Evaluating Bioresonance Impact on Synovial Joint Health
AUG 11, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Bioresonance and Joint Health: Background and Objectives
Bioresonance therapy, a controversial alternative medicine approach, has gained attention in recent years for its potential impact on various health conditions, including synovial joint health. This emerging field combines principles of quantum physics and traditional medicine, aiming to detect and correct energetic imbalances within the body. The concept of bioresonance is rooted in the belief that all cells and organs in the human body emit specific electromagnetic frequencies, and that disruptions in these frequencies can lead to health issues.
The evolution of bioresonance technology can be traced back to the early 20th century, with the work of Dr. Albert Abrams, who developed the concept of radionics. However, it wasn't until the 1970s that Dr. Franz Morell and his son-in-law, Erich Rasche, developed the first bioresonance device, known as MORA therapy. Since then, the technology has undergone several iterations and refinements, leading to the current generation of bioresonance devices used in clinical settings.
In the context of synovial joint health, bioresonance therapy aims to address various conditions such as osteoarthritis, rheumatoid arthritis, and other inflammatory joint disorders. The underlying principle suggests that by identifying and correcting abnormal frequency patterns associated with joint tissues, it may be possible to alleviate pain, reduce inflammation, and potentially improve overall joint function.
The primary objective of evaluating bioresonance impact on synovial joint health is to determine the efficacy and safety of this alternative therapy in managing joint-related conditions. This assessment seeks to explore the potential mechanisms by which bioresonance may influence joint health, including its effects on inflammation, tissue repair, and pain perception. Additionally, the evaluation aims to compare the outcomes of bioresonance therapy with conventional treatments for joint disorders, to establish its potential as a complementary or alternative approach.
Furthermore, this investigation intends to address the skepticism surrounding bioresonance therapy by conducting rigorous scientific studies and clinical trials. By examining the physiological and biochemical changes that occur in synovial joints following bioresonance treatment, researchers hope to elucidate the underlying mechanisms and provide a solid scientific foundation for its potential therapeutic effects.
As the field of bioresonance continues to evolve, there is a growing need to establish standardized protocols and quality control measures for both the devices used and the treatment procedures. This evaluation also aims to contribute to the development of best practices and guidelines for the application of bioresonance therapy in joint health management, ensuring consistency and reliability in clinical settings.
The evolution of bioresonance technology can be traced back to the early 20th century, with the work of Dr. Albert Abrams, who developed the concept of radionics. However, it wasn't until the 1970s that Dr. Franz Morell and his son-in-law, Erich Rasche, developed the first bioresonance device, known as MORA therapy. Since then, the technology has undergone several iterations and refinements, leading to the current generation of bioresonance devices used in clinical settings.
In the context of synovial joint health, bioresonance therapy aims to address various conditions such as osteoarthritis, rheumatoid arthritis, and other inflammatory joint disorders. The underlying principle suggests that by identifying and correcting abnormal frequency patterns associated with joint tissues, it may be possible to alleviate pain, reduce inflammation, and potentially improve overall joint function.
The primary objective of evaluating bioresonance impact on synovial joint health is to determine the efficacy and safety of this alternative therapy in managing joint-related conditions. This assessment seeks to explore the potential mechanisms by which bioresonance may influence joint health, including its effects on inflammation, tissue repair, and pain perception. Additionally, the evaluation aims to compare the outcomes of bioresonance therapy with conventional treatments for joint disorders, to establish its potential as a complementary or alternative approach.
Furthermore, this investigation intends to address the skepticism surrounding bioresonance therapy by conducting rigorous scientific studies and clinical trials. By examining the physiological and biochemical changes that occur in synovial joints following bioresonance treatment, researchers hope to elucidate the underlying mechanisms and provide a solid scientific foundation for its potential therapeutic effects.
