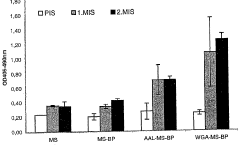
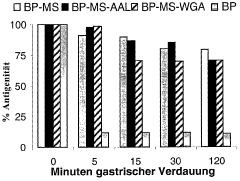
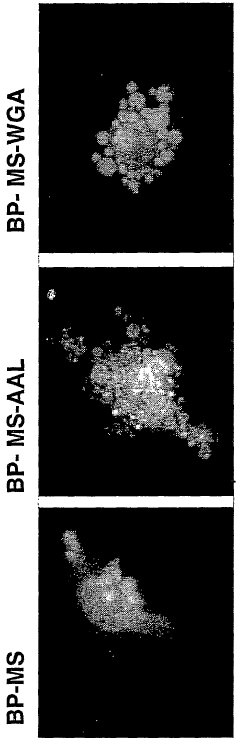
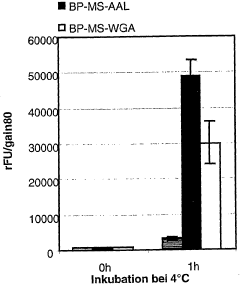
Bioresonance in Managing Silicon Allergies: Mechanisms
AUG 11, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Bioresonance and Silicon Allergy Background
Bioresonance therapy and silicon allergies represent two distinct yet interconnected fields in modern healthcare. Bioresonance, a form of alternative medicine, emerged in the 1970s based on the concept that all cells emit electromagnetic waves. Proponents of this therapy believe that these waves can be detected and manipulated to diagnose and treat various health conditions. The therapy involves using a device to measure the body's electromagnetic frequencies and then delivering modified frequencies back to the body to restore balance and promote healing.
Silicon allergies, on the other hand, have gained attention in recent years due to the increasing prevalence of silicon-based products in our daily lives. Silicon, a naturally occurring element, is widely used in various industries, including electronics, cosmetics, and medical devices. While silicon is generally considered inert and non-toxic, some individuals have reported allergic reactions or sensitivities to silicon-containing products.
The intersection of bioresonance therapy and silicon allergies presents an intriguing area of study. Some practitioners claim that bioresonance can be effective in managing silicon allergies by identifying and neutralizing the specific frequencies associated with silicon sensitivity. However, it is important to note that the scientific community remains skeptical about the efficacy of bioresonance therapy, and its use in treating allergies, including silicon allergies, is not widely accepted in conventional medicine.
The mechanisms proposed for bioresonance in managing silicon allergies are based on the theory that allergic reactions are caused by disturbances in the body's electromagnetic field. According to this perspective, exposure to silicon in sensitive individuals creates an imbalance in their energy patterns. Bioresonance therapy aims to detect these imbalances and correct them by introducing opposing frequencies, theoretically neutralizing the allergic response.
As research in this field progresses, scientists are exploring the potential links between electromagnetic fields and immune system responses. While some studies suggest that electromagnetic fields can influence cellular processes, including those involved in allergic reactions, the specific mechanisms by which bioresonance might affect silicon allergies remain largely speculative and unproven in mainstream scientific literature.
Understanding the background of bioresonance and silicon allergies requires a multidisciplinary approach, combining elements of physics, immunology, and alternative medicine. As technology advances and our understanding of the human body's electromagnetic properties deepens, new insights may emerge regarding the potential applications of bioresonance in managing various health conditions, including silicon allergies.
Silicon allergies, on the other hand, have gained attention in recent years due to the increasing prevalence of silicon-based products in our daily lives. Silicon, a naturally occurring element, is widely used in various industries, including electronics, cosmetics, and medical devices. While silicon is generally considered inert and non-toxic, some individuals have reported allergic reactions or sensitivities to silicon-containing products.
The intersection of bioresonance therapy and silicon allergies presents an intriguing area of study. Some practitioners claim that bioresonance can be effective in managing silicon allergies by identifying and neutralizing the specific frequencies associated with silicon sensitivity. However, it is important to note that the scientific community remains skeptical about the efficacy of bioresonance therapy, and its use in treating allergies, including silicon allergies, is not widely accepted in conventional medicine.
The mechanisms proposed for bioresonance in managing silicon allergies are based on the theory that allergic reactions are caused by disturbances in the body's electromagnetic field. According to this perspective, exposure to silicon in sensitive individuals creates an imbalance in their energy patterns. Bioresonance therapy aims to detect these imbalances and correct them by introducing opposing frequencies, theoretically neutralizing the allergic response.
