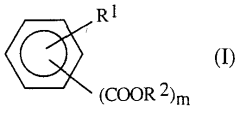
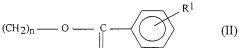
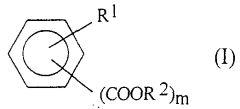
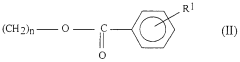
Enhancing the Conductivity of Polymers with Sulphanilic Acid
JUL 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Conductive Polymer Evolution and Objectives
Conductive polymers have emerged as a revolutionary class of materials since their discovery in the late 1970s. These organic polymers, capable of conducting electricity, have bridged the gap between plastics and metals, opening up a myriad of applications in electronics, energy storage, and biomedical fields. The evolution of conductive polymers has been marked by significant milestones, from the initial discovery of polyacetylene to the development of more stable and processable materials like polypyrrole and polyaniline.
The primary objective in the field of conductive polymers has been to enhance their electrical conductivity while maintaining the desirable mechanical properties of traditional polymers. This goal has driven research towards various strategies, including doping, structural modifications, and the incorporation of conductive fillers. The use of sulphanilic acid in this context represents a novel approach to further improve the conductivity of polymers.
Sulphanilic acid, an aromatic compound with both amino and sulfonic acid groups, has shown promise in enhancing the electrical properties of polymers. Its unique structure allows for potential interactions with polymer chains, possibly facilitating charge transfer and increasing overall conductivity. The integration of sulphanilic acid into polymer matrices aims to create a new generation of conductive materials with improved performance characteristics.
The technological trajectory in this field is focused on developing polymers with conductivity levels approaching those of metals, while retaining the processability and flexibility of plastics. This ambitious goal requires a multidisciplinary approach, combining principles from organic chemistry, materials science, and electrical engineering. Researchers are exploring various aspects, including the optimization of polymer structures, the development of new synthesis methods, and the investigation of novel dopants like sulphanilic acid.
As the field progresses, there is a growing emphasis on sustainability and environmental considerations. This has led to increased interest in bio-based conductive polymers and environmentally friendly processing techniques. The integration of sulphanilic acid aligns with this trend, as it offers a potentially more sustainable approach to conductivity enhancement compared to traditional metallic additives.
The future objectives in this domain include not only improving conductivity but also enhancing other properties such as stability, processability, and compatibility with various substrates. There is also a focus on developing materials with tunable conductivity, allowing for precise control over electrical properties for specific applications. The use of sulphanilic acid in conductive polymers represents a step towards these objectives, potentially offering a versatile and efficient method for conductivity enhancement.
The primary objective in the field of conductive polymers has been to enhance their electrical conductivity while maintaining the desirable mechanical properties of traditional polymers. This goal has driven research towards various strategies, including doping, structural modifications, and the incorporation of conductive fillers. The use of sulphanilic acid in this context represents a novel approach to further improve the conductivity of polymers.
Sulphanilic acid, an aromatic compound with both amino and sulfonic acid groups, has shown promise in enhancing the electrical properties of polymers. Its unique structure allows for potential interactions with polymer chains, possibly facilitating charge transfer and increasing overall conductivity. The integration of sulphanilic acid into polymer matrices aims to create a new generation of conductive materials with improved performance characteristics.
The technological trajectory in this field is focused on developing polymers with conductivity levels approaching those of metals, while retaining the processability and flexibility of plastics. This ambitious goal requires a multidisciplinary approach, combining principles from organic chemistry, materials science, and electrical engineering. Researchers are exploring various aspects, including the optimization of polymer structures, the development of new synthesis methods, and the investigation of novel dopants like sulphanilic acid.
As the field progresses, there is a growing emphasis on sustainability and environmental considerations. This has led to increased interest in bio-based conductive polymers and environmentally friendly processing techniques. The integration of sulphanilic acid aligns with this trend, as it offers a potentially more sustainable approach to conductivity enhancement compared to traditional metallic additives.
