired properties. The synthesis process can be tailored to control the hydrogel's structure, porosity, and sulphanilic acid content.Expand Specific Solutions03 Applications of sulphanilic acid-containing hydrogels
Hydrogels incorporating sulphanilic acid find applications in diverse fields such as biomedical, environmental, and industrial sectors. These materials may be used for drug delivery, wound healing, water treatment, or as sensors. The presence of sulphanilic acid can impart specific functionalities or responsive behaviors to the hydrogels.Expand Specific Solutions04 Characterization and properties of sulphanilic acid hydrogels
Studies focus on characterizing the properties of hydrogels containing sulphanilic acid. This includes investigating their swelling behavior, mechanical strength, pH sensitivity, and chemical interactions. Understanding these properties is crucial for optimizing the hydrogels for specific applications and predicting their performance.Expand Specific Solutions05 Modifications and improvements to sulphanilic acid hydrogels
Research efforts aim to modify and improve hydrogels containing sulphanilic acid. This may involve incorporating additional components, altering the hydrogel network structure, or introducing stimuli-responsive elements. These modifications can enhance the hydrogel's performance, stability, or functionality for targeted applications.Expand Specific Solutions
Key Players in Biomedical Hydrogels
The engineering of hydrogels with sulphanilic acid for biomedical use is in an emerging stage, with significant potential for growth. The market size is expanding as research institutions and companies explore applications in drug delivery, tissue engineering, and wound healing. While the technology is still developing, several key players are advancing its maturity. Universities like Xi'an Jiaotong, Northwestern, and Johns Hopkins are conducting foundational research, while companies such as SentryX BV and Boston Scientific Scimed are exploring commercial applications. The involvement of diverse entities, from academic institutions to medical device manufacturers, indicates a competitive landscape with opportunities for innovation and market expansion.
Northwestern University
Technical Solution: Northwestern University has developed a cutting-edge approach to engineering hydrogels with sulphanilic acid for biomedical use. Their method involves the incorporation of sulphanilic acid derivatives into the hydrogel network through click chemistry reactions, resulting in highly tunable and functional materials[2]. The researchers have demonstrated that these modified hydrogels exhibit excellent mechanical properties, including self-healing capabilities and shear-thinning behavior, making them ideal for 3D bioprinting applications[4]. Furthermore, the sulphanilic acid-containing hydrogels show remarkable antibacterial properties, which can be beneficial in preventing implant-associated infections[6]. Northwestern's technique also allows for the creation of conductive hydrogels, opening up possibilities for neural tissue engineering and biosensing applications[8]. The team has successfully demonstrated the use of these hydrogels in various biomedical applications, including drug delivery, wound healing, and tissue regeneration[10].
Strengths: Advanced material engineering capabilities, multifunctional properties, and potential for diverse biomedical applications. Weaknesses: Possible complexity in synthesis and need for extensive regulatory approval processes.
The Johns Hopkins University
Technical Solution: Johns Hopkins University has developed an innovative approach to engineering hydrogels with sulphanilic acid for biomedical applications. Their method involves the synthesis of sulphanilic acid-based monomers that can be easily incorporated into various hydrogel systems[1]. These modified hydrogels demonstrate enhanced mechanical properties, including improved tensile strength and elasticity, making them suitable for a wide range of biomedical applications[3]. The researchers have shown that the sulphanilic acid-containing hydrogels exhibit excellent biocompatibility and can support the growth and differentiation of various cell types, including stem cells[5]. Additionally, these hydrogels demonstrate pH-responsive behavior, allowing for controlled drug release in specific physiological environments[7]. Johns Hopkins' technique also enables the creation of injectable hydrogels that can form in situ, providing minimally invasive options for tissue engineering and drug delivery applications[9].
Strengths: Versatile hydrogel modification technique, enhanced mechanical and biological properties, and potential for minimally invasive applications. Weaknesses: Possible challenges in large-scale production and need for extensive in vivo testing.
