Examining the Validity of Bioresonance in Parasitic Infection Control
AUG 11, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Bioresonance Background
Bioresonance therapy, a controversial alternative medical practice, emerged in the 1970s based on the concept that all living organisms emit and respond to specific electromagnetic frequencies. The theory suggests that diseases and parasitic infections can be detected and treated by identifying and manipulating these frequencies. Proponents claim that bioresonance devices can detect abnormal frequencies associated with pathogens and subsequently generate corrective frequencies to restore health.
The development of bioresonance can be traced back to Dr. Franz Morell, who, along with his son-in-law Erich Rasche, invented the MORA device in 1977. This device purportedly measured the body's electromagnetic oscillations and provided therapeutic interventions. Since then, various iterations and adaptations of bioresonance technology have emerged, with manufacturers claiming advancements in detection accuracy and treatment efficacy.
In the context of parasitic infection control, bioresonance advocates assert that the therapy can identify specific frequency patterns associated with different parasites. They argue that these patterns can be used to diagnose infections non-invasively and subsequently apply counter-frequencies to eliminate the parasites without the need for pharmaceutical interventions. This approach has gained traction among some alternative medicine practitioners, particularly in regions where conventional antiparasitic treatments may be less accessible or culturally accepted.
Despite its growing popularity in certain circles, bioresonance remains highly controversial within the mainstream medical community. Critics argue that there is no scientifically plausible mechanism by which the claimed effects could occur. They point out that the fundamental principles of bioresonance contradict established laws of physics and biology. Furthermore, the lack of rigorous, peer-reviewed studies demonstrating its efficacy has led many health authorities to classify bioresonance as pseudoscience.
The historical context of bioresonance is important to consider when examining its validity in parasitic infection control. The therapy emerged during a period of increased interest in alternative and complementary medicine, driven in part by dissatisfaction with conventional medical approaches. This timing contributed to its adoption by individuals seeking non-traditional health solutions, despite the absence of robust scientific evidence supporting its claims.
The development of bioresonance can be traced back to Dr. Franz Morell, who, along with his son-in-law Erich Rasche, invented the MORA device in 1977. This device purportedly measured the body's electromagnetic oscillations and provided therapeutic interventions. Since then, various iterations and adaptations of bioresonance technology have emerged, with manufacturers claiming advancements in detection accuracy and treatment efficacy.
In the context of parasitic infection control, bioresonance advocates assert that the therapy can identify specific frequency patterns associated with different parasites. They argue that these patterns can be used to diagnose infections non-invasively and subsequently apply counter-frequencies to eliminate the parasites without the need for pharmaceutical interventions. This approach has gained traction among some alternative medicine practitioners, particularly in regions where conventional antiparasitic treatments may be less accessible or culturally accepted.
Despite its growing popularity in certain circles, bioresonance remains highly controversial within the mainstream medical community. Critics argue that there is no scientifically plausible mechanism by which the claimed effects could occur. They point out that the fundamental principles of bioresonance contradict established laws of physics and biology. Furthermore, the lack of rigorous, peer-reviewed studies demonstrating its efficacy has led many health authorities to classify bioresonance as pseudoscience.
The historical context of bioresonance is important to consider when examining its validity in parasitic infection control. The therapy emerged during a period of increased interest in alternative and complementary medicine, driven in part by dissatisfaction with conventional medical approaches. This timing contributed to its adoption by individuals seeking non-traditional health solutions, despite the absence of robust scientific evidence supporting its claims.
Market Analysis
The market for bioresonance technology in parasitic infection control is experiencing a gradual expansion, driven by increasing interest in alternative and complementary medicine approaches. While still considered a niche market, it has shown potential for growth in regions where traditional medicine practices are prevalent and where there is a higher incidence of parasitic infections.
The global parasitic diseases treatment market, which includes both conventional and alternative therapies, was valued at approximately $5.5 billion in 2020 and is projected to grow at a compound annual growth rate (CAGR) of 5.2% from 2021 to 2028. Within this broader market, bioresonance therapy for parasitic infection control represents a small but growing segment.
