Environmental Implications of Perchloric Acid Usage in Industrial Settings
AUG 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Perchloric Acid Usage Background and Objectives
Perchloric acid, a powerful oxidizing agent, has been utilized in various industrial applications for decades. Its unique properties make it invaluable in certain processes, particularly in analytical chemistry and as a component in rocket propellants. However, the environmental implications of its usage have become a growing concern in recent years, prompting a comprehensive review of its applications and impacts.
The historical development of perchloric acid usage can be traced back to the early 20th century when it was first synthesized on a large scale. Initially, its applications were limited due to its highly reactive nature. As industrial processes evolved, the demand for strong oxidizing agents increased, leading to a wider adoption of perchloric acid in various sectors.
In the context of industrial settings, perchloric acid has found applications in electropolishing of metals, etching of printed circuit boards, and as a catalyst in organic synthesis. Its ability to form stable perchlorates has also made it useful in the production of certain explosives and pyrotechnics. However, these same properties that make it industrially valuable also contribute to its potential environmental hazards.
The primary objective of this technical research is to thoroughly examine the environmental implications of perchloric acid usage in industrial settings. This includes assessing its impact on air quality, water resources, soil contamination, and potential effects on ecosystems. Additionally, the study aims to evaluate current handling and disposal practices, identifying areas where improvements can be made to minimize environmental risks.
Another crucial aspect of this research is to explore the technological trends in perchloric acid usage. This involves analyzing emerging alternatives that may offer similar industrial benefits with reduced environmental impact. The study will also investigate advancements in containment, treatment, and remediation technologies specifically designed for perchloric acid and its byproducts.
Furthermore, this research seeks to understand the regulatory landscape surrounding perchloric acid usage. As environmental concerns have grown, many countries have implemented stricter regulations on the use, storage, and disposal of perchloric acid. Analyzing these regulatory frameworks and their effectiveness in mitigating environmental risks is essential for developing future strategies.
Lastly, the research aims to provide a forward-looking perspective on the future of perchloric acid in industrial applications. This includes predicting potential shifts in usage patterns, anticipating technological breakthroughs that could alter its environmental impact, and proposing sustainable practices for industries that rely on this chemical. By comprehensively addressing these aspects, this study will contribute to a more environmentally conscious approach to perchloric acid usage in industrial settings.
The historical development of perchloric acid usage can be traced back to the early 20th century when it was first synthesized on a large scale. Initially, its applications were limited due to its highly reactive nature. As industrial processes evolved, the demand for strong oxidizing agents increased, leading to a wider adoption of perchloric acid in various sectors.
In the context of industrial settings, perchloric acid has found applications in electropolishing of metals, etching of printed circuit boards, and as a catalyst in organic synthesis. Its ability to form stable perchlorates has also made it useful in the production of certain explosives and pyrotechnics. However, these same properties that make it industrially valuable also contribute to its potential environmental hazards.
The primary objective of this technical research is to thoroughly examine the environmental implications of perchloric acid usage in industrial settings. This includes assessing its impact on air quality, water resources, soil contamination, and potential effects on ecosystems. Additionally, the study aims to evaluate current handling and disposal practices, identifying areas where improvements can be made to minimize environmental risks.
Another crucial aspect of this research is to explore the technological trends in perchloric acid usage. This involves analyzing emerging alternatives that may offer similar industrial benefits with reduced environmental impact. The study will also investigate advancements in containment, treatment, and remediation technologies specifically designed for perchloric acid and its byproducts.
Furthermore, this research seeks to understand the regulatory landscape surrounding perchloric acid usage. As environmental concerns have grown, many countries have implemented stricter regulations on the use, storage, and disposal of perchloric acid. Analyzing these regulatory frameworks and their effectiveness in mitigating environmental risks is essential for developing future strategies.