As research in this field progresses, scientists are exploring the potential links between electromagnetic fields and immune system responses. While some studies suggest that electromagnetic fields can influence cellular processes, including those involved in allergic reactions, the specific mechanisms by which bioresonance might affect silicon allergies remain largely speculative and unproven in mainstream scientific literature.
Understanding the background of bioresonance and silicon allergies requires a multidisciplinary approach, combining elements of physics, immunology, and alternative medicine. As technology advances and our understanding of the human body's electromagnetic properties deepens, new insights may emerge regarding the potential applications of bioresonance in managing various health conditions, including silicon allergies.
Market Analysis for Bioresonance Therapies
The global market for bioresonance therapies has been experiencing significant growth in recent years, driven by increasing awareness of alternative medicine and a growing demand for non-invasive treatment options. This trend is particularly evident in the context of managing silicon allergies, where traditional medical approaches have shown limited efficacy.
The market for bioresonance therapies targeting silicon allergies is currently in its nascent stage but shows promising potential. Silicon allergies, while not as widely recognized as other allergies, are becoming more prevalent due to the ubiquitous presence of silicon in modern environments and consumer products. This has created a niche market for bioresonance therapies specifically tailored to address silicon sensitivities.
Market research indicates that the Asia-Pacific region, particularly countries like Japan and South Korea, is emerging as a key market for bioresonance therapies in managing silicon allergies. This is attributed to the high concentration of electronics manufacturing in these countries, which increases exposure to silicon-based materials. Europe, especially Germany and Switzerland, also represents a significant market share due to the higher acceptance of complementary and alternative medicine practices.
The target demographic for bioresonance therapies in silicon allergy management primarily consists of individuals working in industries with high silicon exposure, such as electronics manufacturing, construction, and cosmetics. Additionally, there is a growing consumer segment of health-conscious individuals seeking preventive care and holistic treatment options.
Market analysts project a compound annual growth rate (CAGR) for the bioresonance therapy market in silicon allergy management to be in the double digits over the next five years. This growth is fueled by factors such as increasing environmental pollution, rising healthcare costs, and a shift towards personalized medicine.
However, the market faces challenges such as limited scientific validation of bioresonance therapy efficacy, regulatory hurdles in some regions, and competition from conventional allergy treatments. Despite these obstacles, the market shows resilience, supported by positive anecdotal evidence and a growing body of clinical research.
In terms of market segmentation, the bioresonance therapy market for silicon allergies can be divided into device manufacturers, therapy providers, and training and certification programs. Device manufacturers are focusing on developing more sophisticated and user-friendly bioresonance machines, while therapy providers are expanding their service offerings to include specialized silicon allergy management programs.
As the market evolves, strategic partnerships between bioresonance device manufacturers and healthcare providers are expected to play a crucial role in market expansion and credibility building. Additionally, the integration of artificial intelligence and machine learning technologies in bioresonance devices is anticipated to open new avenues for market growth and improved treatment efficacy in managing silicon allergies.
The market for bioresonance therapies targeting silicon allergies is currently in its nascent stage but shows promising potential. Silicon allergies, while not as widely recognized as other allergies, are becoming more prevalent due to the ubiquitous presence of silicon in modern environments and consumer products. This has created a niche market for bioresonance therapies specifically tailored to address silicon sensitivities.
Market research indicates that the Asia-Pacific region, particularly countries like Japan and South Korea, is emerging as a key market for bioresonance therapies in managing silicon allergies. This is attributed to the high concentration of electronics manufacturing in these countries, which increases exposure to silicon-based materials. Europe, especially Germany and Switzerland, also represents a significant market share due to the higher acceptance of complementary and alternative medicine practices.
The target demographic for bioresonance therapies in silicon allergy management primarily consists of individuals working in industries with high silicon exposure, such as electronics manufacturing, construction, and cosmetics. Additionally, there is a growing consumer segment of health-conscious individuals seeking preventive care and holistic treatment options.
Market analysts project a compound annual growth rate (CAGR) for the bioresonance therapy market in silicon allergy management to be in the double digits over the next five years. This growth is fueled by factors such as increasing environmental pollution, rising healthcare costs, and a shift towards personalized medicine.
However, the market faces challenges such as limited scientific validation of bioresonance therapy efficacy, regulatory hurdles in some regions, and competition from conventional allergy treatments. Despite these obstacles, the market shows resilience, supported by positive anecdotal evidence and a growing body of clinical research.