Demand for bioresonance technology in parasitic infection control is primarily driven by consumers seeking non-invasive and drug-free treatment options. This demand is particularly strong in regions with high parasitic infection rates, such as parts of Africa, South Asia, and Latin America. Additionally, there is a growing interest in bioresonance therapy among individuals in developed countries who are looking for alternative approaches to managing chronic parasitic infections or those resistant to conventional treatments.
The market for bioresonance devices and services is fragmented, with numerous small-scale manufacturers and practitioners offering various products and treatments. Key market players include BICOM, Rayonex Biomedical, and Sensitiv Imago, among others. These companies are continuously working on improving their technologies and expanding their product offerings to meet the growing demand.
Despite the increasing interest, the market faces significant challenges. The lack of robust scientific evidence supporting the efficacy of bioresonance in parasitic infection control remains a major hurdle for widespread adoption. Regulatory bodies in many countries have not approved bioresonance devices for medical use, limiting their market potential in conventional healthcare settings.
The COVID-19 pandemic has had a mixed impact on the market. While it has increased overall interest in alternative health approaches, it has also led to reduced access to bioresonance treatments due to lockdowns and social distancing measures. However, this has spurred innovation in the sector, with some providers offering remote or at-home bioresonance solutions.
Looking ahead, the market for bioresonance in parasitic infection control is expected to continue its growth trajectory, albeit at a moderate pace. Factors such as increasing consumer awareness, technological advancements in bioresonance devices, and potential breakthroughs in clinical research could significantly influence the market's future development. However, the need for more substantial scientific validation remains crucial for the technology to gain wider acceptance in mainstream medical practice and to unlock its full market potential.
The global parasitic diseases treatment market, which includes both conventional and alternative therapies, was valued at approximately $5.5 billion in 2020 and is projected to grow at a compound annual growth rate (CAGR) of 5.2% from 2021 to 2028. Within this broader market, bioresonance therapy for parasitic infection control represents a small but growing segment.
Demand for bioresonance technology in parasitic infection control is primarily driven by consumers seeking non-invasive and drug-free treatment options. This demand is particularly strong in regions with high parasitic infection rates, such as parts of Africa, South Asia, and Latin America. Additionally, there is a growing interest in bioresonance therapy among individuals in developed countries who are looking for alternative approaches to managing chronic parasitic infections or those resistant to conventional treatments.
The market for bioresonance devices and services is fragmented, with numerous small-scale manufacturers and practitioners offering various products and treatments. Key market players include BICOM, Rayonex Biomedical, and Sensitiv Imago, among others. These companies are continuously working on improving their technologies and expanding their product offerings to meet the growing demand.
Despite the increasing interest, the market faces significant challenges. The lack of robust scientific evidence supporting the efficacy of bioresonance in parasitic infection control remains a major hurdle for widespread adoption. Regulatory bodies in many countries have not approved bioresonance devices for medical use, limiting their market potential in conventional healthcare settings.
The COVID-19 pandemic has had a mixed impact on the market. While it has increased overall interest in alternative health approaches, it has also led to reduced access to bioresonance treatments due to lockdowns and social distancing measures. However, this has spurred innovation in the sector, with some providers offering remote or at-home bioresonance solutions.
Looking ahead, the market for bioresonance in parasitic infection control is expected to continue its growth trajectory, albeit at a moderate pace. Factors such as increasing consumer awareness, technological advancements in bioresonance devices, and potential breakthroughs in clinical research could significantly influence the market's future development. However, the need for more substantial scientific validation remains crucial for the technology to gain wider acceptance in mainstream medical practice and to unlock its full market potential.
Technical Challenges
Bioresonance therapy for parasitic infection control faces several significant technical challenges that hinder its widespread acceptance and implementation. One of the primary obstacles is the lack of robust scientific evidence supporting its efficacy. While proponents claim that bioresonance can detect and eliminate parasites by manipulating electromagnetic frequencies, the underlying mechanisms remain poorly understood and largely unproven within the scientific community.
The absence of standardized protocols and equipment calibration methods poses another substantial challenge. Different bioresonance devices may produce varying results, making it difficult to replicate findings across studies or clinical settings. This inconsistency undermines the reliability and validity of the technique, further complicating efforts to establish its effectiveness in parasitic infection control.