Lastly, the research aims to provide a forward-looking perspective on the future of perchloric acid in industrial applications. This includes predicting potential shifts in usage patterns, anticipating technological breakthroughs that could alter its environmental impact, and proposing sustainable practices for industries that rely on this chemical. By comprehensively addressing these aspects, this study will contribute to a more environmentally conscious approach to perchloric acid usage in industrial settings.
Industrial Demand Analysis for Perchloric Acid
The industrial demand for perchloric acid has been steadily increasing due to its unique properties and versatile applications across various sectors. This strong oxidizing agent plays a crucial role in numerous industrial processes, particularly in the manufacturing of rocket propellants, explosives, and specialized chemicals. The aerospace and defense industries are major consumers of perchloric acid, utilizing it in the production of solid rocket fuels and high-energy materials.
In the electronics sector, perchloric acid is essential for etching and cleaning printed circuit boards, as well as in the production of certain types of batteries. Its ability to dissolve metal oxides makes it valuable in analytical chemistry and metallurgy, where it is used for sample preparation and metal purification processes. The pharmaceutical industry also relies on perchloric acid for the synthesis of certain drugs and as a reagent in various chemical reactions.
The global market for perchloric acid is projected to grow significantly in the coming years, driven by increasing demand from emerging economies and advancements in technology-intensive industries. However, this growth is tempered by stringent environmental regulations and safety concerns associated with its handling and disposal.
Environmental considerations are becoming increasingly important in the industrial use of perchloric acid. As awareness of its potential environmental impacts grows, there is a rising demand for more sustainable production methods and safer handling practices. This has led to the development of closed-loop systems and improved waste management techniques in industries that heavily rely on perchloric acid.
The geographical distribution of perchloric acid demand is closely tied to industrial centers and regions with a strong presence in aerospace, defense, and high-tech manufacturing. North America and Europe have traditionally been major consumers, but rapid industrialization in Asia-Pacific countries is shifting the demand landscape.
Despite its industrial importance, the use of perchloric acid faces challenges due to its corrosive nature and potential environmental hazards. This has spurred research into alternative chemicals and processes that could potentially replace perchloric acid in certain applications. However, for many specialized uses, particularly in aerospace and defense, perchloric acid remains irreplaceable due to its unique properties.
As industries strive to balance performance requirements with environmental responsibility, the demand for perchloric acid is likely to evolve. Future trends may include the development of more efficient and environmentally friendly production methods, as well as innovations in handling and disposal technologies to mitigate its environmental impact.
In the electronics sector, perchloric acid is essential for etching and cleaning printed circuit boards, as well as in the production of certain types of batteries. Its ability to dissolve metal oxides makes it valuable in analytical chemistry and metallurgy, where it is used for sample preparation and metal purification processes. The pharmaceutical industry also relies on perchloric acid for the synthesis of certain drugs and as a reagent in various chemical reactions.
The global market for perchloric acid is projected to grow significantly in the coming years, driven by increasing demand from emerging economies and advancements in technology-intensive industries. However, this growth is tempered by stringent environmental regulations and safety concerns associated with its handling and disposal.
Environmental considerations are becoming increasingly important in the industrial use of perchloric acid. As awareness of its potential environmental impacts grows, there is a rising demand for more sustainable production methods and safer handling practices. This has led to the development of closed-loop systems and improved waste management techniques in industries that heavily rely on perchloric acid.
The geographical distribution of perchloric acid demand is closely tied to industrial centers and regions with a strong presence in aerospace, defense, and high-tech manufacturing. North America and Europe have traditionally been major consumers, but rapid industrialization in Asia-Pacific countries is shifting the demand landscape.
Despite its industrial importance, the use of perchloric acid faces challenges due to its corrosive nature and potential environmental hazards. This has spurred research into alternative chemicals and processes that could potentially replace perchloric acid in certain applications. However, for many specialized uses, particularly in aerospace and defense, perchloric acid remains irreplaceable due to its unique properties.
As industries strive to balance performance requirements with environmental responsibility, the demand for perchloric acid is likely to evolve. Future trends may include the development of more efficient and environmentally friendly production methods, as well as innovations in handling and disposal technologies to mitigate its environmental impact.