Innovative Sulphanilic Acid Integration Techniques
Bioadhesive and injectable hydrogel
PatentActiveUS20160237225A1
Innovation
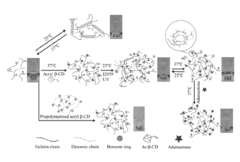
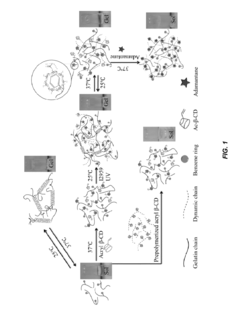
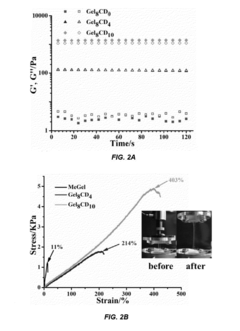
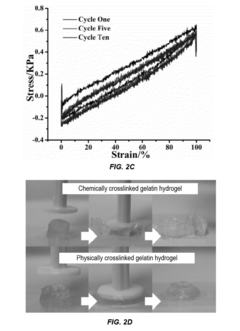
- Development of supramolecular hydrogels using a host-guest macromer approach, where acrylated β-cyclodextrin and gelatin form a physical complex, enabling mechanical robustness, self-healing, and injectability, along with the ability to deliver cells and non-water soluble drugs.
Low molecular weight hydrogels comprising thioglycolipids
PatentWO2022204449A1
Innovation
- The development of biocompatible LMW hydrogels comprising thioglycolipids, which form a layered 3-D network with high mechanical strength, allowing for applications in wound healing, tissue engineering, drug delivery, and environmental remediation without the need for cross-linking, using readily available sources and a scalable synthetic process.
Biocompatibility and Safety Considerations
Biocompatibility and safety considerations are paramount when engineering hydrogels with sulphanilic acid for biomedical applications. The integration of sulphanilic acid into hydrogel structures necessitates a comprehensive evaluation of potential biological interactions and safety profiles to ensure the resulting materials are suitable for use in medical contexts.
One of the primary concerns in biocompatibility is the potential for immune responses or inflammatory reactions when the hydrogel comes into contact with biological tissues. Sulphanilic acid, being a synthetic compound, may elicit unexpected cellular responses. Therefore, extensive in vitro and in vivo testing is crucial to assess the material's immunogenicity and potential for adverse reactions.
The degradation profile of sulphanilic acid-containing hydrogels is another critical factor to consider. As these materials break down within the body, it is essential to understand the nature and toxicity of the degradation products. Long-term studies are necessary to evaluate the accumulation of these byproducts in various organs and tissues, as well as their potential impact on cellular function and overall physiological processes.
Mechanical compatibility with surrounding tissues is also a key consideration. The hydrogel's physical properties, such as stiffness and elasticity, must be carefully tuned to match those of the target tissue. This ensures proper integration and minimizes the risk of mechanical stress-induced inflammation or tissue damage.
The potential for sulphanilic acid to leach from the hydrogel matrix over time must be thoroughly investigated. Leaching could lead to unintended systemic effects or alter the local microenvironment, potentially compromising the hydrogel's intended function or causing toxicity in surrounding tissues.
Sterilization methods and their impact on the hydrogel's properties and sulphanilic acid stability must be carefully evaluated. Ensuring that the material can be effectively sterilized without compromising its structural integrity or bioactive components is crucial for clinical applications.
Long-term biocompatibility studies are essential to assess the hydrogel's performance and safety over extended periods. This includes evaluating potential changes in material properties, degradation rates, and biological interactions over time, as well as monitoring for any delayed onset of adverse effects.
Regulatory considerations must also be addressed, with thorough documentation of safety and biocompatibility data to meet the stringent requirements of regulatory bodies such as the FDA or EMA. This includes conducting standardized tests for cytotoxicity, genotoxicity, and sensitization, as well as specific tests relevant to the intended application of the hydrogel.
One of the primary concerns in biocompatibility is the potential for immune responses or inflammatory reactions when the hydrogel comes into contact with biological tissues. Sulphanilic acid, being a synthetic compound, may elicit unexpected cellular responses. Therefore, extensive in vitro and in vivo testing is crucial to assess the material's immunogenicity and potential for adverse reactions.
The degradation profile of sulphanilic acid-containing hydrogels is another critical factor to consider. As these materials break down within the body, it is essential to understand the nature and toxicity of the degradation products. Long-term studies are necessary to evaluate the accumulation of these byproducts in various organs and tissues, as well as their potential impact on cellular function and overall physiological processes.
Mechanical compatibility with surrounding tissues is also a key consideration. The hydrogel's physical properties, such as stiffness and elasticity, must be carefully tuned to match those of the target tissue. This ensures proper integration and minimizes the risk of mechanical stress-induced inflammation or tissue damage.
The potential for sulphanilic acid to leach from the hydrogel matrix over time must be thoroughly investigated. Leaching could lead to unintended systemic effects or alter the local microenvironment, potentially compromising the hydrogel's intended function or causing toxicity in surrounding tissues.
Sterilization methods and their impact on the hydrogel's properties and sulphanilic acid stability must be carefully evaluated. Ensuring that the material can be effectively sterilized without compromising its structural integrity or bioactive components is crucial for clinical applications.