Another critical issue is the difficulty in accurately detecting and identifying specific parasites using bioresonance. The complex nature of parasitic infections, combined with the subtle electromagnetic signatures they may produce, makes it challenging to develop precise and sensitive detection methods. This limitation raises concerns about potential false positives or negatives, which could lead to misdiagnosis or inappropriate treatment.
The integration of bioresonance technology with existing medical diagnostic tools and treatment protocols presents additional hurdles. Many healthcare professionals remain skeptical of the technique due to its unconventional approach and limited scientific backing. This skepticism creates barriers to adoption and implementation within mainstream medical practice, hindering the potential for collaborative research and development efforts.
Furthermore, the lack of comprehensive safety studies raises concerns about potential long-term effects of repeated exposure to electromagnetic frequencies used in bioresonance therapy. While proponents argue that the technique is non-invasive and harmless, more rigorous research is needed to establish its safety profile, particularly for vulnerable populations such as children, pregnant women, and individuals with pre-existing health conditions.
Regulatory challenges also pose significant obstacles to the advancement of bioresonance in parasitic infection control. Many countries have not yet established clear guidelines or approval processes for bioresonance devices and therapies, leading to a fragmented regulatory landscape. This uncertainty creates difficulties for manufacturers, practitioners, and researchers seeking to develop and validate new applications of the technology.
The absence of standardized protocols and equipment calibration methods poses another substantial challenge. Different bioresonance devices may produce varying results, making it difficult to replicate findings across studies or clinical settings. This inconsistency undermines the reliability and validity of the technique, further complicating efforts to establish its effectiveness in parasitic infection control.
Another critical issue is the difficulty in accurately detecting and identifying specific parasites using bioresonance. The complex nature of parasitic infections, combined with the subtle electromagnetic signatures they may produce, makes it challenging to develop precise and sensitive detection methods. This limitation raises concerns about potential false positives or negatives, which could lead to misdiagnosis or inappropriate treatment.
The integration of bioresonance technology with existing medical diagnostic tools and treatment protocols presents additional hurdles. Many healthcare professionals remain skeptical of the technique due to its unconventional approach and limited scientific backing. This skepticism creates barriers to adoption and implementation within mainstream medical practice, hindering the potential for collaborative research and development efforts.
Furthermore, the lack of comprehensive safety studies raises concerns about potential long-term effects of repeated exposure to electromagnetic frequencies used in bioresonance therapy. While proponents argue that the technique is non-invasive and harmless, more rigorous research is needed to establish its safety profile, particularly for vulnerable populations such as children, pregnant women, and individuals with pre-existing health conditions.
Regulatory challenges also pose significant obstacles to the advancement of bioresonance in parasitic infection control. Many countries have not yet established clear guidelines or approval processes for bioresonance devices and therapies, leading to a fragmented regulatory landscape. This uncertainty creates difficulties for manufacturers, practitioners, and researchers seeking to develop and validate new applications of the technology.
Current Applications
01 Bioresonance measurement and analysis techniques
Various methods and devices for measuring and analyzing bioresonance signals are developed to assess the validity of bioresonance therapy. These techniques involve capturing electromagnetic signals from the body, processing them, and interpreting the results to evaluate physiological conditions or treatment efficacy.- Bioresonance measurement and analysis techniques: Various methods and devices are developed for measuring and analyzing bioresonance signals. These techniques involve capturing electromagnetic signals from the body, processing them, and interpreting the results to assess health conditions or provide therapeutic interventions.
- Bioresonance therapy devices and systems: Specialized equipment and systems are designed for bioresonance therapy applications. These devices typically generate and apply specific electromagnetic frequencies to the body, aiming to restore balance and promote healing based on the principles of bioresonance.
- Integration of bioresonance with other diagnostic methods: Bioresonance techniques are combined with other diagnostic tools and methods to enhance overall assessment capabilities. This integration aims to provide a more comprehensive evaluation of an individual's health status by combining multiple data sources and analytical approaches.
- Software and algorithms for bioresonance data processing: Advanced software solutions and algorithms are developed to process and interpret bioresonance data. These computational tools aim to improve the accuracy and reliability of bioresonance assessments by applying sophisticated data analysis techniques to the captured signals.