Current Challenges in Perchloric Acid Handling
The handling of perchloric acid in industrial settings presents several significant challenges due to its highly reactive and potentially hazardous nature. One of the primary concerns is the risk of explosions when perchloric acid comes into contact with organic materials or dehydrating agents. This necessitates stringent safety protocols and specialized equipment for storage, transportation, and usage.
Corrosion is another major challenge in perchloric acid handling. The acid's strong oxidizing properties can rapidly degrade many common materials used in industrial equipment and storage containers. This requires the use of specialized, corrosion-resistant materials such as high-grade stainless steel or fluoropolymers, which can significantly increase operational costs and complexity.
The volatility of perchloric acid, especially at higher concentrations, poses additional handling difficulties. As the acid evaporates, it can form explosive perchlorates on surfaces, particularly in ventilation systems. This necessitates regular cleaning and decontamination procedures, adding to the operational burden and potential safety risks.
Environmental concerns also complicate perchloric acid handling. The acid and its salts can persist in the environment, potentially contaminating soil and water sources. This requires careful waste management and disposal practices, often involving neutralization or specialized treatment facilities, which can be costly and complex to implement.
Worker safety is a paramount concern in perchloric acid handling. Exposure risks include severe burns, respiratory issues, and potential long-term health effects. This necessitates comprehensive personal protective equipment (PPE) and rigorous safety training programs, adding to operational complexity and costs.
The regulatory landscape surrounding perchloric acid usage presents another challenge. Stringent regulations govern its storage, transportation, and use, requiring extensive documentation, regular inspections, and compliance with evolving standards. This regulatory burden can be particularly challenging for smaller operations or those in regions with rapidly changing environmental policies.
Lastly, the specialized nature of perchloric acid handling often requires dedicated facilities and equipment. This includes fume hoods with wash-down systems, specialized waste treatment facilities, and dedicated storage areas. The cost and space requirements for these specialized setups can be prohibitive for many industrial operations, limiting the widespread adoption of perchloric acid-based processes.
Corrosion is another major challenge in perchloric acid handling. The acid's strong oxidizing properties can rapidly degrade many common materials used in industrial equipment and storage containers. This requires the use of specialized, corrosion-resistant materials such as high-grade stainless steel or fluoropolymers, which can significantly increase operational costs and complexity.
The volatility of perchloric acid, especially at higher concentrations, poses additional handling difficulties. As the acid evaporates, it can form explosive perchlorates on surfaces, particularly in ventilation systems. This necessitates regular cleaning and decontamination procedures, adding to the operational burden and potential safety risks.
Environmental concerns also complicate perchloric acid handling. The acid and its salts can persist in the environment, potentially contaminating soil and water sources. This requires careful waste management and disposal practices, often involving neutralization or specialized treatment facilities, which can be costly and complex to implement.
Worker safety is a paramount concern in perchloric acid handling. Exposure risks include severe burns, respiratory issues, and potential long-term health effects. This necessitates comprehensive personal protective equipment (PPE) and rigorous safety training programs, adding to operational complexity and costs.
The regulatory landscape surrounding perchloric acid usage presents another challenge. Stringent regulations govern its storage, transportation, and use, requiring extensive documentation, regular inspections, and compliance with evolving standards. This regulatory burden can be particularly challenging for smaller operations or those in regions with rapidly changing environmental policies.
Lastly, the specialized nature of perchloric acid handling often requires dedicated facilities and equipment. This includes fume hoods with wash-down systems, specialized waste treatment facilities, and dedicated storage areas. The cost and space requirements for these specialized setups can be prohibitive for many industrial operations, limiting the widespread adoption of perchloric acid-based processes.