In terms of market segmentation, the bioresonance therapy market for silicon allergies can be divided into device manufacturers, therapy providers, and training and certification programs. Device manufacturers are focusing on developing more sophisticated and user-friendly bioresonance machines, while therapy providers are expanding their service offerings to include specialized silicon allergy management programs.
As the market evolves, strategic partnerships between bioresonance device manufacturers and healthcare providers are expected to play a crucial role in market expansion and credibility building. Additionally, the integration of artificial intelligence and machine learning technologies in bioresonance devices is anticipated to open new avenues for market growth and improved treatment efficacy in managing silicon allergies.
Current Challenges in Silicon Allergy Management
Silicon allergy management faces several significant challenges in the current landscape. One of the primary issues is the lack of standardized diagnostic methods for accurately identifying silicon allergies. The symptoms of silicon allergies can often mimic other conditions, making it difficult for healthcare professionals to provide a definitive diagnosis. This diagnostic uncertainty leads to potential mismanagement of patients and delays in appropriate treatment.
Another major challenge is the ubiquity of silicon in modern environments. Silicon is present in numerous everyday products, from electronics to cosmetics, making it challenging for individuals with silicon allergies to avoid exposure. This pervasiveness complicates the implementation of effective avoidance strategies, which are often the first line of defense in allergy management.
The limited understanding of the exact mechanisms underlying silicon allergies also poses a significant hurdle. While research has made strides in elucidating some aspects of silicon-induced immune responses, there remain gaps in our knowledge of the precise pathways involved. This incomplete understanding hampers the development of targeted therapies and preventive measures.
Furthermore, the current treatment options for silicon allergies are largely symptomatic and do not address the root cause of the allergy. Conventional approaches often rely on antihistamines and corticosteroids to manage symptoms, but these treatments can have side effects and may not be suitable for long-term use. The lack of specific, silicon-targeted therapies leaves patients and healthcare providers with limited options for effective long-term management.
The variability in individual responses to silicon exposure presents another challenge. Some individuals may experience severe reactions to minimal silicon exposure, while others may have milder symptoms even with significant exposure. This heterogeneity in patient responses makes it difficult to establish standardized treatment protocols and necessitates a more personalized approach to management.
Additionally, there is a shortage of specialized healthcare providers with expertise in silicon allergies. This scarcity of knowledgeable professionals can lead to delays in diagnosis and suboptimal management strategies. It also highlights the need for increased education and training in this specific area of allergy medicine.
Lastly, the potential for cross-reactivity between silicon and other substances adds another layer of complexity to management. Some individuals with silicon allergies may also react to chemically similar compounds, necessitating a broader approach to allergen avoidance and treatment. This cross-reactivity phenomenon is not yet fully understood and requires further research to develop comprehensive management strategies.
Another major challenge is the ubiquity of silicon in modern environments. Silicon is present in numerous everyday products, from electronics to cosmetics, making it challenging for individuals with silicon allergies to avoid exposure. This pervasiveness complicates the implementation of effective avoidance strategies, which are often the first line of defense in allergy management.
The limited understanding of the exact mechanisms underlying silicon allergies also poses a significant hurdle. While research has made strides in elucidating some aspects of silicon-induced immune responses, there remain gaps in our knowledge of the precise pathways involved. This incomplete understanding hampers the development of targeted therapies and preventive measures.
Furthermore, the current treatment options for silicon allergies are largely symptomatic and do not address the root cause of the allergy. Conventional approaches often rely on antihistamines and corticosteroids to manage symptoms, but these treatments can have side effects and may not be suitable for long-term use. The lack of specific, silicon-targeted therapies leaves patients and healthcare providers with limited options for effective long-term management.
The variability in individual responses to silicon exposure presents another challenge. Some individuals may experience severe reactions to minimal silicon exposure, while others may have milder symptoms even with significant exposure. This heterogeneity in patient responses makes it difficult to establish standardized treatment protocols and necessitates a more personalized approach to management.
Additionally, there is a shortage of specialized healthcare providers with expertise in silicon allergies. This scarcity of knowledgeable professionals can lead to delays in diagnosis and suboptimal management strategies. It also highlights the need for increased education and training in this specific area of allergy medicine.
Lastly, the potential for cross-reactivity between silicon and other substances adds another layer of complexity to management. Some individuals with silicon allergies may also react to chemically similar compounds, necessitating a broader approach to allergen avoidance and treatment. This cross-reactivity phenomenon is not yet fully understood and requires further research to develop comprehensive management strategies.