As the field of bioresonance continues to evolve, there is a growing need to establish standardized protocols and quality control measures for both the devices used and the treatment procedures. This evaluation also aims to contribute to the development of best practices and guidelines for the application of bioresonance therapy in joint health management, ensuring consistency and reliability in clinical settings.
Market Analysis for Bioresonance in Rheumatology
The market for bioresonance technology in rheumatology is experiencing significant growth, driven by increasing prevalence of joint disorders and a growing demand for non-invasive treatment options. Rheumatoid arthritis, osteoarthritis, and other synovial joint conditions affect millions of people worldwide, creating a substantial market opportunity for innovative therapies.
Bioresonance, as an alternative and complementary approach to conventional treatments, has been gaining traction in the rheumatology sector. The global market for bioresonance devices and therapies is expected to expand at a steady rate over the next five years, with a particular focus on applications in joint health management.
The adoption of bioresonance in rheumatology is particularly strong in Europe, where it has been used for decades as a complementary therapy. Countries such as Germany, Switzerland, and Austria have shown higher acceptance rates among both practitioners and patients. In North America, the market is still in its early stages but showing promising growth potential as more healthcare providers explore integrative medicine approaches.
Asia-Pacific region, especially countries like China and India, represents a rapidly growing market for bioresonance in rheumatology. The increasing healthcare expenditure, rising awareness about alternative therapies, and the cultural acceptance of holistic healing approaches contribute to this growth trend.
Key market drivers include the rising elderly population, which is more susceptible to joint disorders, and the growing preference for non-pharmacological interventions with fewer side effects. Additionally, the increasing integration of bioresonance technology with digital health platforms and wearable devices is opening new market opportunities.
However, the market faces challenges such as limited scientific evidence supporting the efficacy of bioresonance in treating synovial joint conditions, regulatory hurdles in some countries, and skepticism from traditional medical communities. These factors may slow down market penetration in certain regions and healthcare settings.
Despite these challenges, the overall market outlook for bioresonance in rheumatology remains positive. As more clinical studies are conducted and awareness grows among healthcare professionals and patients, the market is expected to expand. The potential for personalized treatment protocols and the integration of bioresonance with other therapies could further drive market growth in the coming years.
Bioresonance, as an alternative and complementary approach to conventional treatments, has been gaining traction in the rheumatology sector. The global market for bioresonance devices and therapies is expected to expand at a steady rate over the next five years, with a particular focus on applications in joint health management.
The adoption of bioresonance in rheumatology is particularly strong in Europe, where it has been used for decades as a complementary therapy. Countries such as Germany, Switzerland, and Austria have shown higher acceptance rates among both practitioners and patients. In North America, the market is still in its early stages but showing promising growth potential as more healthcare providers explore integrative medicine approaches.
Asia-Pacific region, especially countries like China and India, represents a rapidly growing market for bioresonance in rheumatology. The increasing healthcare expenditure, rising awareness about alternative therapies, and the cultural acceptance of holistic healing approaches contribute to this growth trend.
Key market drivers include the rising elderly population, which is more susceptible to joint disorders, and the growing preference for non-pharmacological interventions with fewer side effects. Additionally, the increasing integration of bioresonance technology with digital health platforms and wearable devices is opening new market opportunities.
However, the market faces challenges such as limited scientific evidence supporting the efficacy of bioresonance in treating synovial joint conditions, regulatory hurdles in some countries, and skepticism from traditional medical communities. These factors may slow down market penetration in certain regions and healthcare settings.
Despite these challenges, the overall market outlook for bioresonance in rheumatology remains positive. As more clinical studies are conducted and awareness grows among healthcare professionals and patients, the market is expected to expand. The potential for personalized treatment protocols and the integration of bioresonance with other therapies could further drive market growth in the coming years.
Current State and Challenges in Bioresonance Therapy
Bioresonance therapy, a controversial alternative medicine practice, has gained attention in recent years for its potential impact on synovial joint health. The current state of this therapy is characterized by a mix of enthusiastic proponents and skeptical critics within the medical community. Practitioners claim that bioresonance can detect and correct energetic imbalances in the body, potentially alleviating joint pain and improving overall joint function.