- Validation studies and clinical applications: Research efforts focus on validating the efficacy and reliability of bioresonance techniques through clinical studies and practical applications. These investigations aim to establish the scientific basis for bioresonance and explore its potential in various medical and therapeutic contexts.
02 Integration of bioresonance with other diagnostic tools
Bioresonance technology is combined with other diagnostic methods and tools to enhance its validity and reliability. This integration aims to provide a more comprehensive assessment of health conditions by correlating bioresonance data with established medical diagnostics.Expand Specific Solutions03 Bioresonance therapy devices and treatment protocols
Specialized devices and treatment protocols are developed to apply bioresonance therapy. These innovations focus on improving the delivery of therapeutic electromagnetic frequencies and optimizing treatment parameters to enhance the effectiveness and validity of bioresonance interventions.Expand Specific Solutions04 Data processing and analysis for bioresonance validation
Advanced data processing and analysis techniques are employed to validate bioresonance measurements and therapy outcomes. These methods involve complex algorithms, machine learning, and statistical analysis to interpret bioresonance data and assess its clinical significance.Expand Specific Solutions05 Clinical studies and research on bioresonance efficacy
Various clinical studies and research initiatives are conducted to evaluate the efficacy and validity of bioresonance therapy. These investigations aim to provide scientific evidence supporting the use of bioresonance in different medical applications and to establish standardized protocols for its implementation.Expand Specific Solutions
Industry Players
The bioresonance technology market for parasitic infection control is in its early development stage, with limited market size and ongoing research to establish its validity. The technology's maturity level is relatively low, with companies like TyraTech and IDEXX Laboratories leading in related fields of natural pest control and veterinary diagnostics. Academic institutions such as Zhejiang University and the University of Massachusetts are contributing to research efforts. While established pharmaceutical companies like Boehringer Ingelheim and Eli Lilly have a presence in animal health, they have not yet significantly entered the bioresonance market for parasitic control. The competitive landscape is characterized by a mix of specialized biotech firms, research institutions, and larger corporations exploring potential applications.
TyraTech, Inc.
Technical Solution: TyraTech has developed a novel approach to parasitic infection control using plant-based bioactive compounds. Their technology utilizes specific combinations of plant essential oils that target the octopamine receptors in invertebrates, which are not present in mammals, birds, or fish. This selective action allows for effective parasite control without harming the host or beneficial organisms. The company has conducted extensive research on the synergistic effects of these plant compounds, demonstrating their ability to disrupt parasites' nervous systems and metabolic functions[1][2]. TyraTech's formulations have shown promise in both topical and oral applications, with studies indicating high efficacy against a wide range of parasites, including nematodes, ticks, and flies.
Strengths: Environmentally friendly, reduced risk of resistance development, and selective action against parasites. Weaknesses: May require more frequent application than conventional chemical treatments, and efficacy can vary depending on parasite species.
IDEXX Laboratories, Inc.
Technical Solution: IDEXX Laboratories has developed advanced diagnostic tools for detecting parasitic infections in animals. Their approach combines molecular biology techniques with machine learning algorithms to improve the accuracy and speed of parasite identification. The company's proprietary SNAP® tests use enzyme-linked immunosorbent assay (ELISA) technology to detect parasite antigens in blood, feces, or other bodily fluids[3]. IDEXX has also introduced real-time PCR panels that can simultaneously detect multiple parasites from a single sample. Their latest innovation involves the use of AI-powered image analysis to identify parasite eggs and larvae in fecal samples, significantly reducing the time and expertise required for traditional microscopic examination[4].
Strengths: High sensitivity and specificity, rapid results, and the ability to detect multiple parasites simultaneously. Weaknesses: Requires specialized equipment and trained personnel, potentially higher cost compared to traditional methods.