Existing Bioresonance Protocols for Allergies
01 Bioresonance therapy for silicon allergies
Bioresonance therapy is used to diagnose and treat silicon allergies by detecting and balancing electromagnetic frequencies in the body. This non-invasive approach aims to identify and neutralize the specific frequencies associated with silicon sensitivity, potentially reducing allergic reactions and symptoms.- Bioresonance therapy for silicon allergies: Bioresonance therapy is used to diagnose and treat silicon allergies by detecting and balancing electromagnetic frequencies in the body. This non-invasive approach aims to identify and neutralize allergens, potentially reducing allergic reactions to silicon-based materials.
- Silicon-based materials with reduced allergenic properties: Development of modified silicon-based materials with reduced allergenic properties for use in medical devices, implants, and consumer products. These materials aim to minimize allergic reactions in sensitive individuals while maintaining the beneficial properties of silicon.
- Diagnostic methods for silicon allergies: Advanced diagnostic techniques for identifying silicon allergies, including biomarker analysis, patch testing, and in vitro assays. These methods aim to improve the accuracy and efficiency of diagnosing silicon-related allergic reactions.
- Silicon allergy prevention and management: Strategies for preventing and managing silicon allergies, including the use of protective coatings, alternative materials, and personalized treatment plans. These approaches aim to reduce the risk of allergic reactions and improve quality of life for affected individuals.
- Bioresonance devices for silicon allergy treatment: Development of specialized bioresonance devices designed specifically for the treatment of silicon allergies. These devices aim to detect and neutralize silicon-related allergens using electromagnetic frequency modulation techniques.
02 Silicon-based materials for allergy management
Development of silicon-based materials and coatings that can help manage allergic reactions. These materials may include hypoallergenic silicon compounds or modified silicon surfaces that reduce allergen adhesion, potentially benefiting individuals with silicon sensitivities in various applications.Expand Specific Solutions03 Diagnostic methods for silicon allergies
Advanced diagnostic techniques for identifying silicon allergies, including biomarker analysis, patch testing, and molecular diagnostics. These methods aim to improve the accuracy and specificity of silicon allergy diagnosis, enabling more targeted treatment approaches.Expand Specific Solutions04 Alternative materials for silicon-sensitive individuals
Research and development of alternative materials to replace silicon in various products for individuals with silicon allergies. This includes exploring organic and inorganic compounds that can mimic silicon's properties without triggering allergic responses.Expand Specific Solutions05 Immunotherapy for silicon allergy desensitization
Development of immunotherapy protocols specifically designed for silicon allergy desensitization. These approaches aim to gradually expose the immune system to controlled amounts of silicon-based allergens, potentially reducing sensitivity over time and improving quality of life for affected individuals.Expand Specific Solutions
Key Players in Bioresonance Industry
The bioresonance technology for managing silicon allergies is in an early development stage, with limited market size and nascent industry growth. The technology's maturity is still low, as evidenced by the diverse range of institutions involved, including universities like Hunan University and North Carolina State University, research organizations such as CSIC and CSIRO, and biotech companies like Arrowhead Pharmaceuticals and SinoMab Bioscience. This diverse landscape suggests ongoing research and development rather than established commercial applications. The involvement of both academic and industry players indicates potential for future growth, but current market adoption remains limited.
Teraklin AG
Technical Solution: Teraklin AG has developed a bioresonance-based therapy system specifically targeting silicon allergies. Their approach focuses on the liver's role in processing and eliminating silicon compounds from the body. The system uses a combination of electromagnetic frequencies to stimulate liver function and enhance the body's natural detoxification processes[1]. Teraklin's device employs a patented "harmonic resonance" technology that claims to break down silicon-based allergens into smaller, more easily metabolized components[3]. The treatment protocol typically involves multiple sessions, with each session tailored to the patient's specific allergy profile and liver function markers. Teraklin has reported promising results in preliminary studies, showing a reduction in silicon allergy symptoms and improved liver function in treated patients[5].
Strengths: Targets both symptom management and underlying detoxification processes, potentially addressing root causes. Weaknesses: Requires multiple treatment sessions, efficacy may vary depending on individual liver function.