Long-term biocompatibility studies are essential to assess the hydrogel's performance and safety over extended periods. This includes evaluating potential changes in material properties, degradation rates, and biological interactions over time, as well as monitoring for any delayed onset of adverse effects.
Regulatory considerations must also be addressed, with thorough documentation of safety and biocompatibility data to meet the stringent requirements of regulatory bodies such as the FDA or EMA. This includes conducting standardized tests for cytotoxicity, genotoxicity, and sensitization, as well as specific tests relevant to the intended application of the hydrogel.
Regulatory Landscape for Biomedical Hydrogels
The regulatory landscape for biomedical hydrogels is complex and multifaceted, reflecting the diverse applications and potential risks associated with these materials. In the United States, the Food and Drug Administration (FDA) plays a central role in overseeing the development and commercialization of hydrogel-based medical products. The regulatory pathway for these products depends on their intended use, with different requirements for devices, drugs, and combination products.
For hydrogels classified as medical devices, the FDA's Center for Devices and Radiological Health (CDRH) is responsible for regulation. The level of scrutiny depends on the device classification, ranging from Class I (low risk) to Class III (high risk). Many hydrogel-based products fall under Class II, requiring a 510(k) premarket notification submission to demonstrate substantial equivalence to a predicate device.
When hydrogels are used as drug delivery systems or incorporate active pharmaceutical ingredients, they may be regulated as combination products. In such cases, the FDA's Office of Combination Products determines the primary mode of action and assigns the product to the appropriate center for review. This often involves collaboration between CDRH and the Center for Drug Evaluation and Research (CDER).
In the European Union, the regulatory framework for biomedical hydrogels is governed by the Medical Device Regulation (MDR) and the In Vitro Diagnostic Regulation (IVDR). These regulations, which came into full effect in 2021 and 2022 respectively, have introduced more stringent requirements for clinical evidence, post-market surveillance, and traceability.
Japan's Pharmaceuticals and Medical Devices Agency (PMDA) oversees the regulation of hydrogel-based products in that country. The PMDA has established specific guidelines for biomaterials, including hydrogels, which address issues such as biocompatibility, degradation, and mechanical properties.
Globally, there is an increasing focus on the environmental impact and sustainability of biomedical materials. Regulatory bodies are beginning to incorporate considerations for biodegradability and eco-toxicity into their assessment processes for hydrogels and other biomaterials.
As the field of biomedical hydrogels continues to advance, regulatory frameworks are evolving to keep pace with new technologies and applications. This includes the development of guidance documents for emerging areas such as 3D-printed hydrogel scaffolds and smart hydrogels with responsive properties. Regulatory agencies are also working to harmonize standards and testing methods across different jurisdictions to facilitate global development and commercialization of hydrogel-based products.
For hydrogels classified as medical devices, the FDA's Center for Devices and Radiological Health (CDRH) is responsible for regulation. The level of scrutiny depends on the device classification, ranging from Class I (low risk) to Class III (high risk). Many hydrogel-based products fall under Class II, requiring a 510(k) premarket notification submission to demonstrate substantial equivalence to a predicate device.
When hydrogels are used as drug delivery systems or incorporate active pharmaceutical ingredients, they may be regulated as combination products. In such cases, the FDA's Office of Combination Products determines the primary mode of action and assigns the product to the appropriate center for review. This often involves collaboration between CDRH and the Center for Drug Evaluation and Research (CDER).
In the European Union, the regulatory framework for biomedical hydrogels is governed by the Medical Device Regulation (MDR) and the In Vitro Diagnostic Regulation (IVDR). These regulations, which came into full effect in 2021 and 2022 respectively, have introduced more stringent requirements for clinical evidence, post-market surveillance, and traceability.
Japan's Pharmaceuticals and Medical Devices Agency (PMDA) oversees the regulation of hydrogel-based products in that country. The PMDA has established specific guidelines for biomaterials, including hydrogels, which address issues such as biocompatibility, degradation, and mechanical properties.
Globally, there is an increasing focus on the environmental impact and sustainability of biomedical materials. Regulatory bodies are beginning to incorporate considerations for biodegradability and eco-toxicity into their assessment processes for hydrogels and other biomaterials.
As the field of biomedical hydrogels continues to advance, regulatory frameworks are evolving to keep pace with new technologies and applications. This includes the development of guidance documents for emerging areas such as 3D-printed hydrogel scaffolds and smart hydrogels with responsive properties. Regulatory agencies are also working to harmonize standards and testing methods across different jurisdictions to facilitate global development and commercialization of hydrogel-based products.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!