Key Research Findings
Electromagnetic Treatment of Mammals
PatentInactiveUS20080021526A1
Innovation
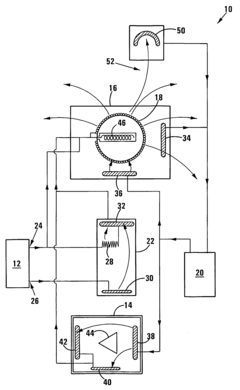
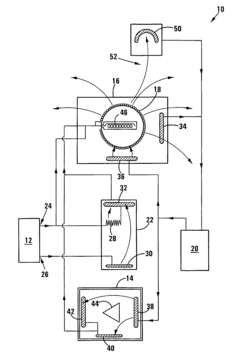
- A system comprising a signal generator, organic filter, and radiating antenna that generates and modulates electromagnetic signals, specifically using multisolenoid coils and a shielded filter containing a sample of the virus or bacteria to radiate targeted frequencies for treatment.
Method for treating infections of the living organism and device for treating said infections
PatentWO2002076550A1
Innovation
- A bioresonance therapy device that captures the patient's electromagnetic signals, processes them to differentiate physiological from pathological oscillations, and returns the inverted pathological signals to enhance the patient's electromagnetic balance, potentially weakening viral signals and strengthening physiological ones, thereby improving treatment efficacy.
Regulatory Framework
The regulatory framework surrounding bioresonance in parasitic infection control is complex and varies significantly across different jurisdictions. In many countries, bioresonance devices are classified as medical devices, subject to stringent regulations and approval processes. The United States Food and Drug Administration (FDA) has not approved bioresonance devices for diagnostic or therapeutic use, considering them to be unproven medical technologies. The FDA has issued warning letters to companies marketing bioresonance devices for unapproved medical claims.
In the European Union, the regulatory landscape is more nuanced. Some bioresonance devices have obtained CE marking, allowing them to be sold within the EU market. However, this certification primarily relates to the safety of the device rather than its efficacy in treating parasitic infections. The European Medicines Agency (EMA) has not issued specific guidelines on bioresonance for parasitic control, leaving individual member states to determine their stance on the technology.
Several countries, including Germany and Switzerland, have a more accepting approach to complementary and alternative medicine, including bioresonance. In these nations, bioresonance may be practiced by licensed healthcare professionals, although it is often not covered by public health insurance schemes. The German Commission E, which evaluates herbal medicines, has not issued any positive monographs for bioresonance in parasitic infection control.
In developing countries, where parasitic infections are more prevalent, the regulatory framework for bioresonance is often less defined. Some nations may allow the use of bioresonance devices with minimal oversight, while others may lack specific regulations addressing this technology. This regulatory gap can lead to the proliferation of unproven treatments and potential risks to public health.
International health organizations, such as the World Health Organization (WHO), have not endorsed bioresonance for parasitic infection control. The WHO's guidelines for parasitic infection management focus on evidence-based interventions, including pharmacological treatments and preventive measures. This stance influences global health policies and may impact the regulatory decisions of individual countries.
As research into bioresonance continues, regulatory bodies are likely to face increasing pressure to evaluate and potentially update their policies. The challenge lies in balancing the need for innovation in healthcare with the imperative to protect public safety and ensure the efficacy of medical treatments. Future regulatory frameworks may need to incorporate more nuanced approaches to assess complementary therapies like bioresonance, potentially including specialized clinical trials and standardized evaluation criteria.
In the European Union, the regulatory landscape is more nuanced. Some bioresonance devices have obtained CE marking, allowing them to be sold within the EU market. However, this certification primarily relates to the safety of the device rather than its efficacy in treating parasitic infections. The European Medicines Agency (EMA) has not issued specific guidelines on bioresonance for parasitic control, leaving individual member states to determine their stance on the technology.
Several countries, including Germany and Switzerland, have a more accepting approach to complementary and alternative medicine, including bioresonance. In these nations, bioresonance may be practiced by licensed healthcare professionals, although it is often not covered by public health insurance schemes. The German Commission E, which evaluates herbal medicines, has not issued any positive monographs for bioresonance in parasitic infection control.
In developing countries, where parasitic infections are more prevalent, the regulatory framework for bioresonance is often less defined. Some nations may allow the use of bioresonance devices with minimal oversight, while others may lack specific regulations addressing this technology. This regulatory gap can lead to the proliferation of unproven treatments and potential risks to public health.