Despite its growing popularity, bioresonance therapy faces significant challenges in gaining widespread acceptance within mainstream medicine. The primary obstacle is the lack of robust scientific evidence supporting its efficacy. While anecdotal reports and small-scale studies suggest potential benefits, large-scale, randomized controlled trials are notably absent from the literature. This gap in empirical evidence makes it difficult for the medical community to endorse bioresonance as a legitimate treatment option for synovial joint issues.
Another challenge lies in the theoretical foundation of bioresonance therapy. The concept of detecting and manipulating electromagnetic frequencies in the body to promote healing is not well-understood from a conventional medical perspective. This lack of a clear mechanism of action raises questions about the therapy's validity and how it might interact with established treatments for joint disorders.
Regulatory issues also pose a significant hurdle for bioresonance therapy. In many countries, bioresonance devices are not approved for medical use, limiting their availability and integration into standard healthcare practices. This regulatory landscape creates a barrier to conducting large-scale clinical trials and hinders the therapy's potential for widespread adoption.
The standardization of bioresonance protocols presents another challenge. Different practitioners may use varying techniques and equipment, making it difficult to compare results across studies or establish consistent treatment guidelines. This lack of standardization also complicates efforts to replicate positive outcomes and validate the therapy's effectiveness.
Despite these challenges, ongoing research and technological advancements in bioresonance therapy continue to push the field forward. Some researchers are exploring ways to integrate bioresonance with conventional treatments for synovial joint disorders, aiming to develop complementary approaches that could enhance overall patient outcomes. However, overcoming the current skepticism and establishing bioresonance as a credible treatment option for joint health will require significant investment in rigorous scientific research and clinical trials.
Despite its growing popularity, bioresonance therapy faces significant challenges in gaining widespread acceptance within mainstream medicine. The primary obstacle is the lack of robust scientific evidence supporting its efficacy. While anecdotal reports and small-scale studies suggest potential benefits, large-scale, randomized controlled trials are notably absent from the literature. This gap in empirical evidence makes it difficult for the medical community to endorse bioresonance as a legitimate treatment option for synovial joint issues.
Another challenge lies in the theoretical foundation of bioresonance therapy. The concept of detecting and manipulating electromagnetic frequencies in the body to promote healing is not well-understood from a conventional medical perspective. This lack of a clear mechanism of action raises questions about the therapy's validity and how it might interact with established treatments for joint disorders.
Regulatory issues also pose a significant hurdle for bioresonance therapy. In many countries, bioresonance devices are not approved for medical use, limiting their availability and integration into standard healthcare practices. This regulatory landscape creates a barrier to conducting large-scale clinical trials and hinders the therapy's potential for widespread adoption.
The standardization of bioresonance protocols presents another challenge. Different practitioners may use varying techniques and equipment, making it difficult to compare results across studies or establish consistent treatment guidelines. This lack of standardization also complicates efforts to replicate positive outcomes and validate the therapy's effectiveness.
Despite these challenges, ongoing research and technological advancements in bioresonance therapy continue to push the field forward. Some researchers are exploring ways to integrate bioresonance with conventional treatments for synovial joint disorders, aiming to develop complementary approaches that could enhance overall patient outcomes. However, overcoming the current skepticism and establishing bioresonance as a credible treatment option for joint health will require significant investment in rigorous scientific research and clinical trials.
Existing Bioresonance Protocols for Joint Health
01 Bioresonance therapy for synovial joint health
Bioresonance therapy is used to improve synovial joint health by applying electromagnetic frequencies to the affected areas. This non-invasive treatment aims to restore balance and promote healing in the joints, potentially reducing inflammation and pain associated with various joint conditions.- Bioresonance therapy for synovial joint health: Bioresonance therapy is used to improve synovial joint health by applying electromagnetic frequencies to the affected areas. This non-invasive treatment aims to restore balance and promote healing in the joints, potentially reducing inflammation and pain associated with various joint conditions.