Existing Safety Protocols for Perchloric Acid Use
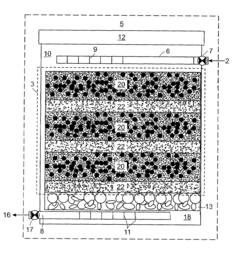
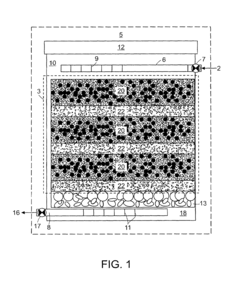
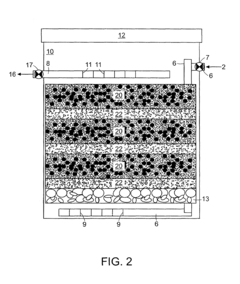
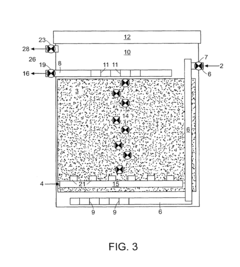
01 Environmental monitoring and detection of perchloric acid
Various methods and devices have been developed for monitoring and detecting perchloric acid in environmental samples. These include advanced analytical techniques and portable detection systems to assess contamination levels in air, water, and soil. Such monitoring is crucial for environmental protection and risk assessment.- Environmental monitoring and detection of perchloric acid: Various methods and devices have been developed for monitoring and detecting perchloric acid in environmental samples. These include advanced analytical techniques and portable sensors that can measure perchloric acid concentrations in air, water, and soil. Such monitoring is crucial for assessing environmental contamination and implementing appropriate remediation strategies.
- Waste treatment and disposal of perchloric acid: Specialized waste treatment and disposal methods have been developed to handle perchloric acid safely and minimize its environmental impact. These include neutralization techniques, chemical decomposition processes, and advanced oxidation methods. Proper disposal is essential to prevent contamination of soil and water resources.
- Remediation of perchloric acid contaminated sites: Various remediation techniques have been developed to clean up sites contaminated with perchloric acid. These may include in-situ chemical reduction, bioremediation, and soil washing methods. The choice of remediation technique depends on the extent of contamination and site-specific conditions.
- Safety measures for handling and storage of perchloric acid: Stringent safety protocols and specialized equipment have been designed for the handling and storage of perchloric acid to prevent accidental releases and minimize environmental risks. These include corrosion-resistant containers, spill containment systems, and personal protective equipment for workers.
- Alternatives and substitutes for perchloric acid: Research has been conducted to develop environmentally friendly alternatives and substitutes for perchloric acid in various applications. These efforts aim to reduce the use of perchloric acid and mitigate its potential environmental impacts. Alternative oxidizing agents and novel chemical processes are being explored to replace perchloric acid in industrial and laboratory settings.
02 Waste treatment and disposal of perchloric acid
Specialized waste treatment processes and disposal methods have been designed to handle perchloric acid safely. These include neutralization techniques, chemical decomposition, and containment strategies to minimize environmental impact. Proper disposal is essential to prevent contamination of water sources and soil.Expand Specific Solutions03 Remediation of perchloric acid contaminated sites
Technologies and methods for remediating sites contaminated with perchloric acid have been developed. These include in-situ and ex-situ treatment processes, such as chemical reduction, bioremediation, and soil washing techniques. The goal is to restore contaminated areas and minimize long-term environmental impacts.Expand Specific Solutions04 Safety measures for handling and storage of perchloric acid
Improved safety protocols and equipment have been designed for the handling and storage of perchloric acid to prevent accidental releases. These include specialized containment systems, personal protective equipment, and emergency response procedures to minimize environmental risks associated with its use in industrial and laboratory settings.Expand Specific Solutions05 Alternatives and substitutes for perchloric acid
Research has been conducted to identify and develop environmentally friendly alternatives or substitutes for perchloric acid in various applications. This includes exploring less hazardous chemicals or processes that can achieve similar results while reducing potential environmental impacts and safety risks.Expand Specific Solutions
Key Industrial Players in Perchloric Acid Production