The future objectives in this domain include not only improving conductivity but also enhancing other properties such as stability, processability, and compatibility with various substrates. There is also a focus on developing materials with tunable conductivity, allowing for precise control over electrical properties for specific applications. The use of sulphanilic acid in conductive polymers represents a step towards these objectives, potentially offering a versatile and efficient method for conductivity enhancement.
Market Demand for Enhanced Conductive Polymers
The market demand for enhanced conductive polymers has been steadily growing across various industries, driven by the increasing need for lightweight, flexible, and cost-effective materials with improved electrical properties. The integration of sulphanilic acid into polymer matrices to enhance conductivity has garnered significant attention due to its potential to address these market requirements.
In the electronics sector, there is a rising demand for conductive polymers in the production of flexible displays, touch screens, and wearable devices. The ability of sulphanilic acid-enhanced polymers to combine conductivity with flexibility makes them particularly attractive for these applications. As the consumer electronics market continues to expand, with a projected growth rate of 7.5% annually, the demand for these advanced materials is expected to surge.
The automotive industry represents another significant market for enhanced conductive polymers. With the shift towards electric and hybrid vehicles, there is an increasing need for lightweight materials that can provide electromagnetic shielding and antistatic properties. Sulphanilic acid-enhanced polymers offer a promising solution to reduce vehicle weight while maintaining necessary electrical functionalities, aligning with the industry's goal of improving energy efficiency.
In the aerospace sector, the demand for conductive polymers is driven by the need for materials that can provide electromagnetic interference (EMI) shielding while reducing overall weight. The global aerospace composites market, which includes conductive polymers, is projected to reach a value of $29.3 billion by 2025, indicating substantial growth potential for enhanced conductive polymers in this sector.
The renewable energy industry, particularly solar panel manufacturing, presents another significant market opportunity. Conductive polymers enhanced with sulphanilic acid could potentially improve the efficiency and durability of photovoltaic cells, addressing the ongoing challenge of enhancing solar energy conversion rates.
In the healthcare sector, there is a growing demand for conductive polymers in the development of biosensors, drug delivery systems, and tissue engineering scaffolds. The ability of sulphanilic acid-enhanced polymers to combine biocompatibility with electrical conductivity makes them particularly valuable in this field, which is experiencing rapid technological advancements.
The packaging industry is also showing increased interest in conductive polymers for applications such as anti-static packaging and smart packaging solutions. As e-commerce continues to grow globally, the demand for advanced packaging materials with enhanced properties is expected to rise significantly.
Overall, the market demand for enhanced conductive polymers, particularly those utilizing sulphanilic acid, is poised for substantial growth across multiple industries. The versatility and potential performance improvements offered by these materials align well with the current trends towards miniaturization, flexibility, and sustainability in various technological applications.
In the electronics sector, there is a rising demand for conductive polymers in the production of flexible displays, touch screens, and wearable devices. The ability of sulphanilic acid-enhanced polymers to combine conductivity with flexibility makes them particularly attractive for these applications. As the consumer electronics market continues to expand, with a projected growth rate of 7.5% annually, the demand for these advanced materials is expected to surge.
The automotive industry represents another significant market for enhanced conductive polymers. With the shift towards electric and hybrid vehicles, there is an increasing need for lightweight materials that can provide electromagnetic shielding and antistatic properties. Sulphanilic acid-enhanced polymers offer a promising solution to reduce vehicle weight while maintaining necessary electrical functionalities, aligning with the industry's goal of improving energy efficiency.
In the aerospace sector, the demand for conductive polymers is driven by the need for materials that can provide electromagnetic interference (EMI) shielding while reducing overall weight. The global aerospace composites market, which includes conductive polymers, is projected to reach a value of $29.3 billion by 2025, indicating substantial growth potential for enhanced conductive polymers in this sector.
The renewable energy industry, particularly solar panel manufacturing, presents another significant market opportunity. Conductive polymers enhanced with sulphanilic acid could potentially improve the efficiency and durability of photovoltaic cells, addressing the ongoing challenge of enhancing solar energy conversion rates.