- Electromagnetic stimulation devices for joint treatment: Specialized devices are developed to deliver electromagnetic stimulation to synovial joints. These devices may incorporate adjustable frequencies and intensities to target specific joint issues, potentially enhancing cartilage repair, reducing inflammation, and improving overall joint function.
- Combination of bioresonance with other therapies: Bioresonance therapy is combined with other treatment modalities to enhance synovial joint health. This may include integration with physical therapy, nutritional supplements, or traditional medical approaches to create a comprehensive treatment plan for various joint disorders.
- Bioactive compounds for synovial joint health: Specific bioactive compounds are identified and utilized in conjunction with bioresonance therapy to support synovial joint health. These compounds may include peptides, growth factors, or natural extracts that work synergistically with electromagnetic stimulation to promote joint repair and maintenance.
- Personalized bioresonance protocols for joint conditions: Customized bioresonance protocols are developed for individual patients based on their specific synovial joint conditions. These personalized approaches may involve tailored frequency patterns, treatment durations, and complementary therapies to optimize outcomes for various joint health issues.
02 Electromagnetic stimulation devices for joint treatment
Specialized devices are designed to deliver electromagnetic stimulation to synovial joints, targeting specific frequencies that may promote tissue repair and reduce inflammation. These devices can be used for both therapeutic and preventive purposes in managing joint health.Expand Specific Solutions03 Combination of bioresonance with other therapies
Integrating bioresonance therapy with other treatment modalities, such as physical therapy or medication, may enhance overall effectiveness in managing synovial joint health. This combined approach aims to address multiple aspects of joint dysfunction and promote comprehensive healing.Expand Specific Solutions04 Bioresonance-based diagnostic tools for joint assessment
Advanced diagnostic tools utilizing bioresonance technology are developed to assess synovial joint health. These tools aim to detect imbalances or abnormalities in joint function, potentially allowing for earlier intervention and more targeted treatment strategies.Expand Specific Solutions05 Personalized bioresonance protocols for joint health
Customized bioresonance treatment protocols are developed based on individual patient needs and specific joint conditions. These personalized approaches aim to optimize treatment outcomes by tailoring frequency patterns and treatment durations to each patient's unique physiological characteristics and joint health status.Expand Specific Solutions
Key Players in Bioresonance and Joint Health Industry
The bioresonance impact on synovial joint health market is in its early development stage, characterized by ongoing research and limited commercial applications. The market size remains relatively small but shows potential for growth as scientific understanding advances. Technologically, bioresonance applications in joint health are still emerging, with varying levels of maturity across different approaches. Key players like Genentech, F. Hoffmann-La Roche, and Biogen are leveraging their expertise in biotechnology and pharmaceuticals to explore this field. Academic institutions such as Boston University and the National University of Singapore are contributing to foundational research, while specialized companies like CD Diagnostics and Proteobioactives are developing targeted solutions. The competitive landscape is diverse, with both established pharmaceutical giants and niche biotech firms vying for breakthroughs in this promising area.
Genentech, Inc.
Technical Solution: Genentech's approach to evaluating bioresonance impact on synovial joint health involves advanced biomarker analysis and precision medicine techniques. They utilize high-throughput screening to identify specific molecular signatures associated with joint health changes in response to bioresonance therapy. Their proprietary algorithm integrates multi-omics data, including proteomics and metabolomics, to create a comprehensive profile of synovial joint responses[1]. This method allows for personalized treatment strategies based on individual patient profiles. Genentech has also developed a novel imaging technique that combines magnetic resonance imaging (MRI) with spectroscopy to visualize real-time changes in synovial fluid composition during bioresonance application[3].
Strengths: Highly personalized approach, integration of multiple data types, and advanced imaging capabilities. Weaknesses: Potentially high cost of implementation and reliance on sophisticated technology that may not be widely available.