The environmental implications of perchloric acid usage in industrial settings represent a complex technological challenge at the intersection of chemical engineering and environmental science. The market is in a growth phase, driven by increasing industrial applications and environmental regulations. The global market size for perchloric acid is projected to expand significantly in the coming years. Technologically, the field is advancing rapidly, with companies like Ecolab USA, Inc. and Fluid Energy Group Ltd. developing innovative solutions for safer handling and disposal. Research institutions such as Zhejiang University and the University of Delaware are contributing to the knowledge base, while industry leaders like China Petroleum & Chemical Corp. are implementing large-scale applications. The competitive landscape is diverse, with a mix of chemical manufacturers, research institutions, and environmental technology firms vying for market share and technological leadership.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed an innovative approach to mitigate the environmental impact of perchloric acid usage in industrial settings. Their method involves a closed-loop recycling system that captures and reprocesses perchloric acid vapors, significantly reducing atmospheric emissions[1]. The system incorporates advanced scrubbing technology to neutralize acid fumes and recover perchloric acid for reuse. Additionally, Sinopec has implemented a water treatment process that effectively removes perchlorate ions from wastewater, ensuring compliance with stringent environmental regulations[3]. This comprehensive approach addresses both air and water pollution concerns associated with perchloric acid use in petrochemical operations.
Strengths: Closed-loop system reduces emissions and waste. Advanced scrubbing technology improves air quality. Effective water treatment process. Weaknesses: High initial implementation costs. Requires specialized training for operators.
Ecolab USA, Inc.
Technical Solution: Ecolab USA, Inc. has developed a suite of environmentally friendly alternatives to perchloric acid for industrial cleaning and oxidation processes. Their approach focuses on replacing perchloric acid with less hazardous substances while maintaining or improving process efficiency. One key innovation is a proprietary blend of organic acids and surfactants that achieves similar cleaning power to perchloric acid but with significantly reduced environmental impact[2]. For oxidation processes, Ecolab has introduced a catalytic system that uses hydrogen peroxide as the primary oxidant, eliminating the need for perchloric acid in many applications[4]. These solutions are complemented by advanced monitoring and dosing systems to optimize chemical usage and minimize waste.
Strengths: Reduced environmental hazards. Improved worker safety. Versatile applications across industries. Weaknesses: May require process modifications for implementation. Potentially higher ongoing operational costs compared to perchloric acid.
Innovative Approaches to Perchloric Acid Management
Process for autotrophic perchlorate reduction using elemental sulfur and mollusk shells
PatentInactiveUS7575686B2
Innovation
- A bioreactor system using elemental sulfur as an electron donor and mollusk shell buffering material, seeded with autotrophic bacteria, which provides a robust and reliable process for perchlorate reduction and nitrogen removal, maintaining higher pH levels and alkalinity, and reducing the need for external carbon sources and frequent backwashing.
Synthetic acids for use in various industrial acrivities
PatentInactiveCA2925635A1
Innovation
- A synthetic acid composition comprising urea and a phosphoric acid derivative in a specific molar ratio, which reduces corrosion and fuming effects, is developed to provide a safer alternative for industrial applications, including chrome-friendly formulations with minimal exothermic reactivity and high salinity tolerance.
Environmental Impact Assessment of Perchloric Acid
The environmental impact assessment of perchloric acid usage in industrial settings is a critical aspect of responsible chemical management. Perchloric acid, a powerful oxidizing agent, has widespread applications in various industries, including electronics manufacturing, rocket propellants, and analytical chemistry. However, its use poses significant environmental risks that require careful evaluation and mitigation strategies.
One of the primary environmental concerns associated with perchloric acid is its potential to contaminate water sources. When released into the environment, perchloric acid can form highly soluble and stable perchlorate ions. These ions can persist in groundwater and surface water for extended periods, potentially affecting drinking water supplies and aquatic ecosystems. The mobility of perchlorates in water systems makes them particularly challenging to contain and remediate once released.