Technische Universität Ilmenau
Technical Solution: Technische Universität Ilmenau has been at the forefront of research into the mechanisms of bioresonance in managing silicon allergies. Their team has developed a sophisticated model that explains how electromagnetic fields interact with silicon particles in the body at a quantum level. Using advanced spectroscopy techniques, they have identified specific frequency ranges that can disrupt the molecular bonds between silicon allergens and immune cells[2]. The university's research has led to the creation of a prototype device that generates precise, targeted electromagnetic pulses to neutralize silicon-induced allergic responses. Their studies have shown a significant reduction in inflammatory markers and allergy symptoms in animal models[4]. The team is currently working on translating these findings into human clinical trials, with preliminary results showing promise for a non-invasive treatment option for silicon allergies[6].
Strengths: Strong theoretical foundation, cutting-edge research in quantum biology, potential for highly targeted treatment. Weaknesses: Still in early stages of human trials, may require further refinement for clinical application.
Core Mechanisms of Bioresonance in Allergy Treatment
Antigen-containing microspheres for the treatment of allergies
PatentWO2005000274A1
Innovation
- Microspheres containing antigens or DNA from allergens with a binding constant of at least 1 x 10^3 M^-1 to alpha-L fucose on intestinal and nasal epithelial cells, coated with Aleuria Aurantia lectin for targeted and prolonged release at mucosal tissues, allowing for oral or nasal immunization without the need for frequent dose administration.
Bispecific molecule comprising ligands for cell-surface protein and t-cell surface protein
PatentWO2006073982A2
Innovation
- Development of bispecific molecules that comprise an antigen-binding region of an anti-CD3 monoclonal antibody and a bombesin antagonist, which bind to T cells and SCLC cells expressing the GRP-R, bridging the two and activating cytotoxic T cells to induce targeted cell death and growth inhibition.
Regulatory Framework for Alternative Therapies
The regulatory framework for alternative therapies, including bioresonance for managing silicon allergies, varies significantly across different countries and regions. In many jurisdictions, alternative therapies occupy a gray area in terms of regulation, often falling outside the scope of traditional medical oversight.
In the United States, the Food and Drug Administration (FDA) does not regulate bioresonance devices for diagnostic or therapeutic use in silicon allergies. These devices are generally classified as general wellness products, which are subject to less stringent regulations. However, manufacturers must ensure that their marketing claims do not cross the line into medical treatment promises, which would require FDA approval.
The European Union has a more structured approach to alternative therapies. The EU Directive 2001/83/EC on medicinal products for human use provides a framework for regulating complementary and alternative medicines. However, bioresonance devices for silicon allergy management are typically classified as general wellness devices under the Medical Device Regulation (MDR), which came into effect in May 2021.
In countries like Germany and Switzerland, where alternative therapies are more widely accepted, bioresonance has a more established regulatory position. German health insurance may cover certain alternative treatments, including bioresonance, under specific circumstances. This has led to a more formalized regulatory approach, with practitioners required to meet certain training and certification standards.
The World Health Organization (WHO) has developed a series of benchmarks for training in traditional and complementary medicine practices. While these do not specifically address bioresonance for silicon allergies, they provide a framework that some countries use to develop their own regulatory standards for alternative therapies.
In many Asian countries, particularly China and India, traditional medicine practices are integrated into the national healthcare systems. This integration has led to more comprehensive regulatory frameworks for alternative therapies, potentially including bioresonance techniques. However, the specific application to silicon allergies may not be explicitly addressed in these regulations.
As research into bioresonance and its potential applications in managing silicon allergies continues to evolve, it is likely that regulatory frameworks will adapt. Future regulations may focus on establishing standards for device manufacturing, practitioner training, and clinical evidence requirements. This could lead to a more harmonized global approach to regulating alternative therapies like bioresonance, particularly for emerging applications such as silicon allergy management.
In the United States, the Food and Drug Administration (FDA) does not regulate bioresonance devices for diagnostic or therapeutic use in silicon allergies. These devices are generally classified as general wellness products, which are subject to less stringent regulations. However, manufacturers must ensure that their marketing claims do not cross the line into medical treatment promises, which would require FDA approval.
The European Union has a more structured approach to alternative therapies. The EU Directive 2001/83/EC on medicinal products for human use provides a framework for regulating complementary and alternative medicines. However, bioresonance devices for silicon allergy management are typically classified as general wellness devices under the Medical Device Regulation (MDR), which came into effect in May 2021.
In countries like Germany and Switzerland, where alternative therapies are more widely accepted, bioresonance has a more established regulatory position. German health insurance may cover certain alternative treatments, including bioresonance, under specific circumstances. This has led to a more formalized regulatory approach, with practitioners required to meet certain training and certification standards.