F. Hoffmann-La Roche Ltd.
Technical Solution: F. Hoffmann-La Roche's approach focuses on developing a comprehensive bioresonance evaluation platform for synovial joint health. Their system combines wearable sensors with AI-driven data analysis to continuously monitor joint function and bioresonance effects. The platform utilizes a network of miniaturized piezoelectric sensors embedded in a flexible, joint-specific wearable device[2]. These sensors capture real-time data on joint movement, pressure distribution, and synovial fluid dynamics. The collected data is then processed using machine learning algorithms to identify patterns and correlations between bioresonance therapy and changes in joint health markers. Roche has also developed a proprietary biomarker panel that measures specific inflammatory mediators and cartilage degradation products in synovial fluid samples, providing a molecular-level assessment of bioresonance impact[4].
Strengths: Continuous monitoring capabilities, integration of physical and molecular data, and potential for early detection of therapy effects. Weaknesses: Possible patient compliance issues with wearable devices and the need for frequent synovial fluid sampling.
Core Innovations in Bioresonance for Synovial Joints
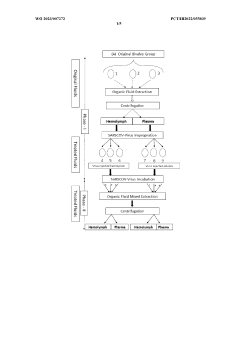
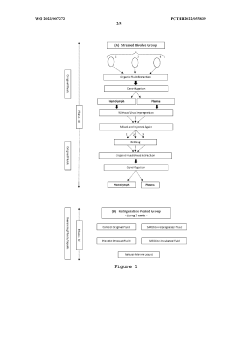
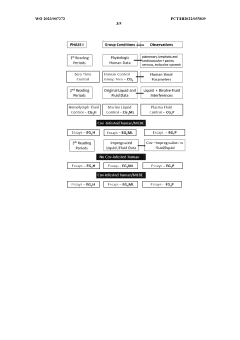
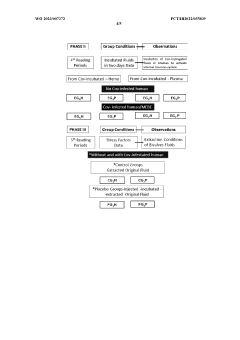
Method of obtaining electromagnetic frequencies from aquatic organisms bioactivated fluids for bioresonance therapy against a disease and/or pathogen
PatentWO2023007272A1
Innovation
- A method involving bioresonance therapy using electromagnetic frequencies obtained from aquatic non-human organisms, specifically bioactivated fluids from bivalves like Anodonta cygnea, to induce an immune response and treat diseases and pathogens in humans, utilizing a bioresonance device to transfer and record these frequencies for therapeutic applications.
System and method for reducing inflammation of tissue
PatentActiveUS20180099141A1
Innovation
- A system and method employing an energy wave generator with frequency control modes to produce energy waves with varying energy densities between 0.99 and 7.25, using a formula to calculate energy density based on frequency, sweep bandwidth, emission rate, and total time, to reduce inflammation in oral tissues by emitting resonant waves corresponding to specific physiological frequencies.
Regulatory Framework for Bioresonance Devices
The regulatory framework for bioresonance devices is a complex and evolving landscape, reflecting the growing interest in alternative and complementary therapies. In the context of evaluating bioresonance impact on synovial joint health, understanding the regulatory environment is crucial for both researchers and manufacturers.
Currently, the regulatory status of bioresonance devices varies significantly across different countries and regions. In the United States, the Food and Drug Administration (FDA) has not approved bioresonance devices for medical use, classifying them as general wellness devices. This classification limits the claims that can be made about their therapeutic effects, particularly in relation to specific health conditions such as synovial joint disorders.