Soil contamination is another significant environmental implication of perchloric acid usage. Perchlorate ions can accumulate in soil, leading to long-term environmental persistence. This accumulation can affect soil microorganisms and plant life, potentially disrupting local ecosystems and agricultural productivity. The remediation of perchlorate-contaminated soil often requires extensive and costly treatment processes.
Air quality is also a concern when handling perchloric acid in industrial settings. Vapors and aerosols generated during production or use can contribute to air pollution if not properly controlled. These emissions may pose risks to both human health and the surrounding environment, necessitating robust air quality management systems and personal protective equipment for workers.
The potential for perchloric acid to form explosive compounds under certain conditions adds another layer of environmental risk. Improper storage or disposal practices can lead to the formation of shock-sensitive perchlorates, which pose significant safety hazards and potential environmental damage in case of accidental detonation.
To mitigate these environmental risks, industries using perchloric acid must implement comprehensive management strategies. These include adopting closed-loop systems to minimize releases, implementing advanced wastewater treatment technologies to remove perchlorates, and ensuring proper storage and handling protocols. Regular environmental monitoring programs are essential to detect and address any potential contamination promptly.
Regulatory compliance is a crucial aspect of managing the environmental impact of perchloric acid. Many jurisdictions have established strict guidelines for its use, storage, and disposal. Industries must stay abreast of these regulations and implement best practices to ensure compliance and minimize environmental risks.
In conclusion, while perchloric acid remains an important industrial chemical, its environmental implications are significant and multifaceted. A thorough environmental impact assessment is essential for any industry utilizing this compound, focusing on water, soil, and air quality protection, as well as safety considerations. By implementing robust management strategies and adhering to regulatory requirements, industries can minimize the environmental footprint of perchloric acid usage and contribute to more sustainable chemical practices.
One of the primary environmental concerns associated with perchloric acid is its potential to contaminate water sources. When released into the environment, perchloric acid can form highly soluble and stable perchlorate ions. These ions can persist in groundwater and surface water for extended periods, potentially affecting drinking water supplies and aquatic ecosystems. The mobility of perchlorates in water systems makes them particularly challenging to contain and remediate once released.
Soil contamination is another significant environmental implication of perchloric acid usage. Perchlorate ions can accumulate in soil, leading to long-term environmental persistence. This accumulation can affect soil microorganisms and plant life, potentially disrupting local ecosystems and agricultural productivity. The remediation of perchlorate-contaminated soil often requires extensive and costly treatment processes.
Air quality is also a concern when handling perchloric acid in industrial settings. Vapors and aerosols generated during production or use can contribute to air pollution if not properly controlled. These emissions may pose risks to both human health and the surrounding environment, necessitating robust air quality management systems and personal protective equipment for workers.
The potential for perchloric acid to form explosive compounds under certain conditions adds another layer of environmental risk. Improper storage or disposal practices can lead to the formation of shock-sensitive perchlorates, which pose significant safety hazards and potential environmental damage in case of accidental detonation.
To mitigate these environmental risks, industries using perchloric acid must implement comprehensive management strategies. These include adopting closed-loop systems to minimize releases, implementing advanced wastewater treatment technologies to remove perchlorates, and ensuring proper storage and handling protocols. Regular environmental monitoring programs are essential to detect and address any potential contamination promptly.
Regulatory compliance is a crucial aspect of managing the environmental impact of perchloric acid. Many jurisdictions have established strict guidelines for its use, storage, and disposal. Industries must stay abreast of these regulations and implement best practices to ensure compliance and minimize environmental risks.
In conclusion, while perchloric acid remains an important industrial chemical, its environmental implications are significant and multifaceted. A thorough environmental impact assessment is essential for any industry utilizing this compound, focusing on water, soil, and air quality protection, as well as safety considerations. By implementing robust management strategies and adhering to regulatory requirements, industries can minimize the environmental footprint of perchloric acid usage and contribute to more sustainable chemical practices.