International health organizations, such as the World Health Organization (WHO), have not endorsed bioresonance for parasitic infection control. The WHO's guidelines for parasitic infection management focus on evidence-based interventions, including pharmacological treatments and preventive measures. This stance influences global health policies and may impact the regulatory decisions of individual countries.
As research into bioresonance continues, regulatory bodies are likely to face increasing pressure to evaluate and potentially update their policies. The challenge lies in balancing the need for innovation in healthcare with the imperative to protect public safety and ensure the efficacy of medical treatments. Future regulatory frameworks may need to incorporate more nuanced approaches to assess complementary therapies like bioresonance, potentially including specialized clinical trials and standardized evaluation criteria.
Ethical Considerations
The ethical considerations surrounding the use of bioresonance in parasitic infection control are multifaceted and require careful examination. One primary concern is the potential for false hope and exploitation of vulnerable individuals seeking alternative treatments. As bioresonance lacks substantial scientific evidence to support its efficacy, there is a risk that patients may forgo proven medical interventions in favor of this unverified method, potentially leading to delayed or inadequate treatment of serious parasitic infections.
Another ethical issue is the informed consent process. Given the limited scientific understanding of bioresonance, it is challenging to provide patients with comprehensive and accurate information about the potential risks and benefits of the treatment. This raises questions about whether patients can truly make informed decisions regarding their healthcare when opting for bioresonance therapy.
The marketing and promotion of bioresonance devices and treatments also present ethical challenges. There is a fine line between providing information about alternative therapies and making unsubstantiated claims that could mislead consumers. Regulatory bodies and healthcare professionals must grapple with how to balance patient autonomy and the right to explore alternative treatments with the need to protect the public from potentially fraudulent or ineffective practices.
Furthermore, the use of bioresonance in parasitic infection control raises concerns about the equitable distribution of healthcare resources. If resources are diverted to researching or implementing unproven therapies, it could potentially detract from efforts to develop and distribute evidence-based treatments, particularly in regions where parasitic infections are endemic and resources are limited.
The ethical implications extend to the broader scientific community as well. Researchers and healthcare providers must consider their responsibility in investigating and potentially validating or refuting the claims made by proponents of bioresonance. This includes designing and conducting rigorous studies to evaluate its efficacy, while also being mindful of the ethical considerations in human subject research, especially when dealing with vulnerable populations affected by parasitic infections.
Lastly, there are ethical considerations regarding the regulation and standardization of bioresonance devices and practices. Without proper oversight, there is a risk of inconsistent quality and safety standards, potentially putting patients at risk. Policymakers and regulatory bodies face the challenge of striking a balance between allowing innovation in alternative therapies and ensuring public safety through appropriate regulation and quality control measures.
Another ethical issue is the informed consent process. Given the limited scientific understanding of bioresonance, it is challenging to provide patients with comprehensive and accurate information about the potential risks and benefits of the treatment. This raises questions about whether patients can truly make informed decisions regarding their healthcare when opting for bioresonance therapy.
The marketing and promotion of bioresonance devices and treatments also present ethical challenges. There is a fine line between providing information about alternative therapies and making unsubstantiated claims that could mislead consumers. Regulatory bodies and healthcare professionals must grapple with how to balance patient autonomy and the right to explore alternative treatments with the need to protect the public from potentially fraudulent or ineffective practices.
Furthermore, the use of bioresonance in parasitic infection control raises concerns about the equitable distribution of healthcare resources. If resources are diverted to researching or implementing unproven therapies, it could potentially detract from efforts to develop and distribute evidence-based treatments, particularly in regions where parasitic infections are endemic and resources are limited.
The ethical implications extend to the broader scientific community as well. Researchers and healthcare providers must consider their responsibility in investigating and potentially validating or refuting the claims made by proponents of bioresonance. This includes designing and conducting rigorous studies to evaluate its efficacy, while also being mindful of the ethical considerations in human subject research, especially when dealing with vulnerable populations affected by parasitic infections.
Lastly, there are ethical considerations regarding the regulation and standardization of bioresonance devices and practices. Without proper oversight, there is a risk of inconsistent quality and safety standards, potentially putting patients at risk. Policymakers and regulatory bodies face the challenge of striking a balance between allowing innovation in alternative therapies and ensuring public safety through appropriate regulation and quality control measures.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!