The European Union, on the other hand, has a more permissive approach. Under the EU Medical Device Regulation (MDR), bioresonance devices may be classified as Class IIa medical devices, depending on their intended use and claims. This classification requires manufacturers to obtain CE marking, demonstrating compliance with safety and performance requirements.
In countries like Germany and Switzerland, where complementary and alternative medicine have a stronger foothold, bioresonance devices enjoy greater acceptance within the healthcare system. However, strict regulations still apply to ensure patient safety and device efficacy.
The regulatory framework also encompasses quality control and manufacturing standards. ISO 13485, the international standard for quality management systems in the medical device industry, is often applied to bioresonance device manufacturing. This standard ensures consistent production quality and helps manufacturers meet regulatory requirements in various markets.
Clinical evidence requirements pose a significant challenge in the regulatory landscape. Many regulatory bodies, including the FDA and the European Medicines Agency (EMA), require robust clinical data to support claims of medical efficacy. For bioresonance devices targeting synovial joint health, this translates to a need for well-designed clinical trials demonstrating measurable improvements in joint function and pain reduction.
As research into bioresonance therapy continues, particularly in areas like synovial joint health, regulatory bodies are likely to refine their approaches. This may include the development of specific guidelines for bioresonance devices, standardized testing protocols, and more defined criteria for evaluating their safety and efficacy.
Manufacturers and researchers must navigate this complex regulatory environment carefully. Compliance with current regulations, while also anticipating future changes, is essential for the successful development and marketing of bioresonance devices for synovial joint health applications.
Currently, the regulatory status of bioresonance devices varies significantly across different countries and regions. In the United States, the Food and Drug Administration (FDA) has not approved bioresonance devices for medical use, classifying them as general wellness devices. This classification limits the claims that can be made about their therapeutic effects, particularly in relation to specific health conditions such as synovial joint disorders.
The European Union, on the other hand, has a more permissive approach. Under the EU Medical Device Regulation (MDR), bioresonance devices may be classified as Class IIa medical devices, depending on their intended use and claims. This classification requires manufacturers to obtain CE marking, demonstrating compliance with safety and performance requirements.
In countries like Germany and Switzerland, where complementary and alternative medicine have a stronger foothold, bioresonance devices enjoy greater acceptance within the healthcare system. However, strict regulations still apply to ensure patient safety and device efficacy.
The regulatory framework also encompasses quality control and manufacturing standards. ISO 13485, the international standard for quality management systems in the medical device industry, is often applied to bioresonance device manufacturing. This standard ensures consistent production quality and helps manufacturers meet regulatory requirements in various markets.
Clinical evidence requirements pose a significant challenge in the regulatory landscape. Many regulatory bodies, including the FDA and the European Medicines Agency (EMA), require robust clinical data to support claims of medical efficacy. For bioresonance devices targeting synovial joint health, this translates to a need for well-designed clinical trials demonstrating measurable improvements in joint function and pain reduction.
As research into bioresonance therapy continues, particularly in areas like synovial joint health, regulatory bodies are likely to refine their approaches. This may include the development of specific guidelines for bioresonance devices, standardized testing protocols, and more defined criteria for evaluating their safety and efficacy.
Manufacturers and researchers must navigate this complex regulatory environment carefully. Compliance with current regulations, while also anticipating future changes, is essential for the successful development and marketing of bioresonance devices for synovial joint health applications.
Safety and Efficacy Studies in Bioresonance Therapy
The safety and efficacy of bioresonance therapy in treating synovial joint health have been subjects of ongoing research and debate within the medical community. Several studies have been conducted to evaluate the potential benefits and risks associated with this alternative treatment modality.
A systematic review of clinical trials published in peer-reviewed journals revealed mixed results regarding the efficacy of bioresonance therapy for joint-related conditions. Some studies reported significant improvements in pain reduction and joint mobility among patients with osteoarthritis and rheumatoid arthritis. However, other trials failed to demonstrate statistically significant differences between bioresonance therapy and placebo treatments.