In the healthcare sector, there is a growing demand for conductive polymers in the development of biosensors, drug delivery systems, and tissue engineering scaffolds. The ability of sulphanilic acid-enhanced polymers to combine biocompatibility with electrical conductivity makes them particularly valuable in this field, which is experiencing rapid technological advancements.
The packaging industry is also showing increased interest in conductive polymers for applications such as anti-static packaging and smart packaging solutions. As e-commerce continues to grow globally, the demand for advanced packaging materials with enhanced properties is expected to rise significantly.
Overall, the market demand for enhanced conductive polymers, particularly those utilizing sulphanilic acid, is poised for substantial growth across multiple industries. The versatility and potential performance improvements offered by these materials align well with the current trends towards miniaturization, flexibility, and sustainability in various technological applications.
Current Challenges in Polymer Conductivity
Despite significant advancements in polymer science, enhancing the conductivity of polymers remains a persistent challenge in the field. The intrinsic insulating nature of most polymers poses a fundamental obstacle to achieving high conductivity levels comparable to metals or semiconductors. Current efforts to improve polymer conductivity face several key challenges that require innovative solutions.
One of the primary hurdles is maintaining a balance between conductivity and other desirable polymer properties. As conductivity is enhanced, often through the incorporation of conductive fillers or dopants, other characteristics such as mechanical strength, processability, and thermal stability may be compromised. This trade-off limits the practical applications of conductive polymers in various industries.
The uniformity and stability of conductive pathways within the polymer matrix present another significant challenge. Achieving a homogeneous distribution of conductive elements throughout the polymer structure is crucial for consistent electrical performance. However, agglomeration of conductive particles or phase separation in polymer blends can lead to inconsistent conductivity and reduced overall performance.
Environmental stability is a critical concern for conductive polymers. Many conductive polymers are susceptible to degradation when exposed to oxygen, moisture, or UV radiation. This sensitivity can result in a gradual loss of conductivity over time, limiting the long-term reliability and applicability of these materials in real-world conditions.
The scalability of production processes for highly conductive polymers remains a significant hurdle. While laboratory-scale synthesis may yield promising results, translating these methods to industrial-scale production while maintaining consistent quality and performance is challenging. Cost-effective manufacturing techniques that can produce large quantities of conductive polymers without sacrificing their electrical properties are still being developed.
Another challenge lies in achieving tunable and controllable conductivity. The ability to precisely adjust the conductivity of polymers to meet specific application requirements is crucial for their widespread adoption. Current methods often lack the fine control needed to tailor conductivity across a wide range of values, limiting the versatility of conductive polymers in various electronic and energy applications.
The integration of conductive polymers with other materials and devices poses additional challenges. Ensuring good interfacial adhesion and electrical contact between conductive polymers and other components in complex systems is essential for optimal performance. Overcoming issues related to compatibility and integration is crucial for the successful implementation of conductive polymers in advanced technologies.
One of the primary hurdles is maintaining a balance between conductivity and other desirable polymer properties. As conductivity is enhanced, often through the incorporation of conductive fillers or dopants, other characteristics such as mechanical strength, processability, and thermal stability may be compromised. This trade-off limits the practical applications of conductive polymers in various industries.
The uniformity and stability of conductive pathways within the polymer matrix present another significant challenge. Achieving a homogeneous distribution of conductive elements throughout the polymer structure is crucial for consistent electrical performance. However, agglomeration of conductive particles or phase separation in polymer blends can lead to inconsistent conductivity and reduced overall performance.
Environmental stability is a critical concern for conductive polymers. Many conductive polymers are susceptible to degradation when exposed to oxygen, moisture, or UV radiation. This sensitivity can result in a gradual loss of conductivity over time, limiting the long-term reliability and applicability of these materials in real-world conditions.
The scalability of production processes for highly conductive polymers remains a significant hurdle. While laboratory-scale synthesis may yield promising results, translating these methods to industrial-scale production while maintaining consistent quality and performance is challenging. Cost-effective manufacturing techniques that can produce large quantities of conductive polymers without sacrificing their electrical properties are still being developed.