Regulatory Framework for Perchloric Acid in Industry
The regulatory framework for perchloric acid in industrial settings is complex and multifaceted, reflecting the potential environmental and safety risks associated with its use. At the federal level in the United States, the Environmental Protection Agency (EPA) regulates perchloric acid under the Toxic Substances Control Act (TSCA) and the Resource Conservation and Recovery Act (RCRA). The TSCA mandates reporting, record-keeping, and testing requirements, while the RCRA governs the disposal of perchloric acid as a hazardous waste.
The Occupational Safety and Health Administration (OSHA) has established specific standards for perchloric acid handling in the workplace. These include permissible exposure limits (PELs) and requirements for personal protective equipment (PPE). OSHA's Hazard Communication Standard also ensures that information about the dangers of perchloric acid is disseminated to workers through proper labeling and safety data sheets.
At the state level, regulations can be more stringent. California, for instance, lists perchloric acid under Proposition 65, requiring businesses to provide warnings about significant exposures to chemicals that can cause cancer, birth defects, or other reproductive harm. Many states have adopted additional requirements for storage, handling, and disposal of perchloric acid.
Internationally, the regulatory landscape varies. The European Union regulates perchloric acid under the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) regulation. This comprehensive framework addresses the production and use of chemical substances and their potential impacts on human health and the environment. In Japan, perchloric acid is controlled under the Poisonous and Deleterious Substances Control Law.
Industry-specific regulations also play a crucial role. In the aerospace industry, for example, NASA has developed detailed guidelines for perchloric acid use in clean rooms and other sensitive environments. These guidelines address ventilation requirements, material compatibility, and decontamination procedures.
Compliance with these regulations often requires significant investment in infrastructure and training. Many facilities using perchloric acid must implement specialized ventilation systems, conduct regular environmental monitoring, and establish rigorous safety protocols. The regulatory framework also drives innovation in safer alternatives and more efficient use of perchloric acid in industrial processes.
As environmental concerns grow, there is a trend towards more stringent regulations. Some jurisdictions are considering bans on certain uses of perchloric acid, particularly in consumer products. This evolving regulatory landscape presents both challenges and opportunities for industries reliant on perchloric acid, necessitating ongoing adaptation and investment in compliance measures.
The Occupational Safety and Health Administration (OSHA) has established specific standards for perchloric acid handling in the workplace. These include permissible exposure limits (PELs) and requirements for personal protective equipment (PPE). OSHA's Hazard Communication Standard also ensures that information about the dangers of perchloric acid is disseminated to workers through proper labeling and safety data sheets.
At the state level, regulations can be more stringent. California, for instance, lists perchloric acid under Proposition 65, requiring businesses to provide warnings about significant exposures to chemicals that can cause cancer, birth defects, or other reproductive harm. Many states have adopted additional requirements for storage, handling, and disposal of perchloric acid.
Internationally, the regulatory landscape varies. The European Union regulates perchloric acid under the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) regulation. This comprehensive framework addresses the production and use of chemical substances and their potential impacts on human health and the environment. In Japan, perchloric acid is controlled under the Poisonous and Deleterious Substances Control Law.
Industry-specific regulations also play a crucial role. In the aerospace industry, for example, NASA has developed detailed guidelines for perchloric acid use in clean rooms and other sensitive environments. These guidelines address ventilation requirements, material compatibility, and decontamination procedures.
Compliance with these regulations often requires significant investment in infrastructure and training. Many facilities using perchloric acid must implement specialized ventilation systems, conduct regular environmental monitoring, and establish rigorous safety protocols. The regulatory framework also drives innovation in safer alternatives and more efficient use of perchloric acid in industrial processes.
As environmental concerns grow, there is a trend towards more stringent regulations. Some jurisdictions are considering bans on certain uses of perchloric acid, particularly in consumer products. This evolving regulatory landscape presents both challenges and opportunities for industries reliant on perchloric acid, necessitating ongoing adaptation and investment in compliance measures.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!