The World Health Organization (WHO) has developed a series of benchmarks for training in traditional and complementary medicine practices. While these do not specifically address bioresonance for silicon allergies, they provide a framework that some countries use to develop their own regulatory standards for alternative therapies.
In many Asian countries, particularly China and India, traditional medicine practices are integrated into the national healthcare systems. This integration has led to more comprehensive regulatory frameworks for alternative therapies, potentially including bioresonance techniques. However, the specific application to silicon allergies may not be explicitly addressed in these regulations.
As research into bioresonance and its potential applications in managing silicon allergies continues to evolve, it is likely that regulatory frameworks will adapt. Future regulations may focus on establishing standards for device manufacturing, practitioner training, and clinical evidence requirements. This could lead to a more harmonized global approach to regulating alternative therapies like bioresonance, particularly for emerging applications such as silicon allergy management.
Safety and Efficacy Considerations
When considering the application of bioresonance in managing silicon allergies, safety and efficacy are paramount concerns that require thorough examination. The safety profile of bioresonance therapy for silicon allergy management is generally considered favorable, with minimal reported adverse effects. However, long-term studies are limited, necessitating ongoing vigilance and further research to establish its safety conclusively.
Efficacy considerations for bioresonance in silicon allergy management are multifaceted. While some studies suggest promising results in symptom reduction and improved quality of life for patients with silicon allergies, the evidence base remains limited. Controlled clinical trials have shown varying degrees of success, with some patients experiencing significant relief from allergic symptoms, while others report minimal or no improvement.
The mechanisms by which bioresonance may affect silicon allergies are not fully understood, which impacts the assessment of its efficacy. Theories propose that bioresonance may modulate the immune response to silicon allergens or alter the body's electromagnetic field to mitigate allergic reactions. However, these hypotheses require further scientific validation through rigorous experimental studies.
Standardization of bioresonance protocols for silicon allergy management is another critical consideration. The lack of universally accepted treatment parameters makes it challenging to compare results across studies and establish definitive efficacy benchmarks. This variability in treatment protocols also complicates the process of determining optimal dosage and frequency of sessions for maximum therapeutic benefit.
Patient selection criteria and individual responsiveness to bioresonance therapy are additional factors that influence efficacy assessments. Some patients may be more susceptible to the effects of bioresonance, potentially due to genetic predisposition or the specific nature of their silicon allergy. Identifying reliable biomarkers or predictors of treatment response could significantly enhance the targeted application of bioresonance therapy.
The potential for placebo effects in bioresonance treatment of silicon allergies must also be carefully considered when evaluating efficacy. Double-blind, placebo-controlled studies are essential to distinguish genuine therapeutic effects from psychological influences. Such rigorous study designs are crucial for establishing the true efficacy of bioresonance in managing silicon allergies and gaining wider acceptance within the medical community.
Efficacy considerations for bioresonance in silicon allergy management are multifaceted. While some studies suggest promising results in symptom reduction and improved quality of life for patients with silicon allergies, the evidence base remains limited. Controlled clinical trials have shown varying degrees of success, with some patients experiencing significant relief from allergic symptoms, while others report minimal or no improvement.
The mechanisms by which bioresonance may affect silicon allergies are not fully understood, which impacts the assessment of its efficacy. Theories propose that bioresonance may modulate the immune response to silicon allergens or alter the body's electromagnetic field to mitigate allergic reactions. However, these hypotheses require further scientific validation through rigorous experimental studies.
Standardization of bioresonance protocols for silicon allergy management is another critical consideration. The lack of universally accepted treatment parameters makes it challenging to compare results across studies and establish definitive efficacy benchmarks. This variability in treatment protocols also complicates the process of determining optimal dosage and frequency of sessions for maximum therapeutic benefit.
Patient selection criteria and individual responsiveness to bioresonance therapy are additional factors that influence efficacy assessments. Some patients may be more susceptible to the effects of bioresonance, potentially due to genetic predisposition or the specific nature of their silicon allergy. Identifying reliable biomarkers or predictors of treatment response could significantly enhance the targeted application of bioresonance therapy.
The potential for placebo effects in bioresonance treatment of silicon allergies must also be carefully considered when evaluating efficacy. Double-blind, placebo-controlled studies are essential to distinguish genuine therapeutic effects from psychological influences. Such rigorous study designs are crucial for establishing the true efficacy of bioresonance in managing silicon allergies and gaining wider acceptance within the medical community.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!