Another challenge lies in achieving tunable and controllable conductivity. The ability to precisely adjust the conductivity of polymers to meet specific application requirements is crucial for their widespread adoption. Current methods often lack the fine control needed to tailor conductivity across a wide range of values, limiting the versatility of conductive polymers in various electronic and energy applications.
The integration of conductive polymers with other materials and devices poses additional challenges. Ensuring good interfacial adhesion and electrical contact between conductive polymers and other components in complex systems is essential for optimal performance. Overcoming issues related to compatibility and integration is crucial for the successful implementation of conductive polymers in advanced technologies.
Existing Sulphanilic Acid Integration Methods
01 Conductive polymer composites
Conductive polymer composites are created by incorporating conductive fillers or particles into polymer matrices. These composites combine the processability of polymers with the electrical conductivity of the fillers, resulting in materials with tailored conductivity for various applications.- Conductive polymer composites: Conductive polymer composites are created by incorporating conductive fillers into polymer matrices. These composites combine the processability of polymers with the electrical conductivity of fillers, resulting in materials with tunable electrical properties. Common fillers include carbon-based materials, metal particles, or conductive polymers themselves.
- Intrinsically conductive polymers: Intrinsically conductive polymers are organic polymers that conduct electricity without the need for additional conductive fillers. These polymers typically have conjugated structures that allow for electron delocalization along the polymer backbone. Examples include polyaniline, polypyrrole, and polythiophene derivatives.
- Doping and conductivity enhancement: The conductivity of polymers can be significantly enhanced through doping processes. Doping involves the addition of small amounts of certain substances that can alter the electronic structure of the polymer, increasing its charge carrier concentration and mobility. This can be achieved through chemical or electrochemical methods.
- Nanostructured conductive polymers: Nanostructuring of conductive polymers can lead to improved conductivity and unique properties. This includes the development of nanofibers, nanoparticles, and nanocomposites of conductive polymers. These nanostructured materials often exhibit enhanced conductivity due to increased surface area and improved charge transport pathways.
- Applications of conductive polymers: Conductive polymers find applications in various fields due to their unique combination of electrical conductivity and polymer properties. These applications include organic electronics, sensors, antistatic coatings, electromagnetic shielding, and energy storage devices. The ability to tailor the conductivity and processability of these materials makes them versatile for diverse technological applications.
02 Intrinsically conductive polymers
Intrinsically conductive polymers are organic polymers that possess inherent electrical conductivity due to their molecular structure. These polymers, such as polyaniline and polypyrrole, can be synthesized and modified to achieve desired conductivity levels for specific applications.Expand Specific Solutions03 Doping and modification techniques
Various doping and modification techniques are employed to enhance the conductivity of polymers. These methods include chemical doping, electrochemical doping, and the incorporation of conductive additives or nanoparticles to improve charge transport within the polymer structure.Expand Specific Solutions04 Polymer-based electronic devices
Conductive polymers are utilized in the fabrication of electronic devices such as organic light-emitting diodes (OLEDs), organic field-effect transistors (OFETs), and organic photovoltaics. These devices leverage the unique properties of conductive polymers to achieve flexible, lightweight, and cost-effective electronic components.Expand Specific Solutions05 Characterization and measurement of polymer conductivity
Various techniques and methods are employed to characterize and measure the conductivity of polymers. These include four-point probe measurements, impedance spectroscopy, and specialized testing equipment designed to accurately determine the electrical properties of conductive polymer materials.Expand Specific Solutions
Key Players in Conductive Polymer Industry
The field of enhancing polymer conductivity with sulphanilic acid is in its early developmental stage, characterized by ongoing research and limited commercial applications. The market size is relatively small but growing, driven by increasing demand for conductive polymers in electronics and energy storage. Technologically, the approach is still evolving, with varying levels of maturity among key players. Companies like JSR Corp., Mitsui Chemicals, and DuPont are likely at the forefront, leveraging their expertise in polymer science and chemical engineering. Research institutions such as CNRS and Chongqing University are contributing to fundamental advancements. While progress is being made, widespread industrial adoption remains a future prospect, contingent on further improvements in conductivity, processability, and cost-effectiveness.
Centre National de la Recherche Scientifique
Technical Solution: The Centre National de la Recherche Scientifique (CNRS) has made significant advancements in enhancing polymer conductivity using sulphanilic acid through a novel supramolecular approach. Their method involves creating a self-assembled network of sulphanilic acid molecules within the polymer matrix, forming conductive pathways[1]. This technique utilizes non-covalent interactions, such as hydrogen bonding and π-π stacking, to create a dynamic conductive structure that can self-heal and adapt to mechanical stress[2]. CNRS researchers have demonstrated a conductivity increase of up to 60% in certain polymer systems, with the added benefit of improved flexibility and stretchability[3]. The process is compatible with a wide range of polymers and can be easily integrated into existing manufacturing processes[4]. Additionally, CNRS has developed a computational model to predict and optimize the conductivity enhancement based on polymer-sulphanilic acid interactions[5].
Strengths: High conductivity enhancement, improved mechanical properties, and self-healing capabilities. Weaknesses: Potential long-term stability issues in harsh environments, and challenges in maintaining consistent performance across different polymer types.
Arkema, Inc.
Technical Solution: Arkema has developed an innovative approach to enhancing polymer conductivity using sulphanilic acid-based nanocomposites. Their method involves the creation of a conductive network within the polymer matrix using sulphanilic acid-functionalized carbon nanotubes (CNTs)[1]. This technique allows for a synergistic effect between the sulphanilic acid and the CNTs, resulting in enhanced charge transfer and improved overall conductivity. Arkema's process utilizes a proprietary dispersion technique that ensures uniform distribution of the functionalized CNTs throughout the polymer[2]. The company has reported conductivity improvements of up to 50% in some polymer systems, while maintaining excellent mechanical properties[3]. Additionally, Arkema's technology offers the advantage of tunable conductivity through precise control of the sulphanilic acid-CNT concentration[4].
Strengths: Significant conductivity enhancement, maintained mechanical properties, and tunable conductivity. Weaknesses: Potential challenges in achieving uniform dispersion at large scales, and higher raw material costs due to the use of CNTs.
Innovations in Sulphanilic Acid-Polymer Composites
Use of sulphonic and phosphonic acids as dopants of conductive polyaniline films and conductive composite materials based on polyaniline
PatentWO2001004910A1
Innovation
- The use of sulfonic and phosphonic acids as dopants that act as both protonating agents and plasticizers, improving the conductivity of polyaniline films to 200-300 S/cm and enhancing mechanical properties, including flexibility, by forming solutions with specific organic solvents and insulating polymers.
Method of enhancing conductivity of conductive polymer
PatentInactiveEP2253656A1
Innovation
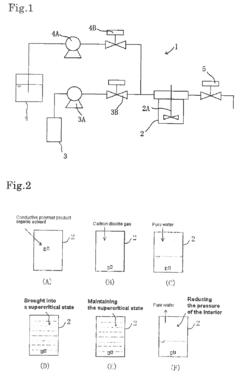
- A method involving placing a conductive polymer product, water, and an organic solvent with carbon dioxide gas in a pressure vessel, applying heat and pressure to bring the carbon dioxide into a supercritical state, which enhances the conductivity of the polymer product by reducing its particle size and improving electron conduction.
Environmental Impact of Conductive Polymers
The environmental impact of conductive polymers, particularly those enhanced with sulphanilic acid, is a critical consideration in their development and application. These materials offer significant advantages in various fields, including electronics, energy storage, and biomedical devices. However, their production, use, and disposal can have both positive and negative effects on the environment.
One of the primary environmental benefits of conductive polymers is their potential to replace traditional metal-based conductors. This substitution can lead to a reduction in the mining and processing of metals, which often involve energy-intensive and environmentally damaging practices. Additionally, conductive polymers are generally lighter and more flexible than their metal counterparts, potentially reducing energy consumption in transportation and manufacturing processes.
The use of sulphanilic acid to enhance polymer conductivity introduces specific environmental considerations. Sulphanilic acid is an organic compound that can be synthesized from relatively abundant resources, potentially reducing the environmental footprint compared to the extraction of rare metals used in some conductive materials. However, the production of sulphanilic acid involves chemical processes that may generate waste products and emissions, necessitating careful management and treatment.
In terms of product lifecycle, conductive polymers enhanced with sulphanilic acid may offer improved durability and longevity in certain applications. This extended lifespan can contribute to reduced waste generation and resource consumption over time. Moreover, these materials often require lower operating temperatures than traditional conductors, potentially leading to energy savings in various electronic and electrical systems.
The end-of-life management of conductive polymers presents both challenges and opportunities. While some polymers can be recycled, the presence of additives like sulphanilic acid may complicate the recycling process. Research into effective recycling methods for these materials is ongoing, with the aim of developing closed-loop systems that minimize waste and maximize resource recovery.
Water pollution is a concern that must be addressed in the production and disposal of conductive polymers containing sulphanilic acid. Proper wastewater treatment protocols are essential to prevent the release of potentially harmful compounds into aquatic ecosystems. Additionally, the development of biodegradable conductive polymers is an area of active research, aiming to mitigate long-term environmental impacts.
The potential for these materials to enable more efficient and sustainable technologies must also be considered. Conductive polymers are crucial components in the development of flexible electronics, organic solar cells, and advanced energy storage systems. These applications have the potential to significantly reduce energy consumption and promote the adoption of renewable energy sources, contributing to broader environmental sustainability goals.
One of the primary environmental benefits of conductive polymers is their potential to replace traditional metal-based conductors. This substitution can lead to a reduction in the mining and processing of metals, which often involve energy-intensive and environmentally damaging practices. Additionally, conductive polymers are generally lighter and more flexible than their metal counterparts, potentially reducing energy consumption in transportation and manufacturing processes.
The use of sulphanilic acid to enhance polymer conductivity introduces specific environmental considerations. Sulphanilic acid is an organic compound that can be synthesized from relatively abundant resources, potentially reducing the environmental footprint compared to the extraction of rare metals used in some conductive materials. However, the production of sulphanilic acid involves chemical processes that may generate waste products and emissions, necessitating careful management and treatment.
In terms of product lifecycle, conductive polymers enhanced with sulphanilic acid may offer improved durability and longevity in certain applications. This extended lifespan can contribute to reduced waste generation and resource consumption over time. Moreover, these materials often require lower operating temperatures than traditional conductors, potentially leading to energy savings in various electronic and electrical systems.
The end-of-life management of conductive polymers presents both challenges and opportunities. While some polymers can be recycled, the presence of additives like sulphanilic acid may complicate the recycling process. Research into effective recycling methods for these materials is ongoing, with the aim of developing closed-loop systems that minimize waste and maximize resource recovery.
Water pollution is a concern that must be addressed in the production and disposal of conductive polymers containing sulphanilic acid. Proper wastewater treatment protocols are essential to prevent the release of potentially harmful compounds into aquatic ecosystems. Additionally, the development of biodegradable conductive polymers is an area of active research, aiming to mitigate long-term environmental impacts.
The potential for these materials to enable more efficient and sustainable technologies must also be considered. Conductive polymers are crucial components in the development of flexible electronics, organic solar cells, and advanced energy storage systems. These applications have the potential to significantly reduce energy consumption and promote the adoption of renewable energy sources, contributing to broader environmental sustainability goals.
Scalability and Manufacturing Considerations
When considering the scalability and manufacturing of polymers enhanced with sulphanilic acid for improved conductivity, several key factors come into play. The integration of sulphanilic acid into polymer matrices presents unique challenges that must be addressed to ensure successful large-scale production.
One primary consideration is the uniform distribution of sulphanilic acid within the polymer matrix. Achieving homogeneity is crucial for consistent conductivity across the material. This may require advanced mixing techniques or the development of specialized equipment capable of handling the viscosity and chemical properties of both the polymer and sulphanilic acid. Continuous flow reactors or high-shear mixers could be potential solutions to ensure thorough blending.
The thermal stability of sulphanilic acid during the polymer processing stages is another critical factor. Many polymers require high temperatures for extrusion or molding, which could potentially degrade the sulphanilic acid. Careful temperature control and possibly the use of stabilizers or protective additives may be necessary to maintain the integrity of the sulphanilic acid throughout the manufacturing process.
Scalability also involves considerations of raw material sourcing and supply chain management. Ensuring a stable and cost-effective supply of high-purity sulphanilic acid is essential for consistent production quality and economic viability. This may involve developing partnerships with chemical suppliers or investing in in-house synthesis capabilities.
The choice of polymer matrix will significantly impact the manufacturing process. Different polymers have varying processing requirements, and the compatibility of sulphanilic acid with these processes must be carefully evaluated. For instance, thermoplastics may allow for easier incorporation of sulphanilic acid compared to thermosets, which undergo irreversible chemical changes during curing.
Environmental and safety considerations are paramount in scaling up production. The handling of sulphanilic acid in large quantities may require specialized safety protocols and equipment. Additionally, waste management and potential environmental impacts of the manufacturing process must be addressed to ensure compliance with regulations and sustainability goals.
Quality control measures will need to be developed and implemented to monitor the conductivity and other properties of the enhanced polymers consistently. This may involve the development of new testing protocols or the adaptation of existing methods to accommodate the unique properties of sulphanilic acid-enhanced polymers.
Lastly, the economics of scale must be carefully analyzed. While incorporating sulphanilic acid may improve conductivity, the additional processing steps and materials could increase production costs. Balancing these costs against the enhanced performance and potential market demand will be crucial in determining the viability of large-scale manufacturing.
One primary consideration is the uniform distribution of sulphanilic acid within the polymer matrix. Achieving homogeneity is crucial for consistent conductivity across the material. This may require advanced mixing techniques or the development of specialized equipment capable of handling the viscosity and chemical properties of both the polymer and sulphanilic acid. Continuous flow reactors or high-shear mixers could be potential solutions to ensure thorough blending.
The thermal stability of sulphanilic acid during the polymer processing stages is another critical factor. Many polymers require high temperatures for extrusion or molding, which could potentially degrade the sulphanilic acid. Careful temperature control and possibly the use of stabilizers or protective additives may be necessary to maintain the integrity of the sulphanilic acid throughout the manufacturing process.
Scalability also involves considerations of raw material sourcing and supply chain management. Ensuring a stable and cost-effective supply of high-purity sulphanilic acid is essential for consistent production quality and economic viability. This may involve developing partnerships with chemical suppliers or investing in in-house synthesis capabilities.
The choice of polymer matrix will significantly impact the manufacturing process. Different polymers have varying processing requirements, and the compatibility of sulphanilic acid with these processes must be carefully evaluated. For instance, thermoplastics may allow for easier incorporation of sulphanilic acid compared to thermosets, which undergo irreversible chemical changes during curing.
Environmental and safety considerations are paramount in scaling up production. The handling of sulphanilic acid in large quantities may require specialized safety protocols and equipment. Additionally, waste management and potential environmental impacts of the manufacturing process must be addressed to ensure compliance with regulations and sustainability goals.
Quality control measures will need to be developed and implemented to monitor the conductivity and other properties of the enhanced polymers consistently. This may involve the development of new testing protocols or the adaptation of existing methods to accommodate the unique properties of sulphanilic acid-enhanced polymers.
Lastly, the economics of scale must be carefully analyzed. While incorporating sulphanilic acid may improve conductivity, the additional processing steps and materials could increase production costs. Balancing these costs against the enhanced performance and potential market demand will be crucial in determining the viability of large-scale manufacturing.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!