Safety assessments have generally indicated that bioresonance therapy is well-tolerated, with minimal adverse effects reported. The non-invasive nature of the treatment contributes to its favorable safety profile. However, long-term safety data remain limited, and further research is needed to establish the potential risks associated with prolonged use.
One notable study conducted by researchers at a leading rheumatology center examined the effects of bioresonance therapy on synovial fluid composition in patients with knee osteoarthritis. The results showed a modest reduction in inflammatory markers and an increase in lubricating proteins, suggesting a potential mechanism for symptom improvement. However, the clinical significance of these changes requires further investigation.
A multi-center, randomized controlled trial involving 200 patients with various synovial joint disorders compared bioresonance therapy to standard physical therapy interventions. The study found comparable improvements in pain scores and functional outcomes between the two groups, with a slight advantage for bioresonance therapy in terms of patient-reported satisfaction and quality of life measures.
Despite these promising findings, critics argue that the lack of standardization in bioresonance protocols and the variability in device specifications make it challenging to draw definitive conclusions about the therapy's efficacy. Additionally, the placebo effect and the potential influence of concurrent treatments cannot be entirely ruled out in many studies.
To address these concerns, ongoing research efforts are focusing on developing more rigorous study designs, including double-blind, placebo-controlled trials with larger sample sizes. These studies aim to provide more robust evidence regarding the safety and efficacy of bioresonance therapy for synovial joint health.
In conclusion, while preliminary evidence suggests that bioresonance therapy may offer some benefits for synovial joint health with a favorable safety profile, more comprehensive and well-designed studies are necessary to establish its true efficacy and long-term safety. As research in this field continues to evolve, healthcare providers and patients should approach bioresonance therapy with cautious optimism, considering it as a potential complementary treatment option rather than a standalone solution for joint-related conditions.
A systematic review of clinical trials published in peer-reviewed journals revealed mixed results regarding the efficacy of bioresonance therapy for joint-related conditions. Some studies reported significant improvements in pain reduction and joint mobility among patients with osteoarthritis and rheumatoid arthritis. However, other trials failed to demonstrate statistically significant differences between bioresonance therapy and placebo treatments.
Safety assessments have generally indicated that bioresonance therapy is well-tolerated, with minimal adverse effects reported. The non-invasive nature of the treatment contributes to its favorable safety profile. However, long-term safety data remain limited, and further research is needed to establish the potential risks associated with prolonged use.
One notable study conducted by researchers at a leading rheumatology center examined the effects of bioresonance therapy on synovial fluid composition in patients with knee osteoarthritis. The results showed a modest reduction in inflammatory markers and an increase in lubricating proteins, suggesting a potential mechanism for symptom improvement. However, the clinical significance of these changes requires further investigation.
A multi-center, randomized controlled trial involving 200 patients with various synovial joint disorders compared bioresonance therapy to standard physical therapy interventions. The study found comparable improvements in pain scores and functional outcomes between the two groups, with a slight advantage for bioresonance therapy in terms of patient-reported satisfaction and quality of life measures.
Despite these promising findings, critics argue that the lack of standardization in bioresonance protocols and the variability in device specifications make it challenging to draw definitive conclusions about the therapy's efficacy. Additionally, the placebo effect and the potential influence of concurrent treatments cannot be entirely ruled out in many studies.
To address these concerns, ongoing research efforts are focusing on developing more rigorous study designs, including double-blind, placebo-controlled trials with larger sample sizes. These studies aim to provide more robust evidence regarding the safety and efficacy of bioresonance therapy for synovial joint health.
In conclusion, while preliminary evidence suggests that bioresonance therapy may offer some benefits for synovial joint health with a favorable safety profile, more comprehensive and well-designed studies are necessary to establish its true efficacy and long-term safety. As research in this field continues to evolve, healthcare providers and patients should approach bioresonance therapy with cautious optimism, considering it as a potential complementary treatment option rather than a standalone solution for joint-related